Disentangling the Effects of Genotype and Environment on Growth and Wood Features of Balfourodendron riedelianum Trees by Common Garden Experiments in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species, Common Gardens, and Sampling
2.2. Wood Anatomical Procedures
2.3. Wood Data Collection
2.4. Scanning Electron Microscopy
2.5. Wood Density
2.6. Statistics and Transfer Functions
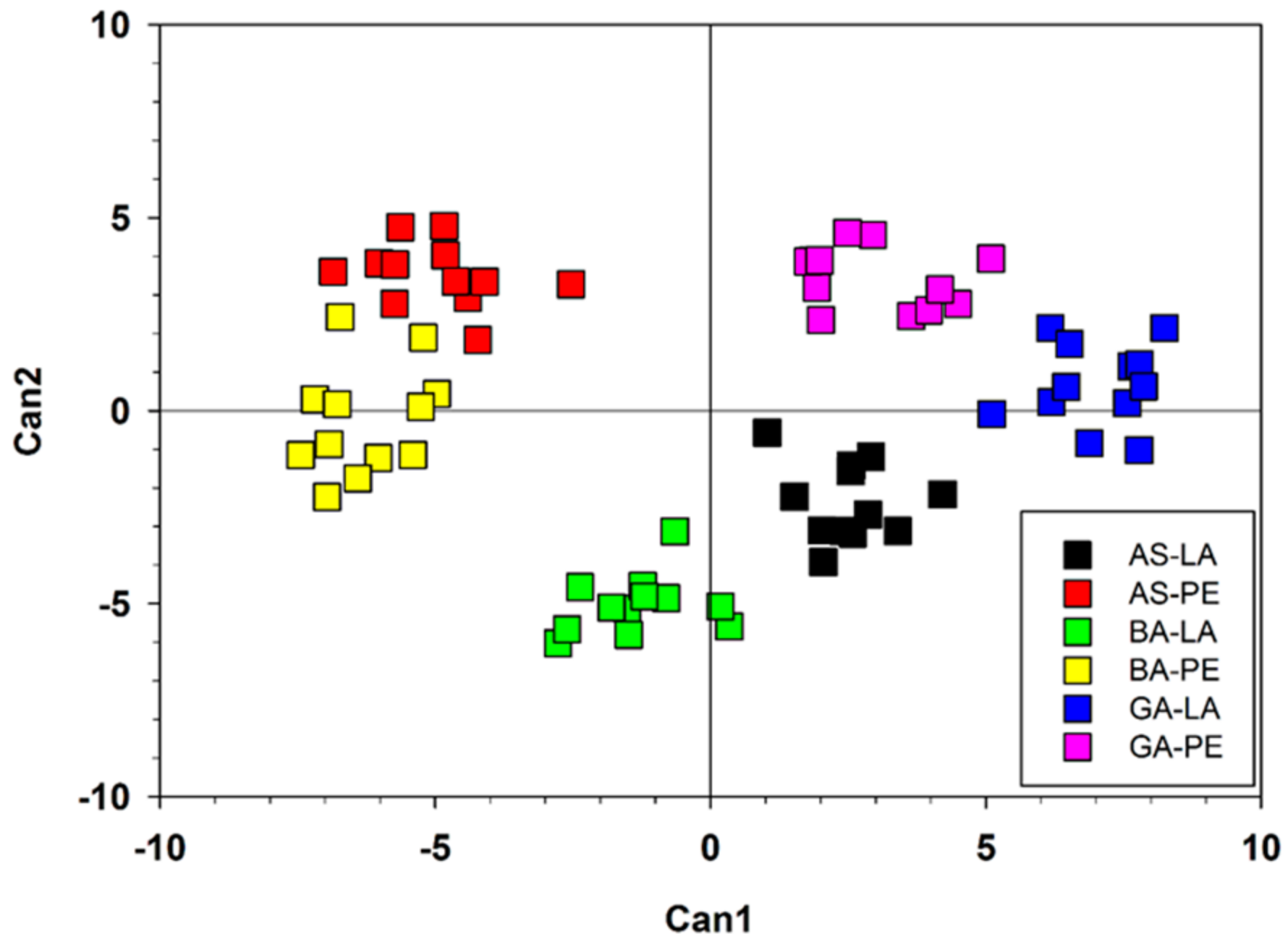
3. Results
3.1. Variation in Wood Features and Growth
3.2. Climate Parameters at the Provenance Origins and Trial Sites
3.3. Transfer Functions
4. Discussion
4.1. Genetic Difference between Populations
4.2. Phenotypic Plasticity of Tree Growth and Wood Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corcuera, L.; Cochard, H.; Gil-Pelegrin, E.; Notivol, E. Phenotypic plasticity in mesic populations of Pinus pinaster improves resistance to xylem embolism (P50) under severe drought. Trees Struct. Funct. 2011, 25, 1033–1042. [Google Scholar] [CrossRef]
- Anderegg, W.R.L. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytol. 2015, 205, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Wortemann, R.; Herbette, S.; Barigah, T.S.; Fumanal, B.; Alia, R.; Ducousso, A.; Gomory, D.; Roeckel-Drevet, P.; Cochard, H. Genotypic variability and phenotypic plasticity of cavitation resistance in Fagus sylvatica L. across Europe. Tree Physiol. 2011, 31, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- López, R.; Heredia, U.L.; Collada, C.; Cano, F.J.; Emerson, B.C.; Cochard, H.; Gil, L. Vulnerability to cavitation, hydraulic efficiency, growth and survival in an insular pine (Pinus canariensis). Ann. Bot. 2013, 111, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.B.; Goldstein, G.; Jones, T.J.; Cordell, S. Wood vessel diameter is related to elevation and genotype in the Hawaiian tree Metrosideros polymorpha (Myrtaceae). Am. J. Bot. 2007, 94, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.G.; Hacke, U.G.; Hamann, A. Variation of xylem vessel diameters across a climate gradient: Insight from a reciprocal transplant experiment with a widespread boreal tree. Funct. Ecol. 2015, 29, 1392–1401. [Google Scholar] [CrossRef]
- Hajek, P.; Kurjak, D.; von Wühlisch, G.; Delzon, S.; Schuldt, B. Intraspecific variation in wood anatomical, hydraulic, and foliar traits in ten European beech provenances differing in growth yield. Front. Plant Sci. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Eilmann, B.; Vries, S.M.G.D.; Ouden, J.D.; Mohren, G.M.J.; Sauren, P.; Sass-Klaassen, U. Origin matters! Difference in drought tolerance and productivity of coastal Douglas-fir (Pseudotsuga menziesii (Mirb.)) provenances. Forest Ecol. Manag. 2013, 302, 133–143. [Google Scholar] [CrossRef]
- O’Brien, E.K.; Mazanec, R.A.; Krauss, S.L. Provenance variation of ecologically important traits of forest trees: Implications for restoration. J. Appl. Ecol. 2007, 44, 583–593. [Google Scholar] [CrossRef]
- Baas, P.; Ewers, F.W.; Davis, S.D.; Wheeler, E.A. Evolution of xylem physiology. In The Evolution of Plant Physiology; Hemley, A.R., Poole, I., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; pp. 273–295. [Google Scholar]
- Carlquist, S. How wood evolves: A new synthesis. Botany 2012, 90, 901–940. [Google Scholar] [CrossRef]
- Baas, P.; Carlquist, S. A comparison of the ecological wood anatomy of the floras of Southern California and Israel. IAWA Bull. 1985, 6, 349–353. [Google Scholar] [CrossRef]
- Barajas-Morales, J. Wood structural differences between trees of two Tropical Forests in Mexico. IAWA J. 1985, 6, 355–364. [Google Scholar] [CrossRef]
- Lindorf, H. Eco-anatomical wood features of species from a very dry tropical forest. IAWA J. 1994, 15, 361–376. [Google Scholar] [CrossRef]
- Carlquist, S.; Hoekman, D.A. Ecological wood anatomy of the woody-Southern Californian flora. IAWA Bull. 1985, 6, 319–347. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S. Functional and ecological xylem anatomy. Perspect. Plant Ecol. Evol. Syst. 2001, 4, 97–115. [Google Scholar] [CrossRef]
- Carlquist, S. Ecological factors in wood evolution: A floristic approach. Am. J. Bot. 1977, 64, 887–895. [Google Scholar] [CrossRef]
- Tyree, M.T.; Davis, S.D.; Cochard, H. Biophysical perspectives of xylem evolution: Is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA J. 1994, 15, 335–360. [Google Scholar] [CrossRef]
- Morris, H.; Gillingham, M.A.F.; Plavcová, L.; Gleason, S.M.; Olson, M.E.; Coomes, D.A.; Fichtler, E.; Klepsch, M.M.; Martínez-Cabrera, H.I.; McGlinn, D.J.; et al. Vessel diameter is related to amount and spatial arrangement of axial parenchyma in woody angiosperms. Plant. Cell Environ. 2018, 41, 245–260. [Google Scholar] [CrossRef]
- Salleo, S.; Lo Gullo, M.A.; Trifiló, P.; Nardini, A. New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant Cell Environ. 2004, 27, 1065–1076. [Google Scholar] [CrossRef]
- Borchert, R.; Pockman, W.T. Water storage capacitance and xylem tension in isolated branches of temperate and tropical trees. Tree Physiol. 2005, 25, 457–466. [Google Scholar] [CrossRef]
- Poorter, L.; McDonald, I.; Alarcón, A.; Fichtler, E.; Licona, J.C.; Peña-Claros, M.; Sterck, F.; Villegas, Z.; Sass-Klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Magrin, G.; Marengo, J.; Boulanger, J.; Buckeridge, M.; Castellanos, E.; Poveda, G.; Scarano, F.R.; Vicuña, S. Central and South America. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1499–1566. [Google Scholar]
- Carvalho, P. Espécies Arbóreas Brasileiras; Colombo: Embrapa Floresta, PR, Brazil, 2003. [Google Scholar]
- Araújo, D.; Sebbenn, A.M.; Zanatto, A.C.S.; Zanata, M.; Morais, E.; de Moraes, M.L.T.; Freitas, M.L.M. Variação genética para caracteres silviculturais em progênies de polinização aberta de Astronium graveolens Jacq. (Anacardiaceae). Cerne 2014, 20, 61–68. [Google Scholar] [CrossRef]
- Kubota, T.Y.; de Moraes, M.A.; da Silva, E.C.B.; Pupin, S.; Aguiar, A.V.; de Moraes, M.L.T.; Freitas, M.L.M.; Sato, A.S.; Machado, J.A.R.; Sebbenn, A.M. Variabilidade genética para caracteres silviculturais em progênies de polinização aberta de Balfourodendron riedelianum (Engler). Sci. For. 2015, 43, 407–415. [Google Scholar]
- Bhering, S.B.; Santos, H.G.; Manzatto, C.V.; Bognola, I.; Carvalho, A.P.; Potter, O.; Aglio, M.L.D.; Silva, J.S.; Chaffin, C.E.; Carvalho Junior, W. Mapa do Solo do Estado do Paraná; Embrapa Solos: Rio de Janeiro, Brazil, 2007. [Google Scholar]
- ESALQ–USP. Parcelas Permanentes em 40 ha de Florestas do Estado de São Paulo: Uma Experiência Multidisciplinar; ESALQ–USP: Piracicaba, Brazil, 2006. [Google Scholar]
- Gurgel-Garrido, L.M.A.; Siqueira, A.C.M.F.; Cruz, S.F.; Romanelli, R.C.; Ettori, L.C.; Crestana, C.S.M.; Silva, A.A.; Morais, E.; Zanatto, A.C.S.; Sato, A.S. Programa de melhoramento genético florestal. IF Série Regist. 1997, 18, 1–53. [Google Scholar]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company: New York, NY, USA, 1940; 528p. [Google Scholar]
- Bukatsch, F. Bemerkungen zur doppelfärbung astrablau-safranin. Mikrokosmost 1972, 61, 33–36. [Google Scholar]
- Roeser, K. Die Nadel der Schwarzkiefer-Massenprodukt und Kunstwerk der Natur. Mikrokosmos 1972, 61, 33–36. [Google Scholar]
- Franklin, G. Preparation of thin sections of synthetic resins and wood-resins composites, and a new macerating method for wood. Nature 1945, 155, 51. [Google Scholar] [CrossRef]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos em Morfologia Vegetal; EDUR: Seropédica, Rio de Janeiro, Brazil, 1997; 198p. [Google Scholar]
- IAWA Committee. List of microscopic features for hardwood identification. IAWA Bull. 1989, 10, 220–332. [Google Scholar]
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S. How to quantify conduits in wood? Front. Plant Sci. 2013, 4, 56. [Google Scholar] [CrossRef]
- Tyree, M.; Zimmermann, M. Xylem Structure and the Ascent of Sap; Springer-Verlag: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Carlquist, S. Vessel grouping in dicotyledon wood: Significance and relationship to imperforate tracheary elements. Aliso 1984, 10, 505–525. [Google Scholar] [CrossRef]
- Williamson, G.B.; Wiemann, M.C. Measuring wood specific gravity…Correctly. Am. J. Bot. 2010, 97, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M.; Beguería, M.S. Package ‘SPEI’. 2017. Available online: https://cran.r-project.org/web/packages/SPEI/SPEI.pdf (accessed on 12 April 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 21 March 2020).
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic predictors for supporting ecological applications in the conterminous United States. US Geol. Surv. Data Ser. 2012, 691, 10. [Google Scholar]
- Pittermann, J.; Sperry, J. Tracheid diameter is the key trait determining the extent of freezing-induced embolism in conifers. Tree Physiol. 2003, 23, 907–914. [Google Scholar] [CrossRef]
- Lens, F.; Tixier, A.; Cochard, H.; Sperry, J.S.; Jansen, S.; Herbette, S. Embolism resistance as a key mechanism to understand adaptive plant strategies. Curr. Opin. Plant Biol. 2013, 16, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Jansen, S.; Choat, B.; Pletsers, A. Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am. J. Bot. 2009, 96, 409–419. [Google Scholar] [CrossRef]
- Carlquist, S. Comparative Wood Anatomy: Systematic, Ecological, and Evolutionary Aspects of Dicotyledon Wood; Springer: Berlin/Heidelberg, Germany, 2001; ISBN 9783642640711. [Google Scholar]
- Lim, D.O.; Soh, W.Y. Cambial development and tracheid length of dwarf pines (Pinus densiflora and P. thunbergii). IAWA J. 1997, 18, 301–310. [Google Scholar] [CrossRef]
- Abe, H.; Nakai, T.; Utsumi, Y.; Kagawa, A. Temporal water deficit and wood formation in Cryptomeria japonica. Tree Physiol. 2003, 23, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.G.; Sperry, J.S.; Wheeler, J.K.; Castro, L. Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol. 2006, 26, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Hölttä, T.; Mencuccini, M.; Nikinmaa, E. Linking phloem function to structure: Analysis with a coupled xylem-phloem transport model. J. Theor. Biol. 2009, 259, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J. Plant Biomechanics: An Engineering Approach to Plant Form and Function; University of Chicago Press: Chicago, IL, USA, 1992. [Google Scholar]
- Poorter, L. The relationships of wood-, gas- and water fractions of tree stems to performance and life history variation in tropical trees. Ann. Bot. 2008, 102, 367–375. [Google Scholar] [CrossRef]
- Augspurger, C.K.; Kelly, C.K. Pathogen mortality of tropical tree seedlings: Experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia 1984, 61, 211–217. [Google Scholar] [CrossRef]





| Trial Sites | |||
|---|---|---|---|
| Luís Antônio (LA) | Pederneiras (PE) | ||
| Mean annual precipitation | 1340 mm | 1260 mm | |
| Mean annual temperature | 23.5 °C | 22.6 °C | |
| Summer (December–February) mean temperature | 31.4 °C | 34.3 °C | |
| Winter (June–August) mean temperature | 11.9 °C | 14.0 °C | |
| Provenances | |||
| Alvorada do Sul (AS) | Bauru (BA) | Gália (GA) | |
| 22°46′49″ S, 51°13′52″ W | 22°18′53″ S, 49°03′38″ W | 22°17′29″ S, 49°33′10″ W | |
| Mean annual precipitation* | 1.368 mm | 1.296 mm | 1.395 mm |
| Mean annual temperature* | 22.1 °C | 22.5 °C | 22.0 °C |
| Altitude | 320 m a.s.l | 530 m a.s.l | 650 m a.s.l |
| Soil type and features ** | Red Nitosol, clayey to very clayey texture, deep, high water holding capacity, well drained, moderately acidic, medium to high natural fertility. | Red Argisols, medium to coarse texture close to sandy, deep, low water holding capacity, low natural fertility. | Dystrophic Red-Yellow Latosol; medium texture (15–25% clay); deep, low water holding capacity; low natural fertility, |
| Site | Provenance | Provenance × site | Progeny | Site × Progeny | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p | F-Value | p | F-Value | p | F-Value | p | F-Value | p | |
| Stem height | 49.19 | <0.0001 | 1.90 | 0.16 | 0.06 | 0.94 | 0.74 | 0.62 | 0.80 | 0.58 |
| Stem diameter at breast height | 12.26 | 0.003 | 2.87 | 0.06 | 1.42 | 0.27 | 1.70 | 0.14 | 0.37 | 0.88 |
| Wood density | 0.42 | 0.52 | 1.85 | 0.17 | 1.41 | 0.27 | 0.57 | 0.75 | 1.23 | 0.34 |
| Potential hydraulic conductivity | 14.96 | 0.0014 | 32.60 | <0.001 | 8.78 | 0.0027 | 0.90 | 0.50 | 2.45 | 0.07 |
| Vessel diameter | 218.32 | <0.001 | 208.3 | <0.001 | 0.28 | 0.75 | 3.28 | 0.01 | 3.16 | 0.03 |
| Vessel wall thickness | 4.84 | 0.04 | 323.05 | <0.001 | 0.65 | 0.53 | 3.74 | 0.005 | 3.82 | 0.01 |
| Vessel density | 0.71 | 0.41 | 17.30 | <0.001 | 3.94 | 0.04 | 1.45 | 0.22 | 1.40 | 0.27 |
| Vessel element length | 27.98 | <0.0001 | 0.02 | 0.98 | 1.21 | 0.32 | 4.27 | 0.002 | 1.06 | 0.42 |
| Vessel grouping index | 5.79 | 0.02 | 19.47 | <0.0001 | 2.75 | 0.09 | 0.78 | 0.58 | 2.10 | 0.11 |
| Intervessel pit area | 13.44 | 0.0032 | 5.77 | 0.01 | 3.89 | 0.04 | -- | -- | -- | -- |
| Intervessel pit aperture area | 9.62 | 0.0092 | 11.39 | 0.001 | 0.23 | 0.79 | -- | -- | -- | -- |
| Fiber length | 1.15 | 0.29 | 2.73 | 0.07 | 1.81 | 0.19 | 0.67 | 0.67 | 2.16 | 0.10 |
| Fiber diameter | 0.22 | 0.64 | 2.70 | 0.07 | 0.38 | 0.68 | 1.52 | 0.19 | 1.86 | 0.15 |
| Fiber lumen diameter | 25.30 | 0.0001 | 1.42 | 0.25 | 6.79 | 0.007 | 0.94 | 0.48 | 1.12 | 0.39 |
| Fiber wall thickness | 5.10 | 0.03 | 2.15 | 0.13 | 4.28 | 0.03 | 0.24 | 0.98 | 1.20 | 0.35 |
| Ray height | 2.46 | 0.13 | 7.93 | 0.001 | 0.20 | 0.82 | 0.84 | 0.54 | 1.45 | 0.25 |
| Ray width | 0.08 | 0.78 | 4.95 | 0.01 | 0.48 | 0.62 | 2.45 | 0.04 | 2.41 | 0.07 |
| Ray density | 91.38 | <0.001 | 10.41 | 0.0002 | 2.25 | 0.13 | 0.66 | 0.68 | 0.95 | 0.48 |
| Vessel fraction | 3.56 | 0.07 | 3.82 | 0.03 | 1.94 | 0.17 | 0.94 | 0.47 | 1.86 | 0.15 |
| Fiber fraction | 0.12 | 0.73 | 3.64 | 0.03 | 1.28 | 0.30 | 0.96 | 0.46 | 1.42 | 0.26 |
| Parenchyma fraction | 7.88 | 0.01 | 4.41 | 0.01 | 0.49 | 0.62 | 0.67 | 0.67 | 1.41 | 0.27 |
| . | Mean Temperature | Precipitation | Precipitation×Mean Temperature | SPEI | ||||
|---|---|---|---|---|---|---|---|---|
| Estimate ±SE | p | Estimate ±SE | p | Estimate ±SE | p | Estimate ±SE | p | |
| Stem height | 0.47 ± 0.52 | 0.36 | 0.01 ± <0.01 | <0.001 | −0.002 ± <0.01 | 0.58 | −43.20 ± 6.87 | <0.001 |
| Stem diameter at breast height | 0.57 ± 0.60 | 0.33 | 0.01 ± <0.01 | 0.03 | 0.001 ± <0.01 | 0.98 | −24.50 ± 7.88 | 0.002 |
| Wood density | −0.01 ± <0.01 | 0.01 | <0.001 ± <0.01 | 0.52 | 0.0001 ± <0.01 | 0.10 | −0.09 ± 0.10 | 0.36 |
| Potential hydraulic conductivity | 2.11 ± 0.11 | 0.44 | 0.08 ± 0.02 | 0.001 | 0.052 ± 0.02 | 0.02 | −86.01 ± 38.15 | 0.03 |
| Vessel diameter | 2.95 ± 1.43 | 0.04 | 0.03 ± 0.01 | 0.01 | −0.005 ± 0.01 | 0.65 | −90.04 ± 17.93 | <0.0001 |
| Vessel wall thickness | 0.46 ± 0.22 | 0.04 | −0.002 ± <0.01 | 0.19 | −0.0008 ± <0.01 | 0.63 | 2.32 ± 3.20 | 0.47 |
| Vessel density | −3.22 ± 3.36 | 0.34 | −0.05 ± 0.03 | 0.09 | −0.043 ± 0.03 | 0.12 | 20.52 ± 46.32 | 0.66 |
| Vessel element length | −1.83 ± 6.23 | 0.77 | 0.11 ± 0.05 | 0.05 | −0.034 ± 0.05 | 0.50 | 376.8 ± 80.77 | <0.001 |
| Vessel grouping index | −0.13 ± 0.06 | 0.03 | <−0.01 ± <0.01 | 0.46 | <0.001 ± <0.01 | 0.86 | 0.669 ± 0.85 | 0.43 |
| Fiber length | 27.80 ± 23.15 | 0.23 | 0.53 ± 0.20 | 0.01 | 0.291 ± 0.19 | 0.13 | −52.77 ± 323.8 | 0.87 |
| Fiber diameter | 0.30 ± 0.14 | 0.03 | <0.01 ± <0.01 | 0.48 | −0.0008 ± 0.001 | 0.46 | −0.212 ± 1.90 | 0.91 |
| Fiber lumen diameter | −0.16 ± 0.90 | 0.08 | <−0.01 ± <0.01 | 0.22 | 0.002 ± <0.01 | 0.004 | 5.634 ± 1.29 | <0.001 |
| Fiber wall thickness | 0.22 ± 0.08 | 0.008 | <0.01 ± <0.01 | 0.82 | −0.001 ± <0.01 | 0.005 | −2.17 ± 1.16 | 0.06 |
| Ray height | 13.17 ± 6.10 | 0.03 | 0.02 ± 0.05 | 0.62 | −0.04 ± 0.05 | 0.35 | −97.53 ± 82.21 | 0.24 |
| Ray width | 1.45 ± 0.75 | 0.06 | <−0.01 ± 0.66 | 0.54 | −0.006 ± 0.006 | 0.29 | 4.08 ± 10.20 | 0.69 |
| Ray density | −0.46 ± 0.14 | 0.001 | <−0.01 ± <0.01 | <0.001 | 0.002 ± 0.001 | 0.02 | 15.19 ± 2.05 | <0.001 |
| Vessel fraction | 1.64 ± 0.89 | 0.07 | 0.01 ± <0.01 | 0.11 | −0.005 ± 0.007 | 0.43 | −17.10 ± 12.3 | 0.17 |
| Fiber fraction | −1.01 ± 1.29 | 0.43 | <0.01 ± 0.01 | 0.76 | 0.01 ± 0.10 | 0.32 | −7.40 ± 17.06 | 0.66 |
| Parenchyma fraction | −0.56 ± 0.70 | 0.42 | −0.01 ± <0.01 | 0.02 | −0.003 ± 0.005 | 0.52 | 22.33 ± 9.19 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.R.d.; Rossi, S.; Khare, S.; Longui, E.L.; Marcati, C.R. Disentangling the Effects of Genotype and Environment on Growth and Wood Features of Balfourodendron riedelianum Trees by Common Garden Experiments in Brazil. Forests 2020, 11, 905. https://doi.org/10.3390/f11090905
Silva JRd, Rossi S, Khare S, Longui EL, Marcati CR. Disentangling the Effects of Genotype and Environment on Growth and Wood Features of Balfourodendron riedelianum Trees by Common Garden Experiments in Brazil. Forests. 2020; 11(9):905. https://doi.org/10.3390/f11090905
Chicago/Turabian StyleSilva, Jane Rodrigues da, Sergio Rossi, Siddhartha Khare, Eduardo Luiz Longui, and Carmen Regina Marcati. 2020. "Disentangling the Effects of Genotype and Environment on Growth and Wood Features of Balfourodendron riedelianum Trees by Common Garden Experiments in Brazil" Forests 11, no. 9: 905. https://doi.org/10.3390/f11090905
APA StyleSilva, J. R. d., Rossi, S., Khare, S., Longui, E. L., & Marcati, C. R. (2020). Disentangling the Effects of Genotype and Environment on Growth and Wood Features of Balfourodendron riedelianum Trees by Common Garden Experiments in Brazil. Forests, 11(9), 905. https://doi.org/10.3390/f11090905







