Abstract
Urban trees provide many ecosystem services essential to city dwellers well-being. Ectomycorrhizal (ECM) fungi are symbionts for trees and may also contribute to urban tree health and thus maintenance of the ecosystem services. However, no studies so far have analysed the composition of the ECM community colonising Crimean linden. In this study we investigated ECM associations of Crimean linden trees in the urban environment, comparing street trees with those growing in a park. Moreover, we studied the ECM community of healthy versus unhealthy linden trees growing along a street. The health status of each tree was assessed using tree health indicators. The smallest degree ECM colonisation was observed in unhealthy street trees (40.5%). Cenococcum geophilum was found to be the most abundant ECM fungal species of the Crimean linden growing in the park (23.9%). Our results suggest that the linden trees growing in the park and those growing along the street but without disease symptoms did not differ in their ECM richness. However, the unhealthy street trees showed less ECM diversity and abundance. Moreover, strong negative correlations between the concentrations of Na and Cl in the soil and ECM colonisation were found. This study describes, for the first time, the ECM fungal community on Tilia “Euchlora” trees grown in Europe. We report novel findings on the ECM associations of Crimean linden trees in an urban ecosystem. Further research should focus on the role of native mycorrhizal fungal communities in nutrient acquisition by urban trees in the presence of salt stress.
1. Introduction
Urban trees and forests are the most prominent elements of the urban landscape (townscape). They provide a large number of beneficial urban ecosystem services, including ecological and aesthetic values e.g., air quality improvement [1,2], carbon sequestration [3,4], storm water attenuation [5], and energy conservation [6]. Trees convey a personal, local, community, and cultural meaning and ensure a large variety of pro-health benefits [7]. However, the city is a difficult living environment for trees. Soil compaction [8], root development limitation [9], urban heat islands [10], drought [11], salinity [12], and many other factors can be stressors and alter soil microenvironments, thereby weakening the health status of trees.
Among soil microorganisms that are important in the development of boreal trees, ectomycorrhizal fungi (ECM) are of particular interest, as they provide the tree with nutrients, protect its root system from microbial pathogens, and enhance its drought tolerance [13]. Therefore, this symbiosis is a crucial factor for tree health [14]. Very few studies have dealt with the ECM fungal community in urban areas, especially as far as mature trees are concerned [15,16,17,18]. Previous studies [19,20] reported that healthy urban trees and forest trees had the highest number of ectomycorrhizal morphotypes in comparison to trees with decreased vitality. ECM fungi play an important role in protection of trees against salt stress in the environment by prevent Na+ and Cl− translocation to leaf tissues and enhance nutrients uptake i.a. phosphorus (P) and nitrogen (N) [21,22].
Soil contamination caused by NaCl used to maintain ice-free roadways and sidewalks in winter is now recognized as one of the major causes of death of urban trees [23,24,25,26]. Ectomycorrhizal fungi can increase plant tolerance to salinity by increasing the levels of N, P, Ca, and K in plant cells [24]. Moreover, in response to salt stress, ectomycorrhizal associations increase both the efficiency of water uptake [27] and the synthesis of antioxidants and thus reduce osmotic stress in the soil [27,28]. However, a high level of soil salinity influences soil properties and the associated microorganisms [29,30,31], such as ECM fungi. The capacity of ECM fungal colonisation and the growth of fungal hyphae in the soil [32] may be disturbed by the increased salinity.
As noted by Turgeman et al. [33] and Ma et al. [34], one of the most frequently reported effects of ECM fungi is increased photosynthetic activity, which is manifested by an increase in chlorophyll content in leaves. Disturbances due to heavy metal and salt stress in the functioning of the photosynthetic apparatus result in deterioration of the overall health of the tree. The first observed symptom of these disturbances is leaf chlorosis, which especially affects Tilia ‘Euchlora’ lime trees [35]. The photosynthetic apparatus is susceptible to various environmental factors [36], e.g., mineral deficiency [37,38].
Lime trees, including Tilia ‘Euchlora’ lime trees, are widely grown as urban plantings along the streets, in parks and squares. The present investigation was inspired by research of Milewska-Hendel et al. [39], who found Crimean linden with and without visible symptoms of decay growing in the same habitat. To the best of our knowledge, this is the first study on the ECM fungal community structure in Tilia ‘Euchlora’. Thus, the aims of this study were (1) to investigate the ECM colonisation and communities in two different locations (street versus park) in the urban environment; (2) to determine the factors that might have driven the ECM colonisation and composition of healthy versus unhealthy linden trees growing along the street.
2. Materials and Methods
2.1. Study Sites
Twenty-four linden trees growing in two different sites in the city of Warsaw (Poland) were studied. The ‘street’ site comprised of 16 roadside linden trees standing in a single lane in a 150-cm wide soil bed located in the centre of Warsaw along Żwirki i Wigury Avenue (52°12′27′′ N, 20°59′13′′ E). The park area with 8 linden trees was located in a park (52°12′15′′ N, 20°59′22′′ E) at a distance of approximately 150 m and separated from the road by a dense strip of bushes and hedges. All the trees planted along the street and in the park were at the same age (over 80 years) and derived from the same nursery plant [12]. The weather conditions, i.e., humidity, temperature, and sun exposure, in both studied locations (street and park) were the same [12].
2.2. Tree Health Indicators
The health status of each tree was assessed by determination of chlorophyll fluorescence and foliar chlorophyll (Chl) content in May 2019 (Table 1). The CCM-200 Chlorophyll Meter was used to determine the chlorophyll content of leaves. This measuring instrument determines the chlorophyll content of leaf tissues using the radiation absorption phenomenon. The foliar chlorophyll (Chl) content in linden trees growing in an urban environment can be a useful and objective diagnostic indicator of plant health [40]. The measurement of the chlorophyll content in leaves allows the determination of the greenness index value. The greenness index value increases with the amount of chlorophyll. The measurement was made 9 times in the central part of a mature leaf blade with an area of 7 mm2. The results were expressed in non-denominated units called SPAD values. An abnormal level may indicate the existence of stress factors such as disease, climate change, deficiency, or excess of nutrients related to the amount of light or water, or the presence of toxic substances [41,42]. Next, chlorophyll fluorescence, which is a measure of photosynthetic efficiency, was measured in each sampled leaf after 25-min dark adaptation using an OS5-p Fluorimeter from ACD Bioscientific Ltd. (Hansatech Instruments, Pentney, UK) to obtain the maximum efficiency of PSII (Fv/Fm) [43]. Nine measurements of each leaf were averaged per tree. Fv/Fm reveals changes in a wide range of plant stresses [43]. In the ‘street’ site, eight trees were classified as street healthy trees (SHT) and the eight other trees were classified as street unhealthy trees (SUT). In the park area, all of the eight park trees (PT) represented the healthy tree group (Table 1).

Table 1.
Chlorophyll fluorescence and foliar chlorophyll (Chl) content used to estimate tree health. Fv = variable fluorescence, Fm = maximum fluorescence.
2.3. Sampling
In September 2019, root and leaf samples were taken from PT, SHT, and SUT (Field Permit Number: OŚ-V.6121.18.2020.MSP). A total of 24 soil samples were collected for mycorrhizal assessment. To assess the root system, each sample was extricated with a cylinder (approximately 25 cm diameter, 25 cm depth) of adjacent substrate and packed in labelled plastic bags. The samples were stored at −20 °C until further processing. The leaves were collected from the external belt of the crown, around its entire circumference, at heights from 2.5 m to 3.5 m. All analyses were done for each tree separately, and the leaf samples were not mixed. The leaves were placed in linen bags, transported to the laboratory, and dried for 12 hours at 70 °C. Two soil samples per tree were collected under PT, SHT, and SUT for chemical analyses (48 soil samples in total).
2.4. Identification of Mycorrhizae
Extracted root fragments were examined under a dissecting microscope at 10–60× magnification. The identified root tips were classified as “non-vital” (NV, scurfy surface and easily detachable cortex, with or without remnants of an ECM mantle), “vital non-ECM” (NM, well-developed, inflated and turgid tip, mantle lacking), and “vital ECM” (VM) [45]. Ectomycorrhizae were classified by morphological, anatomical (colour, shape, texture, and thickness of the mantle, presence and organization of emanating hyphae, rhizomorphs, and other elements), and molecular investigations in accordance with the available literature [46,47]. The degree of mycorrhization of the linden roots and the abundance, relative abundance, and frequency of individual ectomycorrhizal fungal taxa were determined according to Olchowik et al. [48]. Each morphotype was analysed separately during molecular identification and pooled for determination of abundance only when the molecular analysis indicated that the morphotypes belonged to the same taxon. The universal primers ITS1 and ITS4, commonly used in ECM community studies, were used to amplify the ITS-1, 5.8S, and ITS-2 regions of the fungal nuclear ribosomal DNA [49,50] and to sequence using Sanger sequencing the product of the polymerase chain reaction (PCR). The full methods used for molecular identification of mycorrhizae were reported by Olchowik et al. [48]. The best representatives of each unique ITS sequences were deposited in NCBI GenBank under accession numbers MT428415–MT428421.
2.5. Chemical Analysis
Dried leaf samples were ground to powder with an impactor mill (Fritsch 14702). After mineralization of the dry leaves in a muffle furnace (Naberthern L40/11/P320) [51], elements (Ca, K, Mg, Na, and P) were determined by atomic spectrophotometry using a Perkin Elmer 1100B instrument (Perkin Elmer, Germany). The weight of the leaf sample was 2 g. Cl was determined by potentiometric titration using an ion-selective electrode and an ion meter Orion Star Plus (Thermo Scientific, Waltham, MA, USA) [52]. To provide quality control (QC), the elemental content in the plant samples was determined using certified reference materials, including apple leaves from the National Institute of Standards and Technology (USA) and beech leaves (ERM100) from European Reference Materials. The obtained results were in close agreement with the certified values. The recovery range (comparisons of measured and certified concentrations of elements in plants in the certified reference material) was 93.9%–106.3% for all elements. Using the standard methods, basic chemical soil parameters were assessed at the National Chemical and Agricultural Station with its registered office in Warsaw, Poland. The accuracy of the analysis was checked against standard reference materials: international standard soils [53,54,55,56].
2.6. Data Analysis
To evaluate the significant differences in the abundance of root tip types between the examined sites, we used generalized linear models (GLM) with binomial distributions. For this GLM model, we used Tukey’s linear contrast as the post hoc test. The one-way analysis of variance ANOVA with Tukey post hoc test was used to analyses of soil properties, content of mineral elements in leaves, and changes in fluorescence. The assumptions of ANOVA were checked based on Levene’s test and Shapiro-Wilk test. To explore relationships between the soil properties and the abundance of different root tip types, we used canonical correspondence analysis (CCA) and its result presented on biplot figures with scaling for abundance of root tip types (type 2 scaling). Nonmetric multidimensional scaling ordination (NMDS) was used to illustrate the differences (based on the Bray–Curtis distance matrix) between the ECM fungal species from SHT, SUT, and PT. To test these differences, we used analysis of similarities (ANOSIM). Three diversity indices, i.e., Chao-1, Shannon–Wiener’s (H′) and Simpson’s (1/D), were calculated based on the abundance and numbers of the ECM fungal species. Statistical analyses were performed using R version 3.6.1. The vegan package [57] from R software was used for multivariate analysis (CCA and NMDS) and for calculation of diversity indices. The accepted level of significance was p < 0.05.
3. Results
3.1. Ectomycorrhizal community
The ECM colonisation of the SHT roots was significantly higher than in the case of the SUT roots (Table 2). After regrouping and combining based on the results of the molecular analysis, seven fungal taxa were finally detected (Table 2, Figure 1). Six of the seven detected fungal taxa were assigned to the species level and one was assigned to the genus level. The richness estimator, Chao-1, indicated that at least 5.0 ECM fungal taxa were expected to colonise the roots of trees growing in the park area. The calculation of the diversity of the ECM fungal taxa based on the Shannon–Wiener and Simpson diversity indices revealed that the estimated richness was significantly higher in PT and SHT than in SUT. The Crimean linden’s roots in the street and park habitats were colonised by seven ECM species: Cenococcum geophilum Fr., Inocybe geophylla (Sowerby) P. Kumm., Sebacina cystidiata Oberw., Garnica & K. Riess, Tylospora asterophora (Bonord.) Donk, Tuber borchii Vittad., Tuber rufum Pollini and one Tomentella species. C. geophilum was found to be the most abundant (23.9%) ECM fungal species of the Crimean linden growing in the park. The proportion of C. geophilum on the SUT roots was only 6.7%, while T. asterophora was the most frequent species on the roots of SHT.

Table 2.
Estimated species richness, diversity, and occurrence of ectomycorrhizal fungal taxa associated with the roots of Crimean linden.
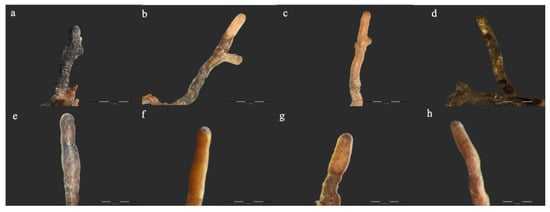
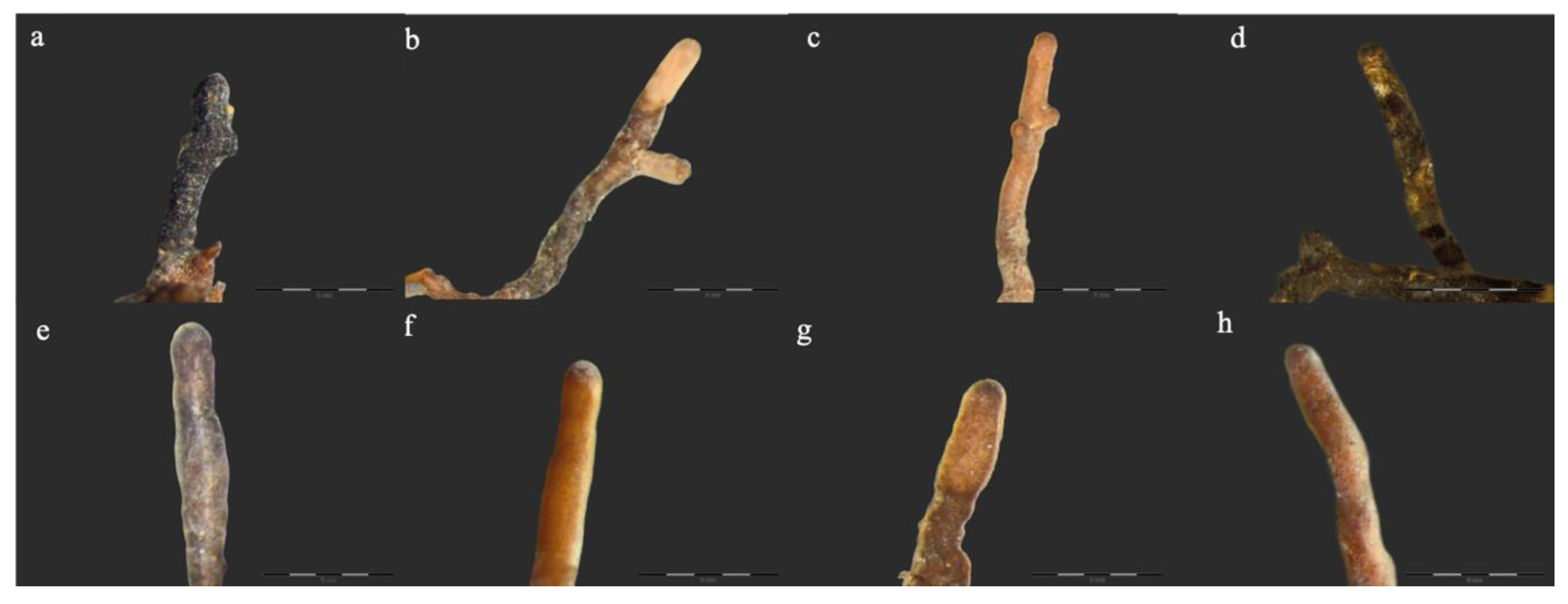
Figure 1.
Ectomycorrhizas observed on Crimean linden: (a) Cenococcum geophilum Fr., (b) Inocybe geophylla (Sowerby) P. Kumm., (c) Sebacina cystidiata Oberw., Garnica & K. Riess, (d) Tomentella sp., (e) Tylospora asterophora (Bonord.) Donk, (f) Tuber borchii Vittad., (g,h) Tuber rufum Picco. Bars in each photograph indicate 0.4 mm length.
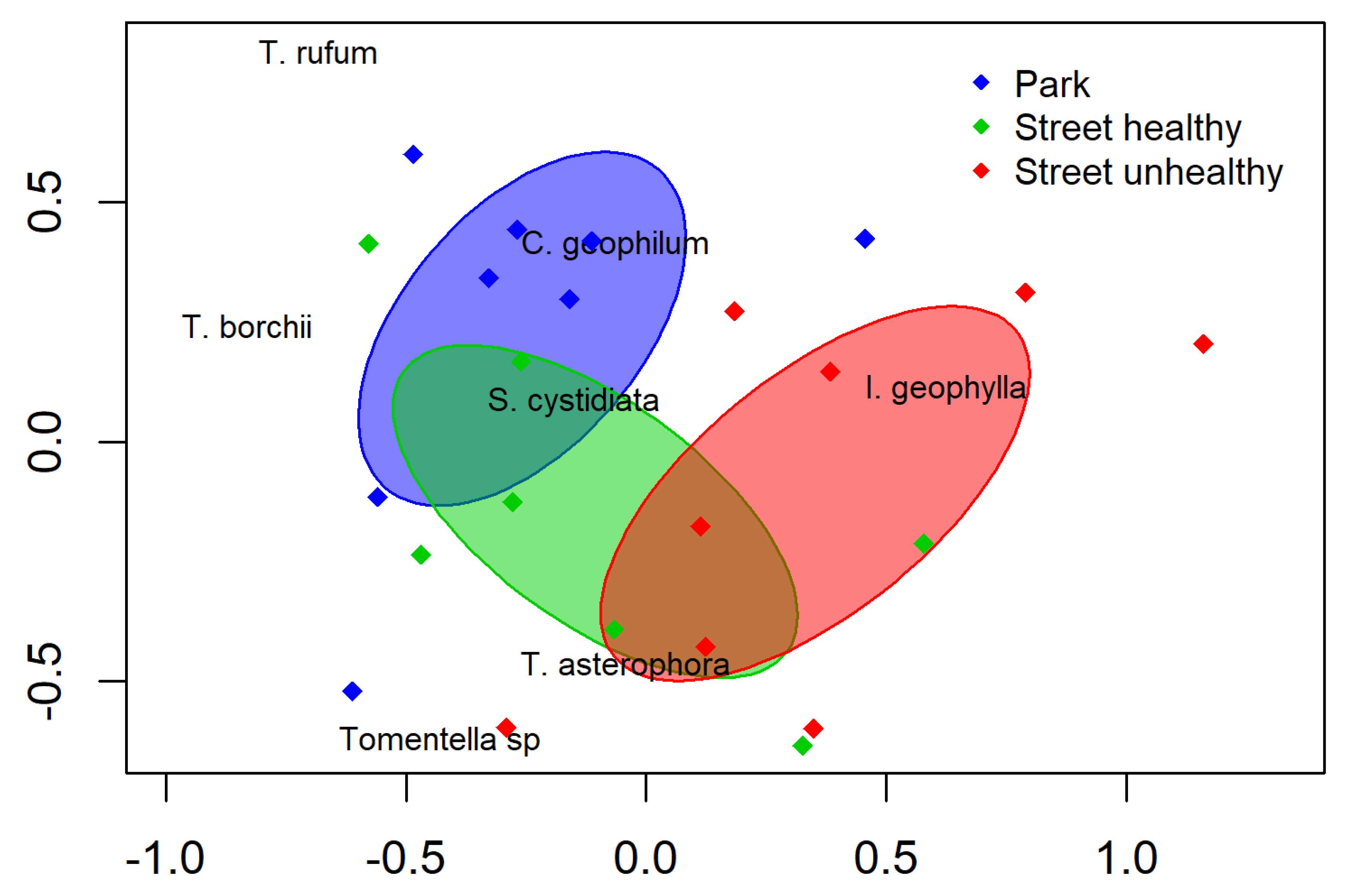
The distribution of the ECM taxa between the street and park trees was not highly differentiated (Figure 2). The NMDS ordination based on the ECM community indicated separation of street unhealthy trees and park trees. The NMDS ordinations of the mycorrhizal communities demonstrated partial separation based on the different sites (Figure 2, stress value 0.19). Despite the partial overlap in the NMDS, ANOSIM revealed a significant difference between the tested variants (global R = 0.52, p <0.0001, permutations = 10,000).
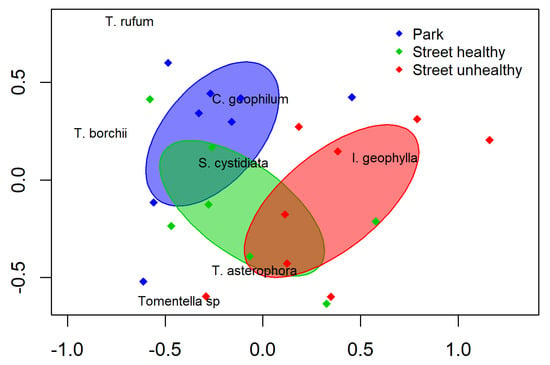
Figure 2.
Non-metric MDS of the street and park trees based on the ectomycorrhizal community. Circles represent individual samples, whilst red crosses represent ectomycorrhizal fungal species. Ellipses indicate the standard deviation around the centroid position of each study treatment.
3.2. Soil and Leaf Parameters
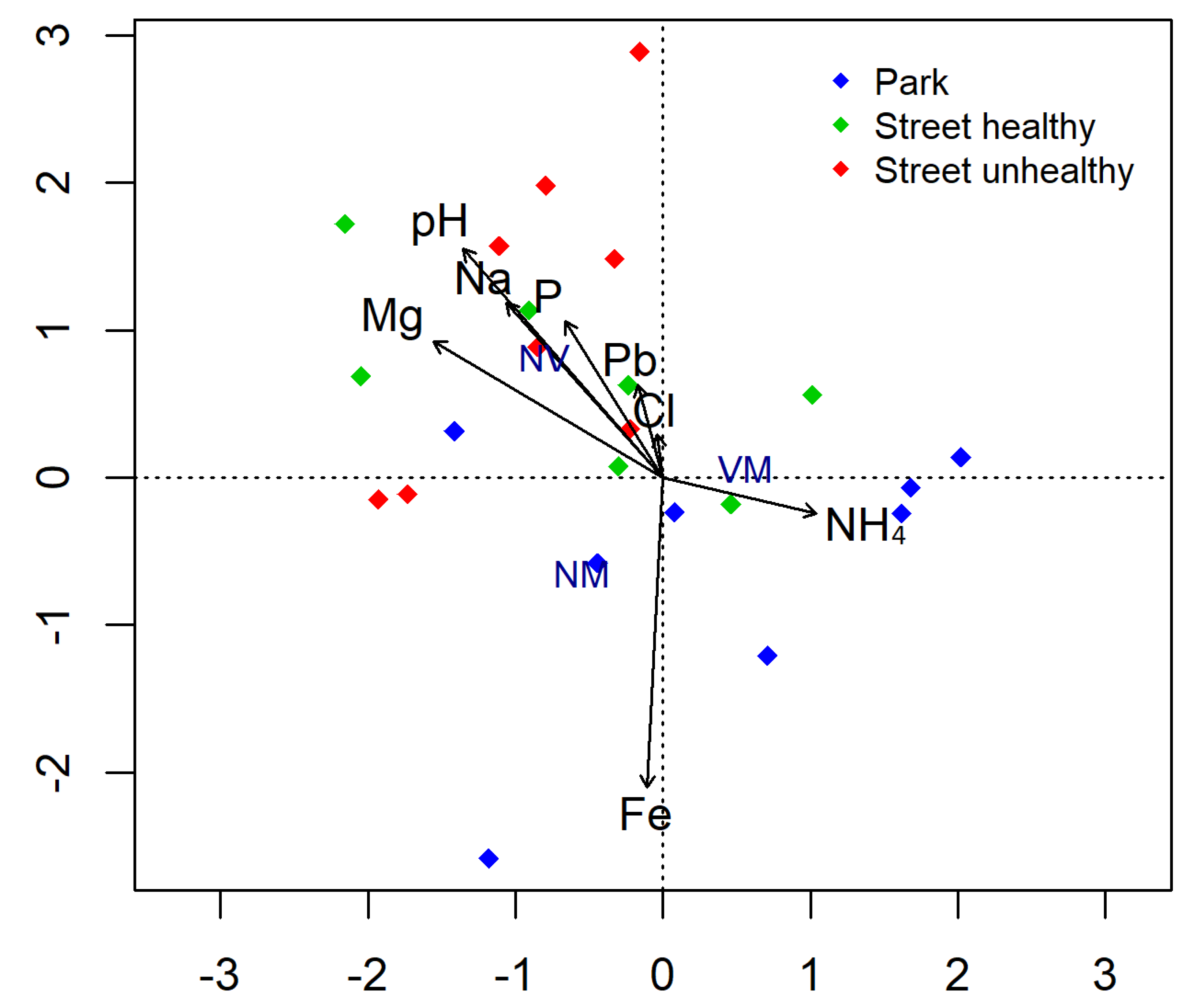
The street soil had clearly higher pH than the park soil (Table 3). The Na content in the street soil was on average approximately twice as high as that in the park habitat. The street soil sampled beneath SHT had the highest amounts of P but very little Fe, compared with the park soil. The content of Zn and Pb was more than twice as high in the soil sampled beneath the linden trees growing in the street than in the case of the park linden (Table 3). Considering non-vital root tips, the value was positively correlated with a heavy metal (Pb) and with pH, Na, and Cl, while correlated negatively with NH4 (Figure 3).

Table 3.
Mean values of selected physical and chemical properties of soils.
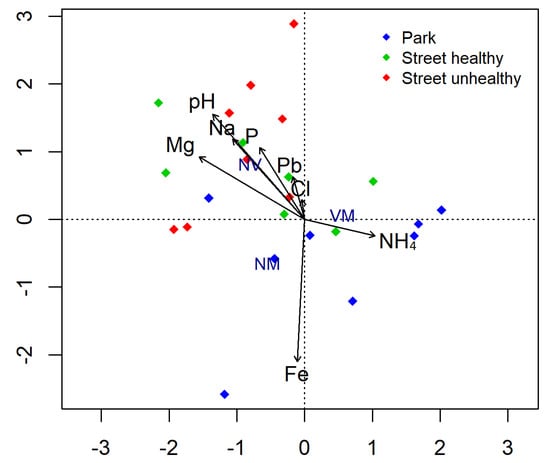
Figure 3.
Biplot for canonical correspondence analysis (CCA) used to show relationship between the abundance of different root tip types (vital mycorrhizal (VM), non-vital (NV), and vital non-mycorrhizal (NM)) and soil properties.
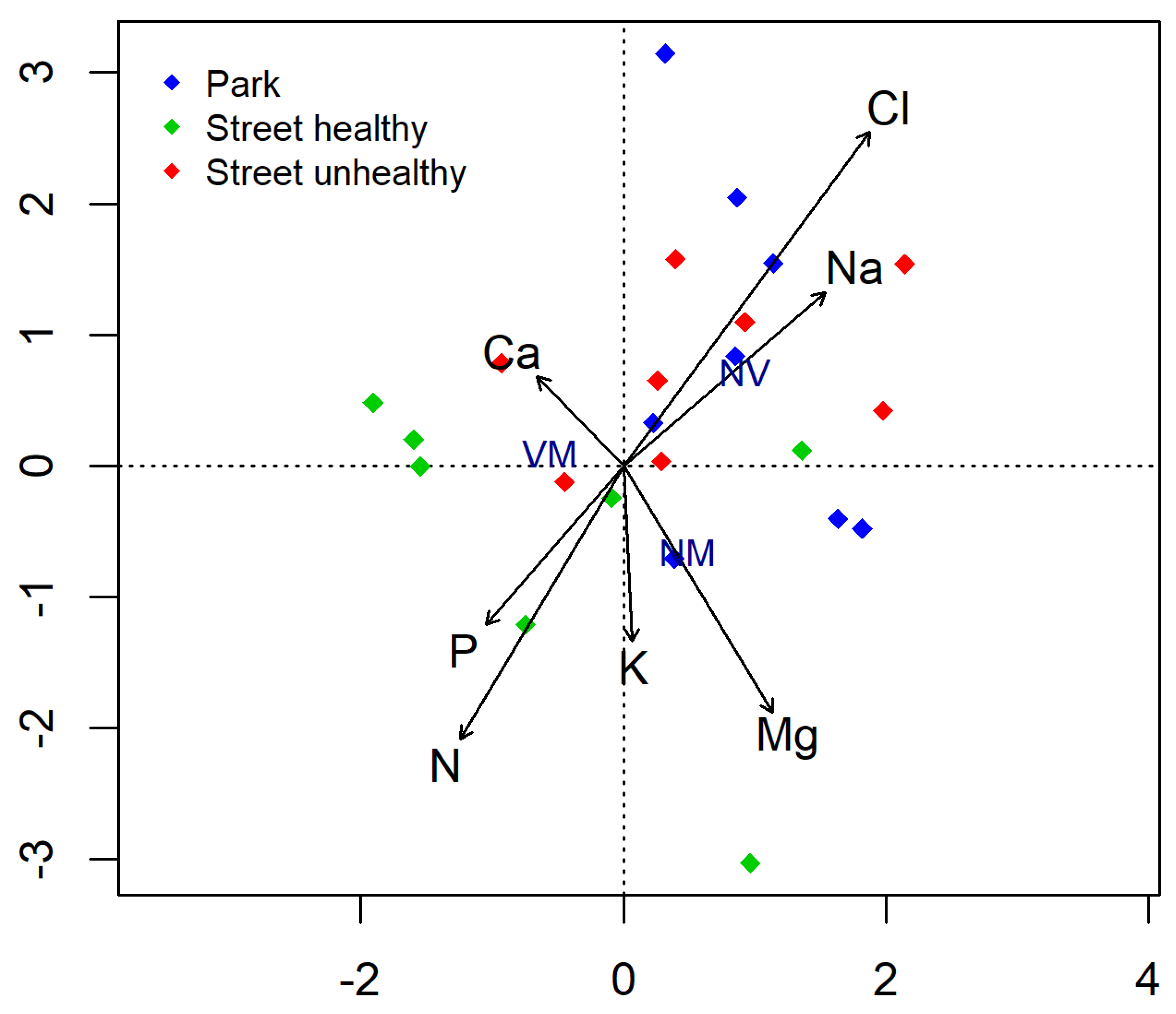
The leaves from the trees growing along the street contained much more Cl and Na than those from the park (Table 4). The SUT leaves contained significantly more Na than SHT and PT. No deficiency was found for elements that are important for basic physiological functions in plants, i.e., nitrogen, phosphorus, and potassium. The increase in the average content of Na and Cl in the leaves of Tilia ‘Euchlora’ was clearly correlated with the non-vital root tips (Figure 4).

Table 4.
Chemical composition of Crimean linden leaves growing in the streets (n = 16) and in the park area (n = 8) as well as p-values based on ANOVA.
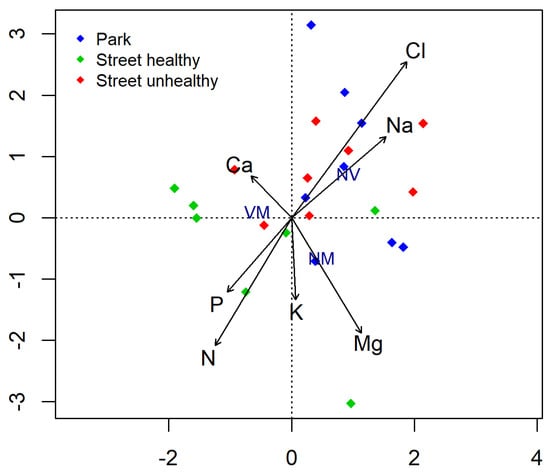
Figure 4.
Biplot for canonical correspondence analysis (CCA) used to show relationship between the abundance of different root tip types (vital mycorrhizal (VM), non-vital (NV), and vital non-mycorrhizal (NM)) and the chemical composition of leaves.
4. Discussion
This study was performed at two different sites of growth of urban linden trees to verify whether environmental variables influence root tip vitality and the ectomycorrhizal community. Our results showed that the linden trees growing in the park and those growing along the street but without disease symptoms did not differ in their ECM richness. However, the unhealthy linden showed significantly lower ECM species richness than the two groups mentioned above. The higher ECM richness in the park trees may be explained by the lower pH level (mean = 5.5), compared to the street unhealthy specimens (mean = 7.7). The higher ECM richness observed in acidic soils is in agreement with a previous study showing that ECM symbiosis is favoured in these conditions [58]. For instance, Aggangan et al. [59] showed that the effectiveness of different ECM taxa in promoting plant growth was reduced at more alkaline pH levels, suggesting that alkaline soils negatively affect the growth of the nutrient-absorbing external hyphae. In a study conducted by Soudzilovskaia et al. [60], a strong negative correlation between soil pH and ECM colonisation was found as well. Moreover, ECM fungi produce enzymes with pH optima between 4 and 6 [61,62], which may explain the higher ECM richness in this pH range. In addition to the differences in the ECM richness, our results also revealed differences in the ECM community composition between the park and street environments. The presence of T. asterophora, i.e., an ectomycorrhizal fungus colonising such coniferous trees as fir [63] and larch [64], in the street indicates that this fungus is not a specialist. It seems that T. asterohora due to it ubiquitous character in high salinity of soil may thrive and colonise roots of linden. Tedersoo et al. [65] suggested that they contribute to wood degradation, enabling them to compete with saprotrophs for resources.
The higher abundance of C. geophilum in roots of linden trees growing in the park than those growing along the street was an unexpected result. This fungus, perhaps the least specialised of ECM fungi with respect to host species and forming ECM with many tree species (it is cosmopolitan), tolerates a wide range of stressors and is widely known for its ability to tolerate water stress [66,67]. Its dominance on roots of 50-year old linden growing in a street habitat was confirmed by Alzetta et al. [68]. Our result may be explained by soil moisture, which paradoxically may have been higher in the street environment than in the park due to street watering and cleansing during summer time. However, we can only speculate on this matter, since we did not measure the soil moisture. On the other hand, in their study, Peter et al. [14] did not observe a significant effect of C. geophilum colonisation on plant physiological parameters under drought stress. Therefore, more studies in this regard are needed to facilitate the identification of drought-adapted strains of C. geophilum, which can be used to support their host trees threatened by pollutants or drought periods in many parts of the world [69].
The health of the street trees was not clearly related to the mycorrhizal diversity, although fewer species were found on the roots of the unhealthy trees; for instance, Tuber borchii and Tuber rufum were not present on these roots (Table 2). Since they require a more stable ecosystem with abundant nutrient supply, these ECM taxa are often regarded as late-stage fungi [70,71]. However, we also found ECM taxa (I. geophylla and Tomentella sp.) representing early-stage fungi, which were common in the park and street habitats. These taxa generally occur in unstable ecosystems (e.g., urban areas) with limited nutrient and water availability [72].
As suggested by Branco et al. [73], ECM fungi, which are not generally salt resistant, can adapt towards tolerance to soil salt. In our study, the composition of the ECM fungal community differed between the street and park habitats, but the key ECM fungi were mainly the same. The NMDS analysis also supports this hypothesis, as the ECM communities demonstrated partial separation based on the different sites (Figure 2). However, the common ECM fungi varied in abundance (Table 2). Since it was exposed to urban disturbances (e.g., salting of the streets during winter, metal pollution), the street soil was characterized by significantly higher amounts of Na, Cl, and Pb than the park soil. In urban environments, mycorrhizal fungal communities affected by high exposure to salt accumulation may reduce their diversity [74]. Moreover, Turpeinen, Kairesalo, and Haggblom [75] showed that heavy metal contamination of soil has a negative effect on fungal communities. In our study, a strong negative correlation between the concentrations of Na, Cl, and Pb in the soil and the ECM colonisation was found as well (Figure 3). The lower degree of the ECM colonisation of the roots in the healthy and unhealthy street trees may be explained by the higher Na and Cl levels in the street soil, compared to the park trees. So far, few studies have shown improved plant salt stress tolerance in ECM fungi [76,77,78]. Our results revealed differences in the content of Na and Cl in leaves between the healthy and unhealthy trees. It is very likely that, in order to protect the photosynthetic apparatus of the trees to maintain the production of carbon compounds, the transfer of toxic Na ions into the root cortex may be retained by ECM fungi [79]. The lower content of Na and Cl observed in the leaves of the healthy trees may be explained by the higher ECM colonisation (VM = 47.7), compared to the unhealthy trees (VM = 40.5). In a study conducted by Muhsin and Zwiazek [78], alleviation of salt stress in white spruce (Picea glauca) seedlings by Hebeloma crustuliniforme was found as well. Moreover, the authors identified the reduction of shoot Na uptake accompanied by increasing N and P absorption as an important salt tolerance mechanism related to ectomycorrhizal symbiosis [78].
The park soil had the highest content of Fe. Iron is more mobile in acidic soil systems than in alkaline systems [80]. Thus, acidic soil in park site favored a higher iron concentration in soil than did the alkaline street soil (Table 3). The salt used to deice roads has a slightly alkalising effect on soil due to the formation of sodium hydroxide [81]. Alkalisation deteriorates the availability of micronutrients (Fe, Mn, Zn, Cu) [81]. Moreover, this may negatively affect the health status of trees [82]. Our results seem to confirm this observation. The average manganese and iron content measured in the unhealthy street soils was the lowest among all sites (Table 3).
5. Conclusions
Our research describes, for the first time, the ECM fungal community on Tilia “Euchlora” trees grown in Europe. Our results suggest that the Crimean linden trees growing in the park and those growing along the street but without disease symptoms did not differ in their ECM richness. However, the ECM colonisation of the park trees roots was significantly higher than in the case of the street healthy trees roots (61.9% and 47.7%, respectively). Increased knowledge of both the soil properties and the cause-effect relationship between the ECM population dynamics and urban tree decline severity enhance the awareness of the importance of the belowground environment for urban trees. Future lines of research should include a detailed description of the role of native mycorrhizal fungal communities in the nutrient acquisition by urban trees in the presence of salt stress.
Author Contributions
Conceptualization: J.O. and M.S. (Marzena Suchocka); Identification of mycorrhizas: J.O.; Chemical analysis of leaves: A.B.-D.; Statistical analysis: M.S. (Marcin Studnicki); molecular analysis: J.O. and T.M.; Writing—original draft: J.O., M.S. (Marzena Suchocka), A.B.-D., and D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Science Centre (Poland) MINIATURA (Grant 2019/03/X/NZ9/00230).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nowak, D.J.; Hirabayashi, S.; Greenfield, E. Tree and forest effects on air quality and human health in the United States. Environ. Pollut. 2014, 193, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, F.J.; Kroeger, T.; Wagner, J.E. Urban forests and pollution mitigation: Analyzing ecosystem services and disservices. Environ. Pollut. 2011, 159, 2078–2087. [Google Scholar] [CrossRef]
- Nowak, D.J.; Dwyer, J.F. Understanding the benefits and costs of urban forest ecosystems. In Urban and Community Forestry in the Northeast, 2nd ed.; Kuser, J.E., Ed.; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Tyrväinen, L.; Pauleit, S.; Seeland, K.; de Vries, S. Benefits and uses of urban forests and trees. In Urban Forests and Trees; Konijnendijk, C.C., Nilsson, K., Randrup, T.B., Schipperijn, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 81–114. [Google Scholar]
- Day, S.D.; Dove, J.E.; Bartens, J.; Harris, J.R. Stormwater management that combines paved surfaces and urban trees. In Geosustainability and Geohazard Mitigation; Reddy, K.R., Khire, M.V., Alshawabkeh, A.N., Eds.; GeoCongres: New Orleans, LA, USA, 2008; pp. 1129–1136. [Google Scholar]
- Akbari, H.; Pomerantz, M.; Taha, H. Cool surfaces and shade trees to reduce energy use and improve air quality in urban areas. Sol. Energy 2001, 70, 295–310. [Google Scholar] [CrossRef]
- Tyrväinen, L.; Ojala, A.; Korpela, K.; Lanki, T.; Tsunetsugu, Y.; Kagawa, T. The influence of urban green environments on stress relief measures: A field experiment. J. Environ. Psychol. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Ampoorter, E.; De Schrijver, A.; De Frenne, P.; Hermy, M.; Verheyen, K. Experimental assessment of ecological restoration options for compacted forest soils. Ecol. Eng. 2011, 37, 1734–1746. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Jiang, B.; Wen, Z.; Yang, N.; Li, L. Tree survival and growth are impacted by increased surface temperature on paved land. Landsc. Urban Plan. 2017, 162, 68–79. [Google Scholar] [CrossRef]
- Arnfield, A.J. Two decades of urban climate research: A review of turbulence, exchanges of energy and water, and the urban heat island. Int. J. Climatol. 2003, 23, 1–26. [Google Scholar] [CrossRef]
- Stojnić, S.; Suchocka, M.; Benito-Garzón, M.; Torres-Ruiz, J.M.; Cochard, H.; Bolte, A.; Cocozza, C.B.; de Luis, M.; Martinez-Vilalta, J. Variation in xylem vulnerability to embolism in European beech from geographically marginal populations. Plant Physiol. 2018, 38, 173–185. [Google Scholar] [CrossRef]
- Sienkiewicz-Paderewska, D.; Dmuchowski, W.; Baczewska, A.H.; Brągoszewska, P.; Gozdowski, D. The effect of salt stress on lime aphid abundance on Crimean linden (Tilia ‘Euchlora’) leaves. Urban For. Urban Green. 2017, 21, 74–79. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Peter, M.; Kohler, A.; Ohm, R.A.; Kuo, A.; Krützmann, J.; Morin, E.; Arend, M.; Barry, K.W.; Binder, M.; Choi, C.; et al. Ectomycorrhizal ecology is imprinted in the genome of the dominant symbiotic fungus Cenococcum Geophilum. Nat. Commun. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Appleton, B.; Koci, J.; French, S.; Leystan, M.; Harris, R. Mycorrhizal fungal inoculation of established street trees. Arboric. J. 2003, 29, 107–111. [Google Scholar]
- Garbaye, J.; Lohou, C.; Laurent, P.; Churin, J.L. Ectomycorrhizal inoculation of avenue trees in Paris. Acta Hortic. 1999, 496, 445–450. [Google Scholar] [CrossRef]
- Garbaye, J.; Churin, J.L. Effect of ectomycorrhizal inoculation at planting on growth and foliage quality of Tilia Tomentosa. Arboric. J. 1996, 22, 29–34. [Google Scholar]
- Nielsen, J.S.; Rasmussen, H.N. Mycorrhizal status and morphotype diversity in Tilia cordata—a pilot study of nurseries and urban habitats. Acta Hortic. 1999, 496, 451–460. [Google Scholar] [CrossRef]
- Allen, M.F.; Swenson, W.; Querejeta, J.I.; Egerton-Warburton, L.M.; Treseder, K.K. Ecology of mycorrhizae: A conceptual framework for complex interactions among plants and fungi. Annu. Rev. Phytopathol. 2003, 41, 271–303. [Google Scholar] [CrossRef]
- Timonen, S.; Kauppinen, P. Mycorrhizal colonisation patterns of Tilia trees in street, nursery and forest habitats in southern Finland. Urban For. Urban Green. 2008, 7, 265–276. [Google Scholar] [CrossRef]
- Tang, M.; Sheng, M.; Chen, H.; Zhang, F. In vitro salinity resistance of three ectomycorrhizal fungi. Soil. Biol. Biochem. 2009, 41, 948–953. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef]
- Fay, L.; Shi, X. Environmental impacts of chemicals for snow and ice control: State of the knowledge. Water Air Soil Pollut. 2012, 223, 2751–2770. [Google Scholar] [CrossRef]
- Chen, S.; Hawighorst, P.; Sun, J.; Polle, A. Salt tolerance in Populus: Significance of stress signaling networks, mycorrhization, and soil amendments for cellular and whole-plant nutrition. J. Exp. Bot. 2014, 107, 113–124. [Google Scholar] [CrossRef]
- Dmuchowski, W.; Brogowski, Z.; Baczewska, A.H. Evaluation of vigour and health of ‘street’ trees using foliar ionic status. Pol. J. Environ. Stud. 2011, 20, 489–496. [Google Scholar]
- Ordóñez-Barona, C.; Sabetski, V.; Millward, A.A.; Steenberg, J. Deicing salt contamination reduces urban tree performance in structural soil cells. Environ. Pollut. 2018, 234, 562–571. [Google Scholar] [CrossRef]
- Cabot, C.; Sibole, J.V.; Barceló, J.; Poschenrieder, C. Lessons from crop plants struggling with salinity. Plant Sci. J. 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.; Oh, S.-H.; Sa, T. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef]
- Calvo Polanco, M.; Zwiazek, J.J.; Voicu, M.C. Responses of ectomycorrhizal American elm (Ulmus americana) seedlings to salinity and soil compaction. Plant Soil 2008, 308, 189–200. [Google Scholar] [CrossRef]
- Yi, H.; Calvo Polanco, M.; MacKinnon, M.D.; Zwiazek, J.J. Responses of ectomycorrhizal Populus tremuloides and Betula papyrifera seedlings to salinity. Environ. Exp. 2008, 62, 357–363. [Google Scholar] [CrossRef]
- Day, S.D.; Amateis, R.L. Predicting canopy and trunk cross-sectional area of silver linden (Tilia tomentosa) in confined planting cutouts. Urban For. Urban Green 2011, 10, 317–322. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Szymanńska, S.; Piernik, A.; Thiem, D. Ectomycorrhizal community structure of Salix and Betula spp. at a saline site in central Poland in relation to the seasons and soil parameters. Water Air Soil Pollut. 2015, 226, 99. [Google Scholar] [CrossRef]
- Turgeman, T.; Ben Asher, J.; Roth-Bejerano, N.; Kagan-Zur, V.; Kapulnik, Y.; Sitrit, Y. Mycorrhizal association between the desert truffle Terfezia boudieri and Helianthemum sessiliflorum alters plant physiology and fitness to arid conditions. Mycorrhiza 2011, 21, 623–630. [Google Scholar] [CrossRef]
- Ma, Y.; He, J.; Ma, C.; Luo, J.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.-B. Ectomycorrhizas with Paxillus involutusenhance cadmium uptake and tolerance in Populus × canescens. Plant Cell Environ. 2014, 37, 627–642. [Google Scholar] [CrossRef]
- Khaleghi, E.; Arzani, K.; Moallemi, N.; Barzegar, M. Evaluation of chlorophyll content and chlorophyll fluorescence parameters and relationships between chlorophyll a,b and chlorophyll content index under water stress in Olea europaea cv. Dezful. World Acad. Sci. Eng. Technol. 2012, 68, 1154–1157. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Goltsev, V.N.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Aleksandrov, V.; Krasteva, V.; Paunov, M.; Chepisheva, M.; Kousmanova, M.; Kalaji, H.M.; Goltsev, V. Deficiency of some nutrient elements in bean and maize plants analyzed by luminescent method. Bulg. J. Agric. Sci. 2014, 20, 24–30. [Google Scholar]
- Kalaji, M.H.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Milewska-Hendel, A.; Baczewska, A.H.; Sala, K.; Dmuchowski, W.; Brągoszewska, P.; Gozdowski, D.; Jozwiak, A.; Chojnacki, T.; Swiezewska, E.; Kurczynska, E. Quantitative and qualitative characteristics of cell wall components and prenyl lipids in the leaves of Tilia x euchlora trees growing under salt stress. PLoS ONE 2017, 12, e0172682. [Google Scholar] [CrossRef]
- Scattolin, L.; Alzetta, C.; Bolzon, P.; Sambo, P.; Accordi, S.M. Linden tree stress detection: Chlorophyll–nitrogen contents and ectomycorrhizal community. Plant Biosyst. 2013, 147, 364–375. [Google Scholar] [CrossRef]
- Burton, K.W.; King, J.B.; Morgan, E. Chlorophyll as an indicator of the upper critical tissue concentration of cadmium in plants. Water Air Soil Pollut. 1986, 27, 147–154. [Google Scholar] [CrossRef]
- Berger, S.; Benediktyová, Z.; Matouš, K.; Bonfig, K.; Mueller, M.J.; Nedbal, L.; Roitsch, T. Visualization of dynamics of plant–pathogen interaction by novel combination of chlorophyll fluorescence imaging and statistical analysis: Differential effects of virulent and avirulent strains of P. syringae and of oxylipins on A. thaliana. J. Exp. Bot. 2007, 58, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Penuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Lichtenthaler, K.; Welburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Agerer, R. Characterization of ectomycorrhizae. In Methods in Microbiology: Techniques for the Study of Mycorrhiza; Norris, J.R., Read, D.J., Varma, A.K., Eds.; Academic Press: London, UK, 1991; pp. 25–73. [Google Scholar]
- Agerer, R. Colour Atlas of Ectomycorrhizae, 1st ed.; Einhorn-Verlag: Munich, Germany, 1987. [Google Scholar]
- Olchowik, J.; Bzdyk, R.; Studnicki, M.; Bednarska-Błaszczyk, M.; Urban, A.; Aleksandrowicz-Trzcinńska, M. The effects of silver and copper nanoparticles on the condition of English oak (Quercus robur L.) seedlings in a container nursery experiment. Forests 2017, 8, 310. [Google Scholar] [CrossRef]
- Olchowik, J.; Hilszczańska, D.; Bzdyk, R.M.; Studnicki, M.; Malewski, T.; Borowski, Z. Effect of Deadwood on Ectomycorrhizal Colonisation of Old-Growth Oak Forests. Forests 2019, 10, 480. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimsha, W.H.N.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford, UK, 1974. [Google Scholar]
- LaCroix, R.L.; Keeney, D.R.; Walsh, L.M. Potentiometric titration of chloride in plant tissue extracts using the chloride ion electrode. Commun. Soil Sci. Plan. 1970, 1, 1–6. [Google Scholar] [CrossRef]
- ISO 13878. Soil Quality. In Determination of Total Nitrogen Content by Dry Combustion (“Elemental Analysis”); International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- ISO 10694. Soil Quality. In Determination of Organic and Total Carbon after Dry Combustion (“Elementary Analysis”); International Organization for Standardization: Geneva, Switzerland, 2002. [Google Scholar]
- ISO 11260. Soil Quality. In Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- ISO 10390. Soil Quality. In Determination of pH; International Organization for Standardization: Geneva, Switzerland, 1997. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B. Vegan: Community Ecology Package. R Package Version 2.4-2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 5 August 2020).
- Read, D.J. Mycorrhizas in ecosystems. Experientia 1991, 47, 376–391. [Google Scholar] [CrossRef]
- Aggangan, N.S.; Dell, B.; Malajczuk, N. Effects of soil pH on the ectomycorrhizal response of Eucalyptus urophylla seedlings. New Phytol. 1996, 134, 539–546. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Douma, J.C.; Akhmetzhanova, A.A.; van Bodegom, P.M.; Cornwell, W.K.; Moens, E.J.; Treseder, K.K.; Tibbett, M.; Wang, Y.-P.; Cornelissen, J.H.C. Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Glob. Ecol. Biogeogr. 2015, 24, 371–382. [Google Scholar] [CrossRef]
- Pritsch, K.; Raidl, S.; Marksteiner, E.; Blaschke, H.; Agerer, R.; Schloter, M.; Hartmann, A. A rapid and highly sensitive method for measuring enzyme activities in single mycorrhizal tips using 4-methylumbelliferone-labelled fluorogenic substrates in a microplate system. J. Microbiol. Methods 2004, 58, 233–241. [Google Scholar] [CrossRef]
- Courty, P.-E.; Pritsch, K.; Schloter, M.; Hartmann, A.; Garbaye, J. Activity profiling of ectomycorrhiza communities in two forest soils using multiple enzymatic tests. New Phytol. 2005, 167, 309–319. [Google Scholar] [CrossRef]
- Rudawska, M.; Pietras, M.; Smutek, I.; Strzeliński, P.; Leski, T. Ectomycorrhizal fungal assemblages of Abies alba Mill. outside its native range in Poland. Mycorrhiza 2016, 26, 57–65. [Google Scholar] [CrossRef]
- Leski, T.; Rudawska, M. Zbiorowiska grzybów ektomykoryzowych modrzewia europejskiego na powierzchni proweniencyjnej w LZD Krynica w Beskidzie Sądeckim. Sylwan 2014, 158, 352–360. [Google Scholar]
- Tedersoo, L.; Kõljalg, U.; Hallenberg, N.; Larsson, K.H. Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol. 2003, 159, 153–165. [Google Scholar] [CrossRef]
- Pigott, C.D. Survival of mycorrhiza formed by Cenococcum geophilum fr. in dry soils. New Phytol. 1982, 92, 513–517. [Google Scholar] [CrossRef]
- Jany, J.L.; Martin, F.; Garbaye, J. Respiration activity of ectomycorrhizas from Cenococcum geophilum and Lactarius sp in relation to soil water potential in five beech forests. Plant Soil 2003, 255, 487–494. [Google Scholar] [CrossRef]
- Alzetta, C.; Scattolin, L.; Scopel, C.; Mutto Accordi, S. The ectomycorrhizal community in urban linden trees and its relationship with soil properties. Trees 2011, 26, 751–767. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Visser, S. Ectomycorrhizal fungal succession in jack pine stands following wildfire. New Phytol. 1995, 129, 389–401. [Google Scholar] [CrossRef]
- Twieg, B.D.; Durall, D.M.; Simard, S.W. Ectomycorrhizal fungal succession in mixed temperate forests. New Phytol. 2007, 176, 437–447. [Google Scholar] [CrossRef]
- Newton, A.C. Towards a functional classification of ectomycorrhizal fungi. Mycorrhiza 1992, 2, 75–79. [Google Scholar] [CrossRef]
- Branco, S.; Gladieux, P.; Ellison, C.E.; Kuo, A.; LaButti, K.; Lipzen, A.; Grigoriev, I.V.; Liao, H.-L.; Vilgalys, R.; Peay, K.G. Genetic isolation between two recently diverged populations of a symbiotic fungus. Mol. Ecol. 2015, 24, 2747–2758. [Google Scholar] [CrossRef]
- Bills, R.J.; Stutz, J.C. AMF Associated with Indigenous and Non-indigenous Plants at Urban and Desert Sites in Arizona. In Mycorrhizas-Functional Processes and Ecological Impact; Azcon-Aguilar, C., Barea, J.S., Gianinazzi, S., Gianinazzi-Pearson, V., Eds.; Springer: Berlin, Germany, 2009; pp. 207–220. [Google Scholar] [CrossRef]
- Turpeinen, R.; Kairesalo, T.; Häggblom, M.M. Microbial community structure and activity in arsenic-, chromium- and copper-contaminated soils. FEMS Microbiol. Ecol. 2004, 47, 39–50. [Google Scholar] [CrossRef]
- Sa, G.; Yao, J.; Deng, C.; Liu, J.; Zhang, Y.; Zhu, Z.; Zhang, Y.; Ma, X.; Zhao, R.; Lin, S.; et al. Amelioration of nitrate uptake under salt stress by ectomycorrhiza with and without a Hartig net. New Phytol. 2019, 222, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin-Nadeau, M.; Gagné, A.; Bissonnette, C.; Bélanger, P.-A.; Fortin, J.A.; Roy, S.; Greer, C.W.; Khasa, D.P. Performance of ectomycorrhizal alders exposed to specific Canadian oil sands tailing stressors under in vivo bipartite symbiotic conditions. Can. J. Microbiol. 2016, 62, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Muhsin, T.M.; Zwiazek, J.J. Colonization with Hebeloma crustuliniforme increases water conductance and limits shoot sodi-um uptake in white spruce (Picea glauca) seedlings. Plant Soil 2002, 238, 217–225. [Google Scholar] [CrossRef]
- Zwiazek, J.J.; Equiza, M.A.; Karst, J.; Senorans, J.; Wartenbe, M.; Calvo-Polanco, M. Role of urban ectomycorrhizal fungi in improving the tolerance of lodgepole pine (Pinus contorta) seedlings to salt stress. Mycorrhiza 2019, 29, 303–312. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, R.E. Soil Microbiology and Biochemistry; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Kleiber, T.; Krzyżaniak, M.; Świerk, D.; Haenel, A.; Gałecka, S. How does the content of nutrients in soil affect the health status of trees in city parks? PLoS ONE 2019, 14, e0221514. [Google Scholar] [CrossRef]
- Breś, W. Anthropopressure factors causing trees to die off in urban landscape. Nauka Przyr. Technol. 2008, 2, 31. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).