Scaling the Roots Mechanical Reinforcement in Plantation of Cunninghamia R. Br in Southwest China
Abstract
1. Introduction
2. Materials and Methods
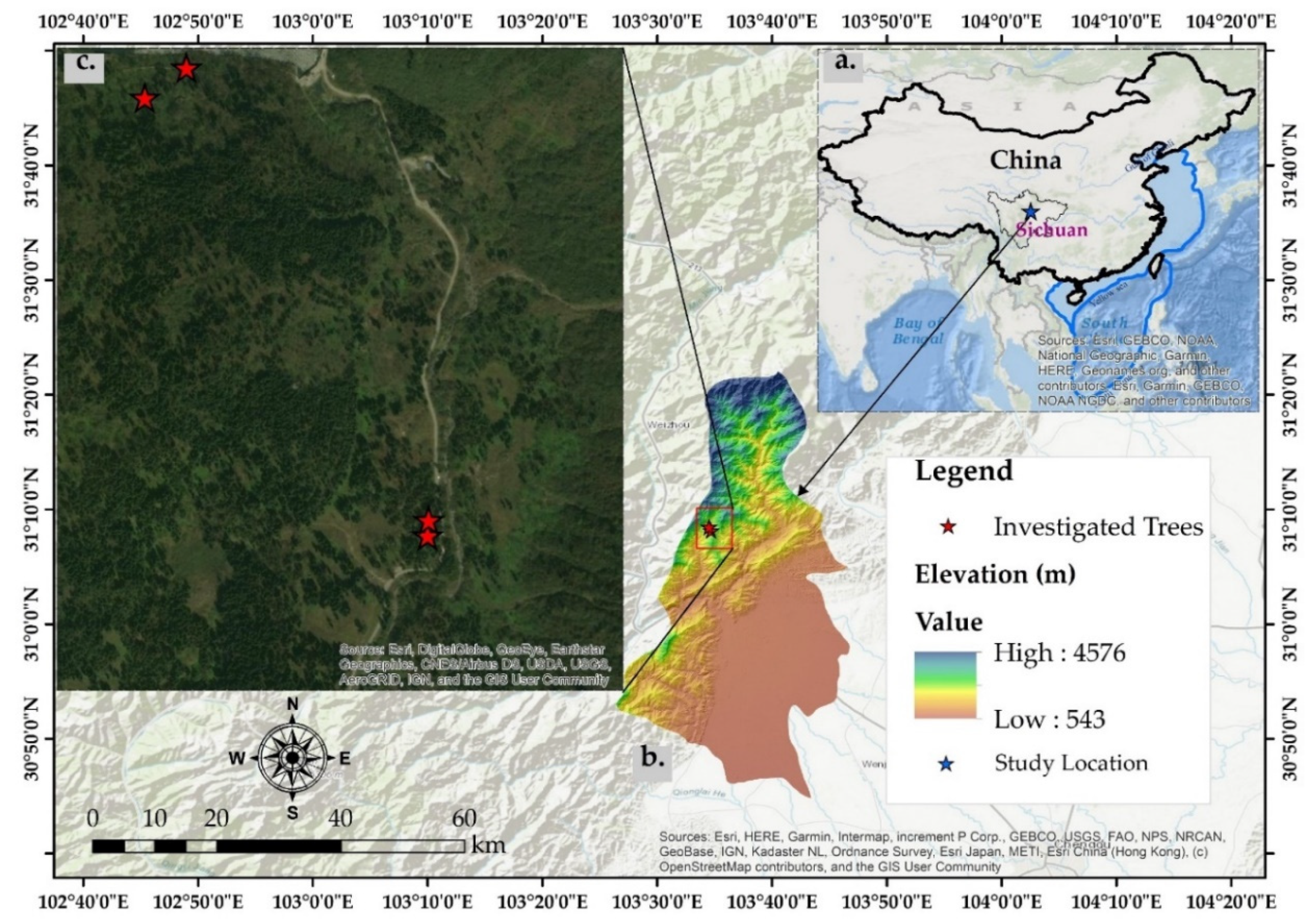
2.1. Study Area
2.2. Selection of Trees and Roots Sampling
2.3. Soil Properties
2.4. Excavation Methodology and Measurement of Root Traits
- The area under the crown of the candidate’s trees for excavation was properly cleaned. All the shrubs and other loose materials were removed.
- An excavation zone for each tree was established with a different diameter ranging between 2.70 m to 3.0 m (boundary marked with red color, Figure 3a).
- The sub-zones dimension was decided by ground slope conditions, tree stem diameter, and distribution of roots. On average, the length of one subzone in the horizontal direction was 20 cm. The excavation zones were carefully marked on the ground with white chalk powder.
- After marking the excavation area, incremental step by step excavation of the sub-zones was done both lateral and in the vertical direction. During excavation, the roots of the shrubs were removed with the excavated soil as the stem of all the shrubs were cut down during the surface cleaning process. Further, the texture of the roots facilitated us whether it belonged to the shrubs or selected tree.
- For each vertical excavation, 10 cm increments were selected below the ground surface until the roots maximum growth depth was reached. In each excavated zone, the roots, which were protruding from the vertical profile (a × h Figure 3b), were counted, and the diameter of each counted root was measured using a digital Vernier caliper. Only those roots were considered, which were in the cross-sectional area (a × h (Figure 3b)), assuming all these roots play a role in providing additional root cohesion. If a root was branched inside the excavated area, the branched roots were not counted, as only the roots crossing the cross-sectional area (a × h) will play a role in root cohesion at that profile [36]. From these roots counting and roots diameter, the roots area ( was calculated. This was used in equation −1 to calculate the roots area ratio (RAR). This calculation of RAR is consistent with the study of [7,18].
- Once the excavation for the vertical increment of 10 cm was completed, all the roots in this trench were cut down and properly bagged in plastic bags for roots biomass calculations before starting excavation for the next 10 cm in the same subzone.
- After excavation of the topsoil layer to a depth of 10 cm (Figure 3b), the next excavation was carried out 10 cm deeper with excavation in the lateral direction unchanged.
- For all the tree excavations, they were carried out in the upslope direction. The orientation of the tree roots was generally upslope and increased soil stability [37]. However, the trees developed different root architecture systems on different sides [38]; therefore, excavation was performed on the same side for all the trees for consistency in result comparison.
2.5. Developing Indices for Root Architectural System
2.6. Root Tensile Strength
2.7. Root Additional Cohesion
2.8. Statistical Analysis
3. Results
3.1. Roots System and Spatial Distribution of Roots
3.2. Distribution of Root Indices with Depth
3.3. Distribution of Root Indices with Horizontal Distance from the Tree Stem
3.4. Root Tensile Strength
3.5. Variation of Root Cohesion with Depth
3.6. Variation of Root Cohesion with Horizontal Distance from the Tree Stem
4. Discussion
4.1. Roots Traits and Architectural Indices
4.2. Roots Tensile Strength
4.3. Roots Cohesion
5. Conclusions
- The tree stem diameter is having a significant impact on the roots indices and root cohesion. The roots indices and root cohesion increase with an increase in tree diameter.
- The tree diameter governs the average root cohesion estimated for the investigated trees. The variation of stem diameter from 220 mm to 460 mm results in increased cohesion from 23 kPa to 63 kPa.
- The maximum depth and lateral distance of the root system for the investigated tree are 50 cm and 300 cm, respectively.
- The values of roots architectural indices are significantly higher in the topsoil depth range (0–20 cm) of the root zone and near to the tree stem in the lateral distance range (0–100 cm).
- Root cohesion estimated by FBM shows the same trend of decrease as that of WWM. However, FBM estimated that cohesion values were less than that of WWM values by the reduction factor of 0.55–0.79.
- The tree with a large stem diameter has more number of fine to medium roots (roots diameter <10 mm) than a tree with smaller stem diameter.
- The same diameter roots class of trees with large stem diameter is having more tensile strength as compared to trees with a smaller diameter. The increase in tree diameter from 220 mm to 468 mm diameter results in the increase of roots tensile strength by 33%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petley, D. Global patterns of loss of life from landslides. Geology 2012, 40, 927–930. [Google Scholar] [CrossRef]
- UN. Sendai Framework for Disaster Risk Reduction 2015–2030; UN: New York, NY, USA, 2015; Available online: http://www.preventionweb.net/files/43291_sendaiframeworkfordrren.pdf (accessed on 5 August 2020).
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development; UN A/RES/70/1; UN: New York, NY, USA, 2015; Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld/publication (accessed on 5 August 2020).
- Wu, T. Slope stabilization. In Slope Stabilization and Erosion Control: A Bioengineering Approach; Morgan, R.P.C., Rickson, R.J., Eds.; E&FN Spon: London, UK, 1995; pp. 233–281. [Google Scholar]
- Van Beek, L.P.H.; Wint, J.; Cammeraat, E.L.H.; Edwards, J.P. Observation and Simulation of Root Reinforcement on Abandoned Mediterranean Slopes. Plant Soil 2005, 278, 55–74. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Moresi, F.V.; Maesano, M.; Matteucci, G.; Romagnoli, M.; Sidle, R.C.; Mugnozza, G.S. Root Biomechanical Traits in a Montane Mediterranean Forest Watershed: Variations with Species Diversity and Soil Depth. Forests 2019, 10, 341. [Google Scholar] [CrossRef]
- Stokes, A.; Douglas, G.B.; Fourcaud, T.; Giadrossich, F.; Gillies, C.; Hubble, T.; Kim, J.H.; Loades, K.W.; Mao, Z.; McIvor, I.R.; et al. Ecological mitigation of hillslope instability: Ten key issues facing researchers and practitioners. Plant Soil 2014, 377, 1–23. [Google Scholar] [CrossRef]
- Giadrossich, F.; Cohen, D.; Schwarz, M.; Seddaiu, G.; Contran, N.; Lubino, M.; Valdés-Rodríguez, O.A.; Niedda, M.; Valdés-Rodrŕguez, O.A. Modeling bio-engineering traits of Jatropha curcas L. Ecol. Eng. 2016, 89, 40–48. [Google Scholar] [CrossRef]
- Graf, F.; Frei, M. Soil aggregate stability related to soil density, root length, and mycorrhiza using site-specific Alnus incana and Melanogaster variegatus s.l. Ecol. Eng. 2013, 57, 314–323. [Google Scholar] [CrossRef]
- Lozanova, L.; Zhiyanski, M.; Vanguelova, E.; Bratanova-Doncheva, S.; Marinov, M.; Lazarova, S. Dynamics and Vertical Distribution of Roots in European Beech Forests and Douglas Fir Plantations in Bulgaria. Forests 2019, 10, 1123. [Google Scholar] [CrossRef]
- Świtała, B.M.; Wu, W. Numerical modelling of rainfall-induced instability of vegetated slopes. Géotechnique 2018, 68, 481–491. [Google Scholar] [CrossRef]
- Waldron, L.; Dakessian, S. Soil reinforcement by roots: Calculation of increased soil shear resistance from root properties. Soil Sci. 1981, 132, 427–435. [Google Scholar] [CrossRef]
- Reubens, B.; Poesen, J.; Danjon, F.; Geudens, G.; Muys, B. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: A review. Trees 2007, 21, 385–402. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Reubens, B.; Wemans, K.; De Baerdemaeker, J.; Muys, B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- Pollen, N.; Simon, A. Estimating the mechanical effects of riparian vegetation on stream bank stability using a fiber bundle model. Water Resour. Res. 2005, 41. [Google Scholar] [CrossRef]
- Genet, M.; Kokutse, N.; Stokes, A.; Fourcaud, T.; Cai, X.; Ji, J.; Mickovski, S. Root reinforcement in plantations of Cryptomeria japonica D. Don: Effect of tree age and stand structure on slope stability. For. Ecol. Manag. 2008, 256, 1517–1526. [Google Scholar] [CrossRef]
- Fu, J.-T.; Hu, X.-S.; Brierley, G.; Qiao, N.; Yu, Q.-Q.; Lu, H.-J.; Li, G.-R.; Zhu, H.-L. The influence of plant root system architectural properties upon the stability of loess hillslopes, Northeast Qinghai, China. J. Mt. Sci. 2016, 13, 785–801. [Google Scholar] [CrossRef]
- Wang, X.; Hong, M.-M.; Huang, Z.; Zhao, Y.-F.; Ou, Y.-S.; Jia, H.-X.; Li, J. Biomechanical properties of plant root systems and their ability to stabilize slopes in geohazard-prone regions. Soil Tillage Res. 2019, 189, 148–157. [Google Scholar] [CrossRef]
- Bischetti, G.B.; Chiaradia, E.A.; Epis, T.; Morlotti, E. Root cohesion of forest species in the Italian Alps. Plant Soil 2009, 324, 71–89. [Google Scholar] [CrossRef]
- Wang, G.-X. Key technique in landslide control and its handling measures. Yanshilixue Yu Gongcheng Xuebao Chin. J. Rock Mech. Eng. 2005, 24, 3818–3827. [Google Scholar]
- Zhang, B.; Zhang, S.; Zhou, W. Investigation and assessment of landslides and debris flows in Sichuan province of China by remote sensing technique. Chin. Geogr. Sci. 2006, 16, 223–228. [Google Scholar] [CrossRef]
- Schmidt, K.; Roering, J.; Stock, J.; Dietrich, W.; Montgomery, D.; Schaub, T. The variability of root cohesion as an influence on shallow landslide susceptibility in the Oregon Coast Range. Can. Geotech. J. 2001, 38, 995–1024. [Google Scholar] [CrossRef]
- Greenwood, J.R. SLIP4EX—A Program for Routine Slope Stability Analysis to Include the Effects of Vegetation, Reinforcement and Hydrological Changes. Geotech. Geol. Eng. 2006, 24, 449–465. [Google Scholar] [CrossRef]
- Chiaradia, E.A.; Vergani, C.; Bischetti, G.B. Evaluation of the effects of three European forest types on slope stability by field and probabilistic analyses and their implications for forest management. For. Ecol. Manag. 2016, 370, 114–129. [Google Scholar] [CrossRef]
- Dietrich, W.; McKean, J.; Bellugi, D.; Perron, T. The prediction of shallow landslide location and size using a multidimensional landslide analysis in a digital terrain model. In Proceedings of the Fourth International Conference on Debris-Flow Hazards Mitigation: Mechanics, Prediction, and Assessment (DFHM-4), Chengdu, China, 10–13 September 2007; Chen, C.L., Major, J.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007. 12p. [Google Scholar]
- Hess, D.M.; Leshchinsky, B.A.; Bunn, M.; Mason, H.B.; Olsen, M.J. A simplified three-dimensional shallow landslide susceptibility framework considering topography and seismicity. Landslides 2017, 14, 1677–1697. [Google Scholar] [CrossRef]
- Milledge, D.; Bellugi, D.; Mckean, J.A.; Densmore, A.L.; Dietrich, W.E. A multidimensional stability model for predicting shallow landslide size and shape across landscapes. J. Geophys. Res. Earth Surf. 2014, 119, 2481–2504. [Google Scholar] [CrossRef]
- Ma, Y.; Li, C. Research on the Debris Flow Hazards after the Wenchuan Earthquake in Bayi Gully, Longchi, Dujiangyan, Sichuan Province, China. In Proceedings of the 2017 International Conference on Advanced Materials Science and Civil Engineering (AMSCE 2017), Phuket, Thailand, 21–22 April 2017. [Google Scholar]
- Chang, M.; Tang, C.; Zhang, D.-D.; Ma, G.-C. Debris flow susceptibility assessment using a probabilistic approach: A case study in the Longchi area, Sichuan province, China. J. Mt. Sci. 2014, 11, 1001–1014. [Google Scholar] [CrossRef]
- Coomes, D.A.; Grubb, P.J. Impacts of root competition in forests and woodlands: A theoretical framework and review of experiments. Ecol. Monogr. 2000, 70, 171–207. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Chen, L.-H.; Liu, Y.-P.; Ji, X.; Liu, X.-P. Triaxial compression test of soil–root composites to evaluate influence of roots on soil shear strength. Ecol. Eng. 2010, 36, 19–26. [Google Scholar] [CrossRef]
- GB/T50123 Standard for Soil Test Method; Ministry of Construction, P.R. China: Beijing, China, 2019. (In Chinese)
- Method C. In ASTM D1557; ASTM: West Conshohocken, PA, USA, 1991.
- Zhang, D.; Cheng, J.; Liu, Y.; Zhang, H.; Ma, L.; Mei, X.; Sun, Y. Spatio-Temporal Dynamic Architecture of Living Brush Mattress: Root System and Soil Shear Strength in Riverbanks. Forests 2018, 9, 493. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Meng, X.; Chen, G.; Zeng, R.; Yang, Q.; Liu, Y.; Wang, B. Evaluation of the Effects of Forest on Slope Stability and Its Implications for Forest Management: A Case Study of Bailong River Basin, China. Sustainability 2020, 12, 6655. [Google Scholar] [CrossRef]
- Yamadera, Y. Shizen Kankyou wo Saisei Suru Midori no Gizyutsu; Nougyoudoboku Jigyou Kyoukai: Tokyo, Japan, 1993; Volume 85. [Google Scholar]
- Nicoll, B.; Berthier, S.; Achim, A.; Gouskou, K.; Danjon, F.; Van Beek, L.P.H. The architecture of Picea sitchensis structural root systems on horizontal and sloping terrain. Trees 2006, 20, 701–712. [Google Scholar] [CrossRef]
- Gregory, P. Roots: Growth, Activity and Interactions with Soils; Wiley-Blackwell Press: Hoboken, NJ, USA, 2006. [Google Scholar]
- Gray, D.H.; Leiser, A.T. Biotechnical Slope Protection and Erosion Control; Van Nostrand Reinhold Company Inc.: New York, NY, USA, 1982. [Google Scholar]
- Nilaweera, N.S.; Nutalaya, P. Role of tree roots in slope stabilisation. Bull. Eng. Geol. Environ. 1999, 57, 337–342. [Google Scholar] [CrossRef]
- Bischetti, G.; Bonfanti, F.; Greppi, M. Misura della resistenza alla trazione delle radici: Apparato sperimentale e metodologia d’analisi. Quad. Idronomia Mont. 2003, 21, 349–360. [Google Scholar]
- Operstein, V.; Frydman, S. The influence of vegetation on soil strength. Proc. Inst. Civ. Eng. Ground Improv. 2000, 4, 81–89. [Google Scholar] [CrossRef]
- Wu, T.H.; McKinnell III, W.P.; Swanston, D.N. Strength of tree roots and landslides on Prince of Wales Island, Alaska. Can. Geotech. J. 1979, 16, 19–33. [Google Scholar] [CrossRef]
- Waldron, L.J. The Shear Resistance of Root-Permeated Homogeneous and Stratified Soil. Soil Sci. Soc. Am. J. 1977, 41, 843–849. [Google Scholar] [CrossRef]
- Burylo, M.; Hudek, C.; Rey, F. Soil reinforcement by the roots of six dominant species on eroded mountainous marly slopes (Southern Alps, France). Catena 2011, 84, 70–78. [Google Scholar] [CrossRef]
- Yen, C. Tree root patterns and erosion control. In Proceedings of the International Workshop on Soil Erosion and Its Countermeasures, Chiang Mai, Thailand, 11–19 November 1984; Soil and Water Conservation Society of Thailand: Bangkok, Thailand, 1987; pp. 92–111. [Google Scholar]
- Böhm, W. Root parameters and their measurement. In Methods of Studying Root Systems; Springer: Berlin/Heidelberg, Germany, 1979; pp. 125–138. [Google Scholar]
- Genet, M.; Stokes, A.; Fourcaud, T.; Norris, J.E. The influence of plant diversity on slope stability in a moist evergreen deciduous forest. Ecol. Eng. 2010, 36, 265–275. [Google Scholar] [CrossRef]
- Simon, A.; Collison, A.J.C. Quantifying the mechanical and hydrologic effects of riparian vegetation on streambank stability. Earth Surf. Process. Landf. 2002, 27, 527–546. [Google Scholar] [CrossRef]
- Pollen-Bankhead, N.; Simon, A. Hydrologic and hydraulic effects of riparian root networks on streambank stability: Is mechanical root-reinforcement the whole story? Geomorphology 2010, 116, 353–362. [Google Scholar] [CrossRef]
- Comino, E.; Druetta, A. The effect of Poaceae roots on the shear strength of soils in the Italian alpine environment. Soil Tillage Res. 2010, 106, 194–201. [Google Scholar] [CrossRef]
- Norris, J.E.; Stokes, A.; Mickovski, S.B.; Cammeraat, E.; van Beek, R.; Nicoll, B.C.; Achim, A. Slope Stability and Erosion Control: Ecotechnological Solutions; Springer: Dordrecht, Netherlands, 2008. [Google Scholar]
- Wu, T.H.; Beal, P.E.; Lan, C. In-situ shear test of soil-root systems. J. Geotech. Eng. 1988, 114, 1376–1394. [Google Scholar] [CrossRef]
- Danjon, F.; Reubens, B. Assessing and analyzing 3D architecture of woody root systems, a review of methods and applications in tree and soil stability, resource acquisition and allocation. Plant Soil 2008, 303, 1–34. [Google Scholar] [CrossRef]
- Ghestem, M.; Veylon, G.; Bernard, A.; Vanel, Q.; Stokes, A. Influence of plant root system morphology and architectural traits on soil shear resistance. Plant Soil 2014, 377, 43–61. [Google Scholar] [CrossRef]
- Sun, H.-L.; Li, S.-C.; Xiong, W.-L.; Yang, Z.-R.; Cui, B.-S.; Yang, T. Influence of slope on root system anchorage of Pinus yunnanensis. Ecol. Eng. 2008, 32, 60–67. [Google Scholar] [CrossRef]
- Chiatante, D.; Scippa, S.G.; Di Iorio, A.; Sarnataro, M. The Influence of Steep Slopes on Root System Development. J. Plant Growth Regul. 2003, 21, 247–260. [Google Scholar] [CrossRef]
- Stokes, A. The Supporting Roots of Trees and Woody Plants: Form, Function and Physiology; Springer: Dordrecht, Netherlands, 2013; Volume 87. [Google Scholar]
- Sudmeyer, R.A.; Speijers, J.; Nicholas, B.D. Root distribution of Pinus pinaster, P. radiata, Eucalyptus globulus and E. kochii and associated soil chemistry in agricultural land adjacent to tree lines. Tree Physiol. 2004, 24, 1333–1346. [Google Scholar] [CrossRef]
- Quine, C.P.; Burnand, A.C.; Coutts, M.P.; Reynard, B.R. Effects of Mounds and Stumps on the Root Architecture of Sitka Spruce on a Peaty Gley Restocking Site. For. Int. J. For. Res. 1991, 64, 385–401. [Google Scholar] [CrossRef]
- Coutts, M.; Nielsen, C.; Nicoll, B. The development of symmetry, rigidity and anchorage in the structural root system of conifers. Plant Soil 1999, 217, 1–15. [Google Scholar] [CrossRef]
- John, B.; Pandey, H.N.; Tripathi, R.S. Vertical distribution and seasonal changes of fine and coarse root mass in Pinus kesiya Royle Ex.Gordon forest of three different ages. Acta Oecol. 2001, 22, 293–300. [Google Scholar] [CrossRef]
- Goss, M.; Miller, M.; Bailey, L.; Grant, C. Root growth and distribution in relation to nutrient availability and uptake. Eur. J. Agron. 1993, 2, 57–67. [Google Scholar] [CrossRef]
- Rundel, P.; Nobel, P. Structure and Function in Desert Root Systems; Blackwell Scientific Publications: Oxford, UK, 1991; pp. 349–378. ISBN 0632027576. [Google Scholar]
- Genet, M.; Stokes, A.; Salin, F.; Mickovski, S.B.; Fourcaud, T.; Dumail, J.-F.; Van Beek, R. The Influence of Cellulose Content on Tensile Strength in Tree Roots. Plant Soil 2005, 278, 1–9. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Chen, L.-H.; Jiang, J. Why fine tree roots are stronger than thicker roots: The role of cellulose and lignin in relation to slope stability. Geomorphology 2014, 206, 196–202. [Google Scholar] [CrossRef]
- Vergani, C.; Schwarz, M.; Cohen, D.; Thormann, J.; Bischetti, G.B. Effects of root tensile force and diameter distribution variability on root reinforcement in the Swiss and Italian Alps. Can. J. For. Res. 2014, 44, 1426–1440. [Google Scholar] [CrossRef]
- Vergani, C.; Chiaradia, E.; Bischetti, G. Variability in the tensile resistance of roots in Alpine forest tree species. Ecol. Eng. 2012, 46, 43–56. [Google Scholar] [CrossRef]
- Hales, T.C.; Cole-Hawthorne, C.; Lovell, L.; Evans, S.L. Assessing the accuracy of simple field based root strength measurements. Plant Soil 2013, 372, 553–565. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wang, Y.; Ma, C. Effects of root spatial distribution on the elastic-plastic properties of soil-root blocks. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Tosi, M. Root tensile strength relationships and their slope stability implications of three shrub species in the Northern Apennines (Italy). Geomorphology 2007, 87, 268–283. [Google Scholar] [CrossRef]
- Docker, B.B.; Hubble, T.C.T. Quantifying root-reinforcement of river bank soils by four Australian tree species. Geomorphology 2008, 100, 401–418. [Google Scholar] [CrossRef]
- O’loughlin, C.; Ziemer, R.R. The importance of root strength and deterioration rates upon edaphic stability in steepland forests. In Proceedings of the IUFRO Workshop P. 1.07-00 Ecology of Subalpine Ecosystems as a Key to Management, Corvallis, OR, USA, 2–3 August 1982; Oregon State University: Corvallis, OR, USA, 1982; pp. 70–78. [Google Scholar]
- Mickovski, S.B.; Van Beek, L.P.H. Root morphology and effects on soil reinforcement and slope stability of young vetiver (Vetiveria zizanioides) plants grown in semi-arid climate. Plant Soil 2009, 324, 43–56. [Google Scholar] [CrossRef]
- Gray, D.H.; Ohashi, H. Mechanics of Fiber Reinforcement in Sand. J. Geotech. Eng. 1983, 109, 335–353. [Google Scholar] [CrossRef]












| Tree Specie | Location | Altitude (m) | DBH (mm) |
|---|---|---|---|
| Cunninghamia R. Br | Latitude: 31°8′9.78″ N Longitude: 103°34′41.01″ E | 1805 | 220 ± 0.3 |
| Latitude: 31°8′30.04″ N Longitude: 103°34′31.62′′E | 1806 | 450 ± 0.7 | |
| Latitude:31°8′10.41″ N Longitude: 103°34′41.13′′E | 1834 | 468 ± 1 | |
| Latitude:31° 8′28.97″ N Latitude:103°34′29.48″ E | 1843 | 320 ± 0.4 |
| Parameter | Tree Diameter = 220 mm | Tree Diameter = 320 mm | Tree Diameter = 450 mm | Tree Diameter = 568 mm |
|---|---|---|---|---|
| Gravel Content (%) | 7 | 11 | 9 | 12 |
| Sand Content (%) | 67 | 64 | 67 | 64 |
| <74 μm grain size content (%) | 27 | 25 | 24 | 24 |
| Moisture Content (%) | 20.56 | 20.08 | 20.66 | 20.06 |
| Bulk Unit Weight gm/cm3 | 1.453 | 1.445 | 1.453 | 1.445 |
| Liquid Limit (%) | 35.56 | 33.25 | 36.24 | 34.15 |
| Plastic Limit (%) | 49.96 | 48.96 | 49.25 | 48.16 |
| Plasticity Index | 14.31 | 15.71 | 13.01 | 14.01 |
| Root Diameter (mm) | With Depth | With Horizontal Distance | ||
|---|---|---|---|---|
| F | p | F | p | |
| d ≤ 1 | 14.17 | <0.001 | 16.752 | <0.001 |
| 1 < d ≤ 2 | 15.63 | <0.001 | 9.708 | 0.001 |
| 2 < d ≤ 5 | 20.68 | <0.001 | 10.31 | 0.001 |
| 5 < d ≤ 10 | 20.94 | <0.001 | 13.103 | <0.001 |
| Tree Diameter (mm) | Root Diameter (mm) | Tensile Strength (MPa) | |||
|---|---|---|---|---|---|
| Min | Max | Max | Min | Mean ± Standard Error | |
| 220 | 0.76 | 9.98 | 40 | 3 | 11.8 ± 2.1 |
| 320 | 0.50 | 9.81 | 107 | 3 | 14.1 ± 2.4 |
| 450 | 0.60 | 9.98 | 77 | 5 | 15.9 ± 2 |
| 468 | 0.67 | 8.53 | 70 | 6 | 19.9 ± 2 |
| Tree Diameter (mm) | Tensile Strength | Tensile Force | ||||
|---|---|---|---|---|---|---|
| a | b | R-Squared | a | b | R-Squared | |
| 220 | 20.369 | 0.522 | 0.37 | 18.890 | 1.364 | 0.80 |
| 320 | 26.470 | 0.636 | 0.47 | 15.980 | 1.478 | 0.82 |
| 450 | 28.930 | 0.644 | 0.52 | 22.710 | 1.476 | 0.83 |
| 468 | 30.640 | 0.523 | 0.36 | 24.050 | 1.478 | 0.81 |
| Depth (cm) | Cunninghamia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tree Dia=220 mm | Tree Dia = 320 mm | Tree Dia = 450 mm | Tree Dia = 468 mm | |||||||||
| cr (kPa) | cfbm (kPa) | cfbm/cr | cr (kPa) | cfbm (kPa) | cfbm/cr | cr (kPa) | cfbm (kPa) | cfbm/cr | cr (kPa) | cfbm (kPa) | cfbm/cr | |
| 10 | 42.36 | 30.03 | 0.71 | 51.69 | 35.02 | 0.68 | 67.43 | 46.20 | 0.69 | 78.23 | 62.0 | 0.79 |
| 20 | 39.17 | 25.46 | 0.65 | 47.16 | 30.97 | 0.66 | 60.95 | 43.38 | 0.71 | 77.4 | 54.1 | 0.70 |
| 30 | 11.85 | 10.54 | 0.89 | 13.59 | 6.91 | 0.51 | 40.03 | 16.64 | 0.42 | 44.1 | 33.6 | 0.76 |
| 40 | 4.52 | 3.32 | 0.74 | 4.86 | 3.40 | 0.70 | 13.94 | 7.26 | 0.52 | 10.9 | 7.5 | 0.69 |
| 50 | - | - | - | - | - | - | 16.38 | 4.54 | 0.28 | 8.9 | 3.9 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehtab, A.; Jiang, Y.-J.; Su, L.-J.; Shamsher, S.; Li, J.-J.; Mahfuzur, R. Scaling the Roots Mechanical Reinforcement in Plantation of Cunninghamia R. Br in Southwest China. Forests 2021, 12, 33. https://doi.org/10.3390/f12010033
Mehtab A, Jiang Y-J, Su L-J, Shamsher S, Li J-J, Mahfuzur R. Scaling the Roots Mechanical Reinforcement in Plantation of Cunninghamia R. Br in Southwest China. Forests. 2021; 12(1):33. https://doi.org/10.3390/f12010033
Chicago/Turabian StyleMehtab, Alam, Yuan-Jun Jiang, Li-Jun Su, Sadiq Shamsher, Jia-Jia Li, and Rahman Mahfuzur. 2021. "Scaling the Roots Mechanical Reinforcement in Plantation of Cunninghamia R. Br in Southwest China" Forests 12, no. 1: 33. https://doi.org/10.3390/f12010033
APA StyleMehtab, A., Jiang, Y.-J., Su, L.-J., Shamsher, S., Li, J.-J., & Mahfuzur, R. (2021). Scaling the Roots Mechanical Reinforcement in Plantation of Cunninghamia R. Br in Southwest China. Forests, 12(1), 33. https://doi.org/10.3390/f12010033






