Earthworms as an Ecological Indicator of Soil Recovery after Mechanized Logging Operations in Mixed Beech Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
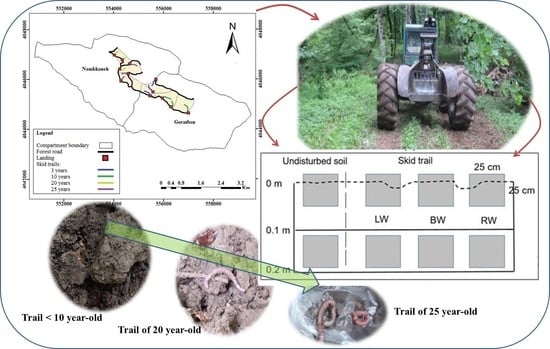
2.2. Experimental Design and Data Collection
2.3. Measurements and Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Earthworm Density and Biomass
3.2. Principal Component Analysis (PCA)
4. Discussion
5. Conclusions
- Selection of advanced technologies such as real-time mapping, sensors, and optimization of skid trails to reduce unnecessary traffic and prevent the passage of machinery on sensitive areas (wet soils, regenerated tree areas) and thus reduce potential soil damage and increase regeneration capacity of forest stands.
- Assessing soil disturbances during harvesting operations using live monitoring techniques such as machine-mounted LiDAR systems or other types of remote sensing technologies to scan the physical environment is a fundamental step to a better understanding of forest soil disturbances.
- Use of high-powered machinery (high engine power) with flexible tires to reduce the number of passes required to complete timber extraction.
- Using techniques such as wide tires or slash mats to reduce soil compaction.
- The use of improvement treatments such as different foliage mulch and tree planting (especially mixed broadleaf species with low C/N ratio) and comparing their performance in terms of the recovery of the soil properties of skid trails to accelerate biological activity (especially earthworms) and the natural regeneration of trees.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, F.Z.; Kang, D.; Han, X.H.; Yang, G.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Soil stoichiometry and carbon storage in long-term afforestation soil affected by understory vegetation diversity. Ecol. Eng. 2015, 74, 415–422. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Goberna, M.; Verdú, M. Using plant functional distances to select species for restoration of mining sites. J. Appl. Ecol. 2019, 56, 2353–2362. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Verdú, M.; Goberna, M. Trait-based selection of nurse plants to restore ecosystem functions in mine tailings. J. Appl. Ecol. 2018, 55, 1195–1206. [Google Scholar] [CrossRef]
- Venanzi, R.; Picchio, R.; Piovesan, G. Silvicultural and logging impact on soil characteristics in Chestnut (Castanea sativa Mill.) Mediterranean coppice. Ecol. Eng. 2016, 96, 82–89. [Google Scholar] [CrossRef]
- Jourgholami, M.; Ghassemi, T.; Labelle, E.R. Soil physio-chemical and biological indicators to evaluate the restoration of compacted soil following reforestation. Ecol. Indic. 2019, 101, 102–110. [Google Scholar] [CrossRef]
- Marchi, E.; Chung, W.; Visser, R.; Abbas, D.; Nordfjell, T.; Mederski, P.S.; McEwan, A.; Brink, M.; Laschi, A. Sustainable Forest Operations (SFO): A new paradigm in a changing world and climate. Sci. Total Environ. 2018, 634, 1385–1397. [Google Scholar] [CrossRef] [Green Version]
- Schweier, J.; Magagnotti, N.; Labelle, E.R.; Athanassiadis, D. Sustainability impact assessment of forest operations: A review. Curr. For. Rep. 2019, 5, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Jourgholami, M.; Khajavi, S.; Labelle, E.R. Recovery of forest soil chemical properties following soil rehabilitation treatments: An assessment six years after machine impact. Croat. J. For. Eng. 2020, 41, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Mitsch, W.J.; Jørgensen, S.E. Ecological engineering: A field whose time has come. Ecol. Eng. 2003, 20, 363–377. [Google Scholar] [CrossRef]
- Jourgholami, M.; Nasirian, A.; Labelle, E. Ecological restoration of compacted soil following the application of different leaf litter mulches on the skid trail over a five-year period. Sustainability 2018, 10, 2148. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, H.; Jourgholami, M.; Tavankar, F.; Venanzi, R.; Picchio, R. Post-harvest evaluation of soil physical properties and natural regeneration growth in steep-slope terrains. Forests 2019, 10, 1034. [Google Scholar] [CrossRef] [Green Version]
- Nikooy, M.; Tavankar, F.; Naghdi, R.; Ghorbani, A.; Jourgholami, M.; Picchio, R. Soil impacts and residual stand damage from thinning operations. Int. J. For. Eng. 2020, 31, 126–137. [Google Scholar]
- Zenner, E.K.; Fauskee, J.T.; Berger, A.L.; Puettmann, K.J. Impacts of skidding traffic intensity on soil disturbance, soil recovery, and aspen regeneration in north-central Minnesota. North. J. Appl. For. 2007, 24, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Ezzati, S.; Najaf, A.; Rab, M.A.; Zenner, E.K. Recovery of soil bulk density, porosity and rutting from ground skidding over a 20-year period after timber harvesting in Iran. Silva Fenn. 2012, 46, 521–538. [Google Scholar] [CrossRef] [Green Version]
- Picchio, R.; Mederski, P.S.; Tavankar, F. How and how much, do harvesting activities affect forest soil, regeneration and stands? Cur. For. Rep. 2020. [CrossRef] [Green Version]
- Picchio, R.; Neri, F.; Petrini, E.; Verani, S.; Marchi, E.; Certini, G. Machinery-induced soil compaction in thinning two pine stands in central Italy. For. Ecol. Manag. 2012, 285, 38–43. [Google Scholar] [CrossRef]
- Jourgholami, M.; Soltanpour, S.; Etehadi Abari, M.; Zenner, E.K. Influence of slope on physical soil disturbance due to farm tractor forwarding in a Hyrcanian forest of northern Iran. iForest 2014, 7, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Jouquet, P.; Bernard-Reversat, F.; Bottinelli, N.; Orange, D.; Rouland-Lefèvre, C.; Duc, T.T.; Podwojewski, P. Influence of changes in land use and earthworm activities on carbon and nitrogen dynamics in a steepland ecosystem in Northern Vietnam. Biol. Fert. Soils 2007, 44, 69–77. [Google Scholar] [CrossRef]
- Jordan, D.; Hubbard, V.C.; Ponder, F., Jr.; Berry, E.C. The influence of soil compaction and the removal of organic matter on two native earthworms and soil properties in an oak-hickory forest. Biol. Fert. Soils 2000, 31, 323–328. [Google Scholar] [CrossRef]
- Picchio, R.; Tavankar, F.; Nikooy, M.; Pignatti, G.; Venanzi, R.; Lo Monaco, A. Morphology, growth and architecture response of beech (Fagus orientalis Lipsky) and maple tree (Acer velutinum Boiss.) seedlings to soil compaction stress caused by mechanized logging operations. Forests 2019, 10, 771. [Google Scholar] [CrossRef] [Green Version]
- Bottinelli, N.; Capowiezc, Y.; Ranger, J. Slow recovery of earthworm populations after heavy traffic in two forest soils in northern France. Appl. Soil Ecol. 2014, 73, 130–133. [Google Scholar] [CrossRef]
- Jourgholami, M.; Labelle, E.R.; Feghhi, J. Efficacy of leaf litter mulch to mitigate runoff and sediment yield following mechanized operations in the Hyrcanian mixed forests. J. Soils Sediments 2019, 19, 2076–2088. [Google Scholar] [CrossRef]
- Jourgholami, M.; Ramineh, A.; Zahedi Amiri, G.; Labelle, E.R. The Influence of slope positions on the recovery response of compacted soil properties and enzyme activity in an Oriental beech stand in the Hyrcanian forests, Iran. Sustainability 2019, 11, 1940. [Google Scholar] [CrossRef] [Green Version]
- Hseu, Z.Y.; Chen, Z.S.; Wu, Z.D. Characterization of placic horizons in two subalpine forest Inceptisols. Soil Sci. Soc. Am. J. 1999, 63, 941–947. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdick, D.F., Stewart, B.A., Eds.; SSSA Special Publication: Madison, WI, USA, 1994; pp. 3–21. [Google Scholar]
- Cambi, M.; Hoshika, Y.; Mariotti, B.; Paoletti, E.; Picchio, R.; Venanzi, R.; Marchi, E. Compaction by a forest machine affects soil quality and Quercus robur L. seedling performance in an experimental field. For. Ecol. Manag. 2017, 384, 406–414. [Google Scholar] [CrossRef]
- Picchio, R.; Venanzi, R.; Tavankar, F.; Luchenti, I.; Bodaghi, A.I.; Latterini, F.; Nikooy, M.; Di Marzio, N.; Naghdi, R. Changes in soil parameters of forests after windstorms and timber extraction. Eur. J. For. Res. 2019, 138, 875–888. [Google Scholar] [CrossRef]
- Picchio, R.; Mercurio, R.; Venanzi, R.; Gratani, L.; Giallonardo, T.; Lo Monaco, A.; Frattaroli, A.R. Strip clear-cutting application and logging typologies for renaturalization of pine afforestation—A case study. Forests 2018, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. In Ecosystem Management; Springer: New York, NY, USA, 1994; pp. 130–147. [Google Scholar]
- Frouz, J.; Livečková, M.; Albrechtová, J.; Chroňáková, A.; Cajthaml, T.; Pižl, V.; Háněl, L.; Starý, J.; Baldrian, P.; Lhotáková, Z.; et al. Is the effect of trees on soil properties mediated by soil fauna? A case study from post-mining sites. For. Ecol. Manag. 2013, 309, 87–95. [Google Scholar] [CrossRef]
- Tondoh, J.E.; Guei, A.M.; Csuzdi, C.; Okoth, P. EX’ffect of land-use on the earthworm assemblages insemi-deciduous forests of Central-West Ivory Coast. Biodivers. Conserv. 2011, 20, 169–184. [Google Scholar] [CrossRef]
- Rab, M.A. Recovery of soil physical properties from compaction and soil profile disturbance caused by logging of native forest in Victorian Central Highlands, Australia. For. Ecol. Manag. 2004, 191, 329–340. [Google Scholar] [CrossRef]
- Ebeling, C.; Fründ, H.; Lang, L.; Gaertig, T. Evidence for increased P availability on wheel tracks 10 to 40 years after forest machinery traffic. Geoderma 2016, 297, 61–69. [Google Scholar] [CrossRef]
- Fründ, H.C.; Averdiek, A. Soil aeration and soil water tension in skidding trails during three years after trafficking. For. Ecol. Manag. 2016, 380, 224–231. [Google Scholar] [CrossRef]
- Klaes, B.; Struck, J.; Schneider, R.; Schüler, G. Middle-term effects after timber harvesting with heavy machinery on a fine-textured forest soil. Eur. J. For. Res. 2016, 135, 1083–1095. [Google Scholar] [CrossRef]
- Greacen, E.L.; Sands, R. Compaction of forest soils. A review. Aust. J. Soil Res. 1980, 18, 163–189. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Soil compaction and growth of woody plants. Scand. J. For. Res. 1999, 14, 596–619. [Google Scholar] [CrossRef]
- Meyer, C.; Lüscher, P.; Schulin, R. Enhancing the regeneration of compacted forest soils by planting black alder in skid lane tracks. Eur. J. For. Res. 2014, 133, 453–465. [Google Scholar] [CrossRef]
- DeArmond, D.; Emmert, F.; Nogueira Lima, A.J.; Higuchi, N. Impacts of soil compaction persist 30 years after logging operations in the Amazon Basin. Soil Till. Res. 2019, 189, 207–216. [Google Scholar] [CrossRef]
- Lee, K.E.; Foster, R.C. Soil fauna and soil structure. Aust. J. Soil Res. 1991, 29, 745–775. [Google Scholar] [CrossRef]
- Lavelle, P.; Melendez, G.; Pashanasi, B.; Schaefer, R. Nitrogen mineralization and reorganization in casts of the geophagous tropical earthworm Pontoscolex corethrurus (Glossoscolecidae). Biol. Fertil. Soil 1992, 14, 49–53. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A. Soil Ecology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; p. 654. [Google Scholar]
- Capowiez, Y.; Samartino, S.; Cadoux, S.; Bouchant, P.; Richard, G.; Boizard, H. Role of earthworms in regenerating soil structure after compaction in reduced tillage systems. Soil Biol. Biochem. 2012, 55, 93–103. [Google Scholar]
- Blanchart, E.; Albrecht, A.; Alegre, J.; Duboisset, A.; Gilot, C.; Pashanasi, B.; Lavelle, P.; Brussaard, L. Effects of earthworms on soil structure and physical properties. In Earthworm Management in Tropical Agroecosystems; CABI Publishing: New York, NY, USA, 1999; pp. 149–171. [Google Scholar]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms, 3rd ed.; Chapman and Hall: London, UK, 1996; p. 426. [Google Scholar]
- Lavelle, P.; Bignell, D.; Lepage, M.; Wolters, V.; Roger, P.A.; Ineson, P.O.W.H.; Heal, O.W.; Dhillion, S. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 1997, 33, 159–193. [Google Scholar]
- Hale, C.M.; Frelich, L.E.; Reich, P.B.; Pastor, J. Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota, USA. Ecosystems 2005, 8, 911–927. [Google Scholar] [CrossRef]
- Ojha, R.B.; Devkota, D. Earthworms: Soil and Ecosystem Engineers–A review. World J. Agric. Res. 2014, 2, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, J.; Vig, A.P. Earthworm as ecological engineers to change the physio-chemical properties of soil: Soil vs vermicast. Ecol. Eng. 2016, 90, 1–5. [Google Scholar] [CrossRef]
- Larink, O.; Werner, D.; Langmaack, M.; Schrader, S. Regeneration of compacted soil aggregates by earthworm activity. Biol. Fert. Soils 2001, 33, 395–401. [Google Scholar]
- Heydari, M.; Poorbabaei, H.; Bazgir, M.; Salehi, A.; Eshaghirad, J. Earthworms as indicators for different forest management types and human disturbance in Ilam oak forest, Iran. Folia For. Pol. 2014, 56, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Jiang, X.J.; Yang, B.; Wu, J.; Rai, A.; Chen, C.; Ahirwal, J.; Wang, P.; Liu, W.; Singh, N. Biological indicators affected by land use change, soil resource availability and seasonality in dry tropics. Ecol. Indic. 2020, 115, 106369. [Google Scholar] [CrossRef]
- Bhadauria, T.; Kumar, P.; Kumar, R.; Maikhuri, R.K.; Rao, K.S.; Saxena, K.G. Earthworm populations in a traditional village landscape in Central Himalaya, India. Appl. Soil Ecol. 2012, 53, 83–93. [Google Scholar] [CrossRef]
- Salehi, A.; Ghorbanzadeh, N.; Kahneh, E. Earthworm biomass and abundance, soil chemical and physical properties under different poplar plantations in the north of Iran. J. For. Sci. 2013, 59, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Sohrabi, H.; Jourgholami, M.; Jafari, M.; Shabanian, N.; Venanzi, R.; Tavankar, F.; Picchio, R. Soil recovery assessment after timber harvesting based on the Sustainable Forest Operation (SFO) perspective in Iranian temperate forests. Sustainability 2020, 12, 2874. [Google Scholar] [CrossRef] [Green Version]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis: Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeney, R.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Jordan, D.; Li, F.; Ponder, F., Jr.; Berry, E.C.; Hubbard, V.C.; Kim, K.Y. The effects of forest practices on earthworm populations and soil microbial biomass in a hardwood forest in Missouri. Appl. Soil Ecol. 1999, 13, 31–38. [Google Scholar] [CrossRef]
- Horn, R.; Vossbrink, J.; Becker, S. Modern forestry vehicles and their impacts on soil physical properties. Soil Till. Res. 2004, 79, 207–219. [Google Scholar] [CrossRef]
- Sautter, K.D.; Brown, G.G.; James, S.W.; Pasini, A.; Nunes, D.H.; Benito, N.P. Present knowledge on earthworm biodiversity in the State of Paraná, Brazil. Eur. J. Soil Biol. 2006, 42, 296–300. [Google Scholar] [CrossRef]
- Ampoorter, E.; De Schrijver, A.; De Frenne, P.; Hermy, M.; Verheyen, K. Experimental assessment of ecological restoration options for compacted forest soils. Ecol. Eng. 2011, 37, 1734–1746. [Google Scholar] [CrossRef]
- Marchi, E.; Picchio, R.; Spinelli, R.; Verani, S.; Venanzi, R.; Certini, G. Environmental impact assessment of different logging methods in pine forests thinning. Ecol. Eng. 2014, 70, 429–436. [Google Scholar] [CrossRef]
- Boström, U. The effect of soil compaction on earthworms (Lumbricidae) in a heavy clay soil. Swed. J. Agric. Res. 1986, 16, 137–141. [Google Scholar]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Edwards, C.A.; Reichle, D.E.; Crossley, D. The role of soil invertebrates in turnover of organic matter and nutrients. In Analysis of Temperate Forest Ecosystems; Springer: Berlin/Heidelberg, Germany, 1973; pp. 147–172. [Google Scholar]
- Schaller, F. Soil Animals; University of Michigan Press: Ann Arbor, MI, USA, 1968. [Google Scholar]
- Sayad, E.; Hosseini, S.M.; Hosseini, V.; Salehe-Shooshtari, M.H. Soil macrofauna in relation to soil and leaf litter properties in tree plantations. J. For. Sci. 2012, 58, 170–180. [Google Scholar] [CrossRef] [Green Version]
- Kooch, Y.; Tavakoli, M.; Akbarinia, M. Tree species could have substantial consequences on topsoil fauna: A feedback of land degradation/restoration. Eur. J. For. Res. 2018, 137, 793–805. [Google Scholar] [CrossRef]
- Zahedi, G.H. Relation between Ground Vegetation and Soil Characteristics in a Mixed Hardwood Stand. Ph.D. Thesis, University of Gent, Ghent, Belgium, 1998. [Google Scholar]
- Pižl, V. Effect of soil compaction on earthworms (Lumbricidae) in apple orchard soil. Soil Biol. Biochem. 1992, 24, 1573–1575. [Google Scholar] [CrossRef]
- Nawaz, M.F.; Bourrie, G.; Trolard, F. Soil compaction impact and modelling, A review. Agron. Sustain. Dev. 2013, 33, 291–309. [Google Scholar] [CrossRef] [Green Version]
- Dexter, A. Tunnelling in soil by earthworms. Soil Biol. Biochem. 1978, 10, 447–449. [Google Scholar] [CrossRef]
- Radford, B.; Wilson-Rummenie, A.; Simpson, G.; Bell, K.; Ferguson, M. Compacted soil affects soil macrofauna populations in a semi-arid environment in central Queensland. Soil Biol. Biochem. 2001, 33, 1869–1872. [Google Scholar] [CrossRef]
- Navarro-Cano, J.A.; Horner, B.; Goberna, M.; Verdú, M. Additive effects of nurse and facilitated plants on ecosystem functions. J. Ecol. 2019, 107, 2587–2597. [Google Scholar] [CrossRef]
- Jourgholami, M.; Fathi, K.; Labelle, E.R. Effects of litter and straw mulch amendments on compacted soil properties and Caucasian alder (Alnus subcordata) growth. New For. 2020, 51, 349–365. [Google Scholar] [CrossRef]
- Lloyd, R.A.; Lohse, K.A.; Ferré, T.P.A. Influence of road reclamation techniques on forest ecosystem recovery. Front. Ecol. Environ. 2013, 11, 75–81. [Google Scholar] [CrossRef]
- Jourgholami, M.; Feghhi, J.; Picchio, R.; Tavankar, F.; Venanzi, R. Efficiency of leaf litter mulch in the restoration of soil physiochemical properties and enzyme activities in temporary skid roads in mixed high forests. Catena 2020, 105012. [Google Scholar] [CrossRef]
- Andriuzzi, W.S.; Pulleman, M.M.; Schmidt, O.; Faber, J.H.; Brussaard, L. Anecic earthworms (Lumbricus terrestris) alleviate negative effects of extreme rainfall events on soil and plants in field mesocosms. Plant Soil 2015, 397, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Spurgeon, D.J.; Keith, A.M.; Schmidt, O.; Lammertsma, D.R.; Faber, J.H. Land-use and land-management change: Relationships with earthworm and fungi communities and soil structural properties. BMC Ecology 2013, 13, 46. [Google Scholar] [CrossRef] [Green Version]







| Source of Variable | d.f. | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|
| BD (g cm−3) | TP (%) | OC (%) | N (%) | C/N ratio | Ew D (ind. m−2) | Ew B (mg m−2) | ||
| A | 3 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 |
| T | 3 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | 0.422 | 0.429 |
| S | 2 | ≤0.01 | ≤0.01 | ≤0.05 | ≤0.01 | ≤0.01 | 0.090 | 0.115 |
| D | 1 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.01 | ≤0.05 | ≤0.05 |
| A × T | 9 | ≤0.01 | ≤0.01 | ≤0.05 | ≤0.01 | ≤0.01 | 0.824 | 0.817 |
| A × S | 6 | ≤0.01 | ≤0.05 | 0.770 | 0.148 | ≤0.01 | 0.903 | 0.940 |
| A × D | 3 | ≤0.05 | ≤0.01 | 0.889 | 0.735 | ≤0.01 | ≤0.01 | ≤0.01 |
| T × S | 6 | ≤0.05 | ≤0.05 | 0.522 | ≤0.01 | ≤0.05 | 0.798 | 0.828 |
| T × D | 3 | ≤0.01 | ≤0.01 | 0.420 | 0.530 | 0.059 | 0.843 | 0.897 |
| S × D | 2 | ≤0.05 | ≤0.01 | 0.544 | 0.870 | 0.216 | 0.757 | 0.728 |
| A × T × S | 18 | ≤0.05 | ≤0.05 | 0.847 | ≤0.01 | ≤0.05 | 0.982 | 0.980 |
| A × T × D | 9 | ≤0.05 | ≤0.05 | 0.452 | 0.918 | 0.188 | 0.933 | 0.917 |
| A × S × D | 6 | ≤0.05 | 0.418 | 0.155 | 0.656 | 0.562 | 0.915 | 0.908 |
| T × S × D | 6 | ≤0.05 | 0.082 | 0.524 | 0.768 | 0.117 | 0.867 | 0.848 |
| A × T × S × D | 18 | 0.117 | 0.316 | 0.914 | 0.920 | ≤0.05 | 0.832 | 0.855 |
| Soil Properties | Recovery Time (Skid Trails) | ||||
|---|---|---|---|---|---|
| 3 Years | 10 Years | 20 Years | 25 Years | Undisturbed Area | |
| BD (g cm−3) | 1.26 ± 0.01 a | 1.21 ± 0.01 b | 1.18 ± 0.03 b | 1.08 ± 0.01 c | 0.95 ± 0.03 d |
| TP (%) | 52.45 ± 0.24 c | 54.34 ± 0.24 c | 55.47 ± 0.24 c | 59.25 ± 0.24 b | 64.15 ± 0.15 a |
| OC (%) | 2.31 ± 0.13 d | 2.78 ± 0.13 c | 2.9 ± 0.13 c | 3.12 ± 0.13 b | 4.04 ± 0.09 a |
| N (%) | 0.2 ± 0.05 d | 0.27 ± 0.05 c | 0.36 ± 0.05 b | 0.41 ± 0.05 b | 0.67 ± 0.01 a |
| C/N ratio | 11.55 ± 0.07 a | 10.29 ± 0.07 a | 8.05 ± 0.07 b | 7.61 ± 0.07 b | 6.03 ± 0.13 c |
| Traffic Intensity | |||||
|---|---|---|---|---|---|
| Recovery Time (Skid Trails) | Depth (cm) | Undisturbed Area | Low | Medium | High |
| 3 years | 0–10 | 0.78 ± 0.24 a | 0.33 ± 0.22 b | 0.30 ± 0.20 b | 0.22 ± 0.20 c |
| 10–20 | 0.33 ± 0.24 a | 0.15 ± 0.22 b | 0.11 ± 0.19 c | 0.11 ± 0.19 c | |
| 10 years | 0–10 | 0.89 ± 0.24 a | 0.44 ± 0.20 b | 0.44 ± 0.20 b | 0.33 ± 0.20 c |
| 10–20 | 0.33 ± 0.24 a | 0.22 ± 0.19 b | 0.22 ± 0.19 b | 0.11 ± 0.19 c | |
| 20 years | 0–10 | 0.78 ± 0.24 a | 0.56 ± 0.20 b | 0.33 ± 0.20 c | 0.33 ± 0.20 c |
| 10–20 | 0.67 ± 0.24 a | 0.44 ± 0.19 b | 0.33 ± 0.19 bc | 0.22 ± 0.19 c | |
| 25 years | 0–10 | 0.89 ± 0.24 a | 0.58 ± 0.20 b | 0.56 ± 0.20 b | 0.33 ± 0.20 c |
| 10–20 | 0.68 ± 0.24 a | 0.33 ± 0.19 b | 0.33 ± 0.19 b | 0.30 ± 0.19 b | |
| Slope Classes | |||||
|---|---|---|---|---|---|
| Recovery Time (Skid Trails) | Depth (cm) | Undisturbed Area | 0–10% | 10–20% | 20–30% |
| 3 years | 0–10 | 0.78 ± 0.24 a | 0.33 ± 0.31 b | 0.30 ± 31 b | 0.22 ± 0.31 c |
| 10–20 | 0.33 ± 0.24 a | 0.11 ± 0.26 b | 0.15 ± 0.26 b | 0.11 ± 0.26 b | |
| 10 years | 0–10 | 0.89 ± 0.24 a | 0.44 ± 0.31 b | 0.44 ± 0.31 b | 0.33 ± 0.31 c |
| 10–20 | 0.33 ± 0.24 a | 0.11 ± 0.26 b | 0.11 ± 0.26 b | 0.11 ± 0.26 b | |
| 20 years | 0–10 | 0.78 ± 0.24 a | 0.56 ± 0.31 b | 0.56 ± 0.31 b | 0.11 ± 0.31 c |
| 10–20 | 0.67 ± 0.24 a | 0.44 ± 0.26 b | 0.44 ± 0.26 b | 0.11 ± 0.26 c | |
| 25 years | 0–10 | 0.89 ± 0.24 a | 0.58 ± 0.31 b | 0.56 ± 0.31 b | 0.33 ± 0.31 c |
| 10–20 | 0.68 ± 0.24 a | 0.56 ± 0.26 b | 0.22 ± 0.26 b | 0.22 ± 0.26 b | |
| Soil Properties | BD | TP | OC | N | C/N | Ew D | Ew B |
|---|---|---|---|---|---|---|---|
| BD | 1 | −0.96 ** | −0.95 ** | −0.92 ** | 0.40 ns | −0.55 * | −0.54 * |
| TP | 1 | 0.92 ** | 0.90 ** | −0.39 ns | 0.67 ** | 0.66 ** | |
| OC | 1 | 0.99 ** | −0.67 ** | 0.98 ** | 0.98 ** | ||
| N | 1 | −0.82 ** | 0.97 ** | 0.96 ** | |||
| C/N | 1 | −0.90 ** | −0.88 ** | ||||
| Ew D | 1 | 0.99 ** | |||||
| Ew B | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohrabi, H.; Jourgholami, M.; Jafari, M.; Tavankar, F.; Venanzi, R.; Picchio, R. Earthworms as an Ecological Indicator of Soil Recovery after Mechanized Logging Operations in Mixed Beech Forests. Forests 2021, 12, 18. https://doi.org/10.3390/f12010018
Sohrabi H, Jourgholami M, Jafari M, Tavankar F, Venanzi R, Picchio R. Earthworms as an Ecological Indicator of Soil Recovery after Mechanized Logging Operations in Mixed Beech Forests. Forests. 2021; 12(1):18. https://doi.org/10.3390/f12010018
Chicago/Turabian StyleSohrabi, Hadi, Meghdad Jourgholami, Mohammad Jafari, Farzam Tavankar, Rachele Venanzi, and Rodolfo Picchio. 2021. "Earthworms as an Ecological Indicator of Soil Recovery after Mechanized Logging Operations in Mixed Beech Forests" Forests 12, no. 1: 18. https://doi.org/10.3390/f12010018