Mice and Habitat Complexity Attract Carnivorans to Recently Burnt Forests
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Focal Species
2.3. Sampling Design and Field Methods
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
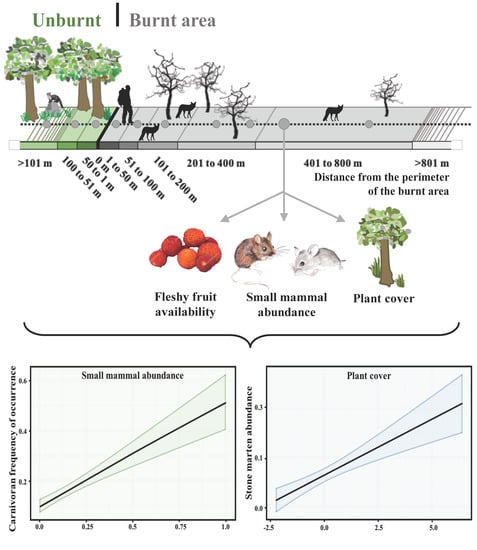
- Immediately after fire, carnivorans were more frequent close to the burnt area perimeter, where fire severity was low, and in places with greater small mammal abundance.
- Red fox occurrence in the burnt area surroundings increased with increasing small mammal abundance and plant cover, and with time-since-fire in the burnt area.
- Stone martens were found around the burnt area perimeter, probably because of their preference for high plant cover, and they were not significantly affected by small mammal abundance.
- The red fox apparently did not affect stone marten occurrence.
- Red fox and stone marten occurrence were not significantly related to fleshy fruit availability.
- Carnivoran responses to fire may be influenced, directly and indirectly, by habitat structure and resource availability.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bond, W.J.; Keeley, J.E. Fire as a global ‘herbivore’: The ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Pastro, L.A.; Dickman, C.R.; Letnic, M. Fire type and hemisphere determine the effects of fire on the alpha and beta diversity of vertebrates: A global meta-analysis. Glob. Ecol. Biogeogr. 2014, 23, 1146–1156. [Google Scholar] [CrossRef] [Green Version]
- Pausas, J.G.; Keeley, J.E. A burning story: The role of fire in the history of life. BioScience 2009, 59, 593–601. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Hostetler, J.A.; Oli, M.K.; Conner, L.M. Effects of predation, fire, and supplemental feeding on populations of two species of Peromyscus mice. J. Mammal. 2011, 92, 934–944. [Google Scholar] [CrossRef] [Green Version]
- Pausas, J.G. Generalized fire response strategies in plants and animals. Oikos 2019, 128, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Pons, P.; Bas, J.M. Open-habitat birds in recently burned areas: The role of the fire extent and species’ habitat breadth. Ardeola 2005, 52, 119–131. [Google Scholar]
- Santos, X.; Mateos, E.; Bros, V.; Brotons, L.; De Mas, E.; Herraiz, J.A.; Herrando, S.; Miño, À.; Olmo-Vidal, J.M.; Quesada, J. Is response to fire influenced by dietary specialization and mobility? A comparative study with multiple animal assemblages. PLoS ONE 2014, 9, e88224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geary, W.L.; Doherty, T.S.; Nimmo, D.G.; Tulloch, A.I.; Ritchie, E.G. Predator responses to fire: A global systematic review and meta-analysis. J. Anim. Ecol. 2019, 89, 955–971. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, D.A.; Lindenmayer, D.B.; Bennett, A.F.; Bode, M.; Bradstock, R.A.; Cary, G.J.; Clarke, M.F.; Dexter, N.; Fensham, R.; Friend, G. Fire management for biodiversity conservation: Key research questions and our capacity to answer them. Biol. Conserv. 2010, 143, 1928–1939. [Google Scholar] [CrossRef]
- Spies, T.A.; Lindenmayer, D.B.; Gill, A.M.; Stephens, S.L.; Agee, J.K. Challenges and a checklist for biodiversity conservation in fire-prone forests: Perspectives from the Pacific Northwest of USA and Southeastern Australia. Biol. Conserv. 2012, 145, 5–14. [Google Scholar] [CrossRef]
- Winnie, J.A.; Creel, S. The many effects of carnivores on their prey and their implications for trophic cascades, and ecosystem structure and function. Food Webs 2017, 12, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Lino, S.; Sillero, N.; Torres, J.; Santos, X.; Álvares, F. The role of fire on wolf distribution and breeding-site selection: Insights from a generalist carnivore occurring in a fire-prone landscape. Landsc. Urban Plan. 2019, 183, 111–121. [Google Scholar] [CrossRef]
- Birtsas, P.; Sokos, C.; Exadactylos, S. Carnivores in burned and adjacent unburned areas in a Mediterranean ecosystem. Mammalia 2012, 76, 407–415. [Google Scholar] [CrossRef]
- Ruiz-Capillas, P.; Mata, C.; Malo, J.E. Community response of mammalian predators and their prey to motorways: Implications for predator–prey dynamics. Ecosystems 2013, 16, 617–626. [Google Scholar] [CrossRef]
- Rost, J.; Pons, P.; Bas, J.M. Seed dispersal by carnivorous mammals into burnt forests: An opportunity for non-indigenous and cultivated plant species. Basic Appl. Ecol. 2012, 13, 623–630. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Kirkendall, L.; Ballard, W. Gray fox and coyote abundance and diet responses after a wildfire in central Arizona. West. N. Am. Nat. 2006, 66, 169–181. [Google Scholar] [CrossRef]
- Thompson, C.M.; Augustine, D.J.; Mayers, D.M. Swift fox response to prescribed fire in shortgrass steppe. West. N. Am. Nat. 2008, 68, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Torre, I.; Díaz, M. Small mammal abundance in Mediterranean post-fire habitats: A role for predators? Acta Oecol. 2004, 25, 137–142. [Google Scholar] [CrossRef]
- Monimeau, L.; Mouillot, D.; Fons, R.; Prodon, R.; Marchand, B. Impact of prescribed burning on the survival rates of the wood mouse (Apodemus sylvaticus). Acta Oecol. 2002, 23, 51–58. [Google Scholar] [CrossRef]
- Puig-Gironès, R.; Clavero, M.; Pons, P. Importance of internal refuges and external unburnt area perimeter on the recovery of rodent populations after wildfire. Int. J. Wildland Fire 2018, 27, 425–436. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.; Baer-Keeley, M. Determinants of postfire recovery and succession in Mediterranean-climate shrublands of California. Ecol. Appl. 2005, 15, 1515–1534. [Google Scholar] [CrossRef] [Green Version]
- Puig-Gironès, R.; Brotons, L.; Pons, P. Aridity influences the recovery of Mediterranean shrubland birds after wildfire. PLoS ONE 2017, 12, e0173599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGregor, H.W.; Legge, S.; Jones, M.E.; Johnson, C.N. Extraterritorial hunting expeditions to intense fire scars by feral cats. Sci. Rep. 2016, 6, 22559. [Google Scholar] [CrossRef] [Green Version]
- McGregor, H.W.; Legge, S.; Jones, M.E.; Johnson, C.N. Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS ONE 2014, 9, e109097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eby, S.; Mosser, A.; Swanson, A.; Packer, C.; Ritchie, M. The impact of burning on lion Panthera leo habitat choice in an African savanna. Curr. Zool. 2013, 59, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Persson, L.; Eklov, P. Prey refuges affecting interactions between piscivorous perch and juvenile perch and roach. Ecology 1995, 76, 70–81. [Google Scholar] [CrossRef]
- Janssen, A.; Sabelis, M.W.; Magalhães, S.; Montserrat, M.; van der Hammen, T. Habitat structure affects intraguild predation. Ecology 2007, 88, 2713–2719. [Google Scholar] [CrossRef]
- Conner, L.M.; Castleberry, S.B.; Derrick, A.M. Effects of mesopredators and prescribed fire on hispid cotton rat survival and cause-specific mortality. J. Wildl. Manag. 2011, 75, 938–944. [Google Scholar] [CrossRef]
- Leahy, L.; Legge, S.M.; Tuft, K.; McGregor, H.W.; Barmuta, L.A.; Jones, M.E.; Johnson, C.N. Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildl. Res. 2016, 42, 705–716. [Google Scholar] [CrossRef]
- Puig-Gironès, R.; Imbeau, L.; Clavero, M.; Rost, J.; Pons, P. Does post-fire salvage logging affect foraging activity by rodents? Eur. J. Forest Res. 2020, 1–14. [Google Scholar] [CrossRef]
- Díaz-Ruiz, F.; Delibes-Mateos, M.; García-Moreno, J.L.; María López-Martín, J.; Ferreira, C.; Ferreras, P. Biogeographical patterns in the diet of an opportunistic predator: The red fox Vulpes vulpes in the Iberian Peninsula. Mammal Rev. 2013, 43, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Vergara, M.; Cushman, S.A.; Urra, F.; Ruiz-González, A. Shaken but not stirred: Multiscale habitat suitability modeling of sympatric marten species (Martes martes and Martes foina) in the northern Iberian Peninsula. Landsc. Ecol. 2016, 31, 1241–1260. [Google Scholar] [CrossRef]
- Cabezas-Díaz, S.; Virgós, E.; Lozano, J.; Mangas, J. Spatial distribution models in a frugivorous carnivore, the stone marten (Martes foina): Is the fleshy-fruit availability a useful predictor? Anim. Biol. 2010, 60, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Baghli, A.; Engel, E.; Verhagen, R. Feeding habits and trophic niche overlap of two sympatric Mustelidae, the polecatMustela putorius and the beech martenMartes foina. Z. Jagdwiss. 2002, 48, 217–225. [Google Scholar] [CrossRef]
- Rosalino, L.M.; Santos-Reis, M. Fruit consumption by carnivores in Mediterranean Europe. Mammal Rev. 2009, 39, 67–78. [Google Scholar] [CrossRef]
- Padial, J.; Avila, E.; Sanchez, J. Feeding habits and overlap among red fox (Vulpes vulpes) and stone marten (Martes foina) in two Mediterranean mountain habitats. Mamm. Biol. 2002, 67, 137–146. [Google Scholar] [CrossRef]
- Herrera, J.M.; de Sá Teixeira, I.; Rodríguez-Pérez, J.; Mira, A. Landscape structure shapes carnivore-mediated seed dispersal kernels. Landsc. Ecol. 2016, 31, 731–743. [Google Scholar] [CrossRef] [Green Version]
- Fernández, C.; Acosta, F.J.; Abellá, G.; López, F.; Díaz, M. Complex edge effect fields as additive processes in patches of ecological systems. Ecol. Model. 2002, 149, 273–283. [Google Scholar] [CrossRef]
- Departament d’Agricultura. Incendis Forestals Ocorreguts Entre El 1986–2019; Departament d’Agricultura a l’Institut Cartogràfic i Geològic de Catalunya: Barcelona, Spain, 2020. [Google Scholar]
- Sikes, R.S.; Gannon, W.L.; Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the american society of mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Slade, N.A.; Blair, S.M. An empirical test of using counts of individuals captured as indices of population size. J. Mammal. 2000, 81, 1035–1045. [Google Scholar] [CrossRef]
- Torre, I.; Arrizabalaga, A. Habitat preferences of the bank vole Myodes glareolus in a Mediterranean mountain range. Acta Theriol. 2008, 53, 241–250. [Google Scholar] [CrossRef]
- Prodon, R.; Lebreton, J.-D. Breeding avifauna of a Mediterranean succession: The holm oak and cork oak series in the eastern Pyrenees, 1. Analysis and modelling of the structure gradient. Oikos 1981, 37, 21–38. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology With R; Gail, M., Krickeberg, K., Samet, J.M., Tsiatis, A., Wong, W., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Fox, J.; Monette, G. Generalized collinearity diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.; Farrell, S. AIC model selection using Akaike weights. Psychon. B Rev. 2004, 11, 192–196. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation: Vienna, Austria, 2017. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bartoń, K. Multi-Model Inference (MuMIn), R package version 1.15.6; R Foundation: Vienna, Austria, 2016. [Google Scholar]
- Rosseel, Y. Lavaan: An R package for structural equation modeling and more. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Delibes-Mateos, M.; De Simon, J.F.; Villafuerte, R.; Ferreras, P. Feeding responses of the red fox (Vulpes vulpes) to different wild rabbit (Oryctolagus cuniculus) densities: A regional approach. Eur. J. Wildl. Res. 2008, 54, 71–78. [Google Scholar] [CrossRef]
- Ruiz-Olmo, J.; Palazon, S. Diet of the stone marten (Martes foina Erxleben, 1777) in northeastern Spain. Acta Vertebr. 1993, 20, 59–67. [Google Scholar]
- Posłuszny, M.; Pilot, M.; Goszczyński, J.; Gralak, B. Diet of sympatric pine marten (Martes martes) and stone marten (Martes foina) identified by genotyping of DNA from faeces. Ann. Zool. Fennici. 2007, 44, 269–284. [Google Scholar]
- Patton, D.R.; Gordon, J. Fire, Habitats, and Wildlife; USDA Forest Service: Flagstaff, AZ, USA, 1995; p. 85.
- Parsons, M.A.; Bridges, A.S.; Biteman, D.S.; Garcelon, D.K. Precipitation and prey abundance influence food habits of an invasive carnivore. Anim. Conserv. 2019. [Google Scholar] [CrossRef] [Green Version]
- Hradsky, B.A.; Penman, T.D.; Ababei, D.; Hanea, A.; Ritchie, E.G.; York, A.; Di Stefano, J. Bayesian networks elucidate interactions between fire and other drivers of terrestrial fauna distributions. Ecosphere 2017, 8, e01926. [Google Scholar] [CrossRef] [Green Version]
- Payne, C.J.; Ritchie, E.G.; Kelly, L.T.; Nimmo, D.G. Does fire influence the landscape-scale distribution of an invasive mesopredator? PLoS ONE 2014, 9, e107862. [Google Scholar] [CrossRef]
- Prigioni, C.; Balestrieri, A.; Remonti, L.; Cavada, L. Differential use of food and habitat by sympatric carnivores in the eastern Italian Alps. Ital. J. Zool. 2008, 75, 173–184. [Google Scholar] [CrossRef]
- Papakosta, M.; Bakaloudis, D.; Kitikidou, K.; Vlachos, C.; Chatzinikos, E. Dietary overlap among seasons and habitats of red fox and stone marten in Central Greece. Eur. J. Sci. Res. 2010, 45, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Barrull, J.; Mate, I.; Salicrú, M.; Palet, J.; Casanovas, J.; Gosàlbez, J.; Ruiz-Olmo, J. Differential response of a carnivore community to predator control: A spatio-temporal observational study. Ital. J. Zool. 2014, 81, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Pons, P.; Rost, J.; Tobella, C.; Puig-Gironès, R.; Bas, J.M.; Franch, M.; Mauri, E. Towards better practices of salvage logging for reducing the ecosystem impacts in Mediterranean burned forests. iForest 2020, in press. [Google Scholar] [CrossRef]
- Rollan, À.; Real, J. Effect of wildfires and post-fire forest treatments on rabbit abundance. Eur. J. Wildl. Res. 2011, 57, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, T.P.; Sullivan, D.S.; Lindgren, P.M.; Ransome, D.B. If we build habitat, will they come? Woody debris structures and conservation of forest mammals. J. Mammal. 2012, 93, 1456–1468. [Google Scholar] [CrossRef]
- Seip, C.R.; Hodder, D.P.; Crowley, S.M.; Johnson, C.J. Use of constructed coarse woody debris corridors in a clearcut by American martens (Martes americana) and their prey. Forestry 2018, 91, 506–513. [Google Scholar] [CrossRef]



| Species | Carnivoran Community | Red Fox (Vulpes Vulpes) | Stone Marten (Martes Foina) | Common Genet (Genetta Genetta) | Least Weasel (Mustela Nivalis) | European Badger (Males Meles) | |
|---|---|---|---|---|---|---|---|
| Number per Type | Feces | 189 | 103 | 85 | 1 | ||
| Active Latrines | 10 | 10 | |||||
| Footprints | 4 | 3 | 1 | ||||
| Captures | 4 | 4 | |||||
| Observations | 5 | 5 | |||||
| Number of Total Evidences | 212 | 111 | 85 | 10 | 4 | 2 | |
| Explanatory Variables | Carnivoran Community | Red Fox (Vulpes vulpes) | Stone Marten (Martes foina) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient ± SE | p | ω+ | Coefficient ± SE | p | ω+ | Coefficient ± SE | p | ω+ | |
| Intercept | −1.96 ± 0.21 | *** | −2.25 ± 0.24 | *** | −2.7 ± 0.29 | *** | |||
| Time-since-fire | −0.08 ± 0.04 | * | 1.0 | −0.25 ± 0.06 | *** | 1.0 | |||
| Distance from the burnt area perimeter | −0.29 ± 0.07 | *** | 1.0 | −0.26 ± 0.09 | ** | 1.0 | −0.16 ± 0.06 | ** | 1.0 |
| Time-distance interaction | 0.06 ± 0.01 | *** | 1.0 | 0.08 ± 0.02 | *** | 1.0 | |||
| Distance from closest refuge | −0.06 ± 0.07 | ns | 0.17 | ||||||
| Small-mammal abundance | 1.46 ± 0.35 | *** | 1.0 | 2.26 ± 0.47 | *** | 1.0 | 0.82 ± 0.58 | ns | 0.49 |
| Fleshy fruit availability | 0.02 ± 0.01 | ns | 0.53 | 0.008 ± 0.02 | ns | 0.21 | |||
| Plant cover (PC1) | 0.28 ± 0.08 | ** | 1.0 | ||||||
| Height of vegetation (PC2) | 0.14 ± 0.11 | ns | 0.37 | ||||||
| Red fox frequency of occurrence | 0.25 ± 0.25 | ns | 0.30 | ||||||
| Response Variable | Explanatory Variables | Standardized Effects | p |
|---|---|---|---|
| Flesh fruits availability | Distance from the burnt area perimeter → Plant cover | −0.10 | *** |
| Time-Since-Fire → Plant cover | 0.16 | *** | |
| Small-mammal abundance | Distance from the burnt area perimeter → Plant cover | −0.12 | *** |
| Time-Since-Fire → Plant cover | 0.19 | *** | |
| Carnivoran occurrence | Distance from the burnt area perimeter → Plant cover | −0.08 | ** |
| Distance from the burnt area perimeter → Small-mammals | −0.03 | * | |
| Time-Since-Fire → Plant cover | 0.12 | ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puig-Gironès, R.; Pons, P. Mice and Habitat Complexity Attract Carnivorans to Recently Burnt Forests. Forests 2020, 11, 855. https://doi.org/10.3390/f11080855
Puig-Gironès R, Pons P. Mice and Habitat Complexity Attract Carnivorans to Recently Burnt Forests. Forests. 2020; 11(8):855. https://doi.org/10.3390/f11080855
Chicago/Turabian StylePuig-Gironès, Roger, and Pere Pons. 2020. "Mice and Habitat Complexity Attract Carnivorans to Recently Burnt Forests" Forests 11, no. 8: 855. https://doi.org/10.3390/f11080855
APA StylePuig-Gironès, R., & Pons, P. (2020). Mice and Habitat Complexity Attract Carnivorans to Recently Burnt Forests. Forests, 11(8), 855. https://doi.org/10.3390/f11080855