Variability among Sites and Climate Models Contribute to Uncertain Spruce Growth Projections in Denmark
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampled Sites and Trees
2.2. Tree-Ring Width Measurements and Analysis
2.3. Climate-Growth Relationships
2.4. Multiple Regression Models to Assess the Climatic Response
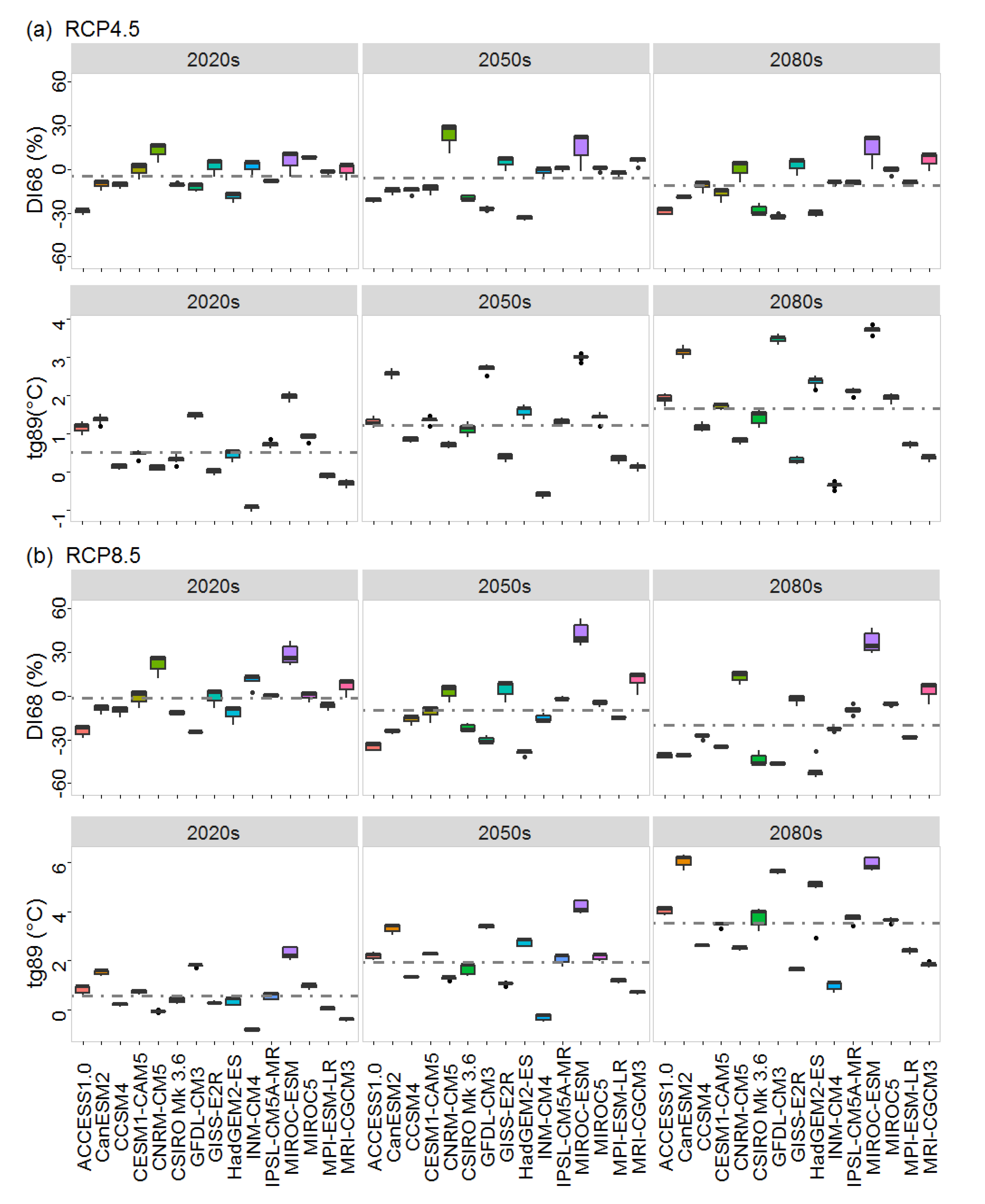
2.5. Prediction of Future Growth of Norway Spruce
3. Results
3.1. Annual Growth Rates, Common Signals, Main Climatic Drivers and Stand/Trial-Climatic Relationship Variation
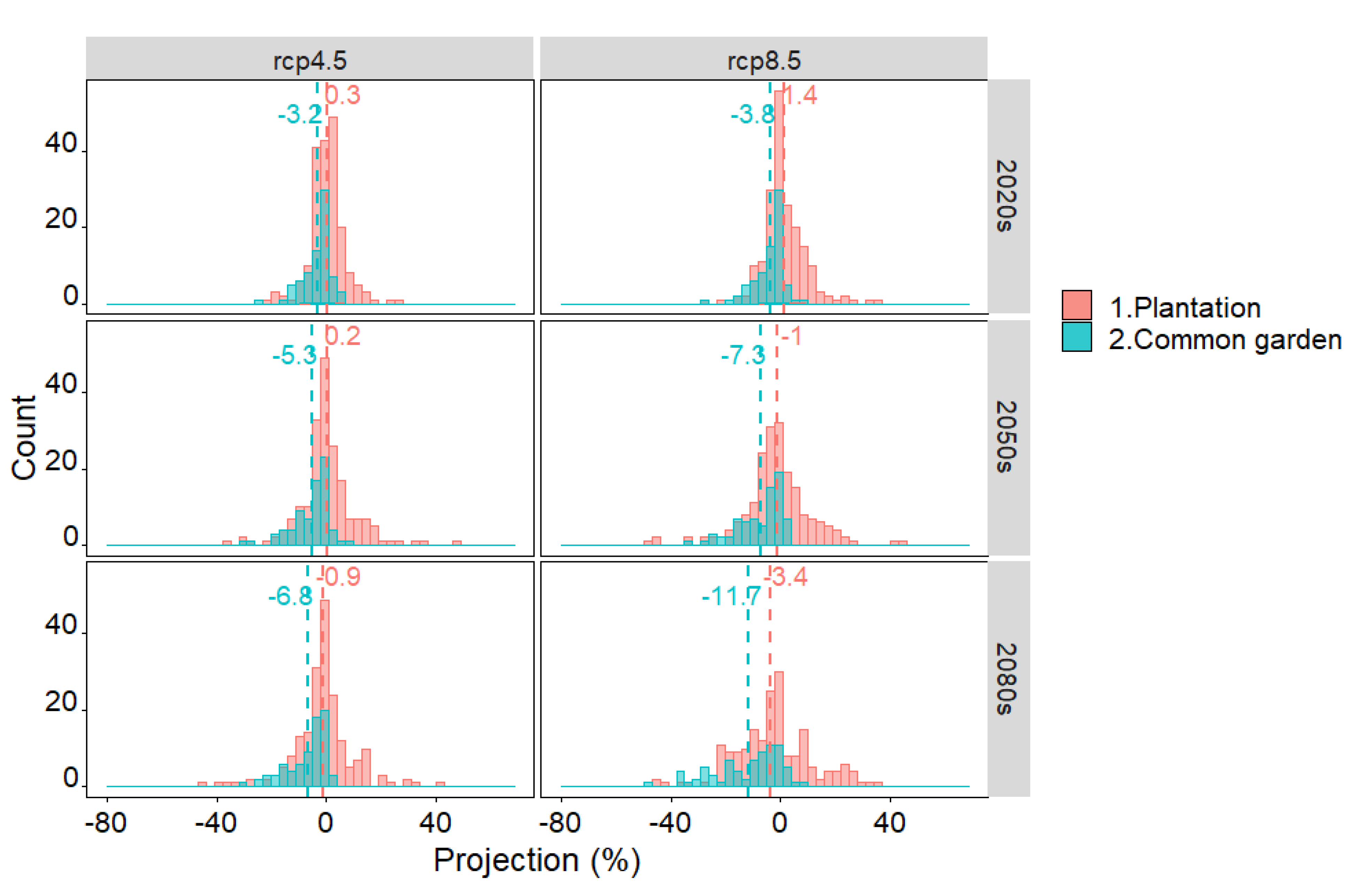
3.2. Future Growth, Common Tendency and Dissimilarity
4. Discussion
4.1. Spruce Growth is Mainly Determined by Summer Drought, Previous Autumn and Spring Warmth
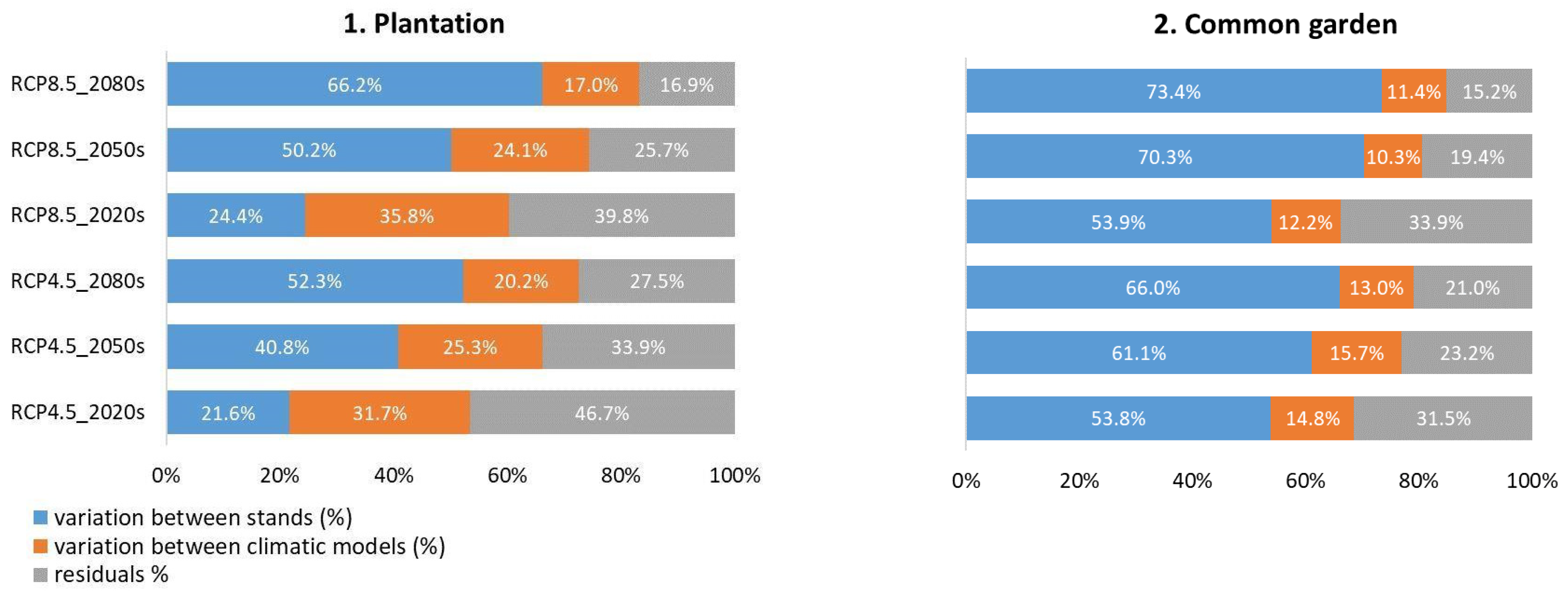
4.2. Growth Projections and Sources of Variation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buser, C.M.; Künsch, H.R.; Lüthi, D.; Wild, M.; Schär, C. Bayesian multi-model projection of climate: Bias assumptions and interannual variability. Clim. Dyn. 2009, 33, 849–868. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Duan, Q.; Sun, Q.; Huang, Y.; Kong, D.; Yang, T.; Ye, A.; Di, Z.; Gong, W. Assessment of CMIP5 climate models and projected temperature changes over Northern Eurasia. Environ. Res. Lett. 2014, 9, 055007. [Google Scholar] [CrossRef]
- Knutti, R.; Sedláček, J. Robustness and uncertainties in the new CMIP5 climate model projections. Nat. Clim. Chang. 2013, 3, 369–373. [Google Scholar] [CrossRef]
- McKenney, D.W.; Pedlar, J.H.; Lawrence, K.; Campbell, K.; Hutchinson, M.F. Potential impacts of climate change on the distribution of North American trees. BioScience 2007, 57, 939–948. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; De Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change—Range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.; Nabuurs, G.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 458–497. [Google Scholar] [CrossRef]
- Huang, J.; Bergeron, Y.; Berninger, F.; Zhai, L.; Tardif, J.C.; Denneler, B. Impact of future climate on radial growth of four major boreal tree species in the eastern Canadian boreal forest. PLoS ONE 2013, 8, e56758. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Fonti, P.; Larsen, J.B.; Ræbild, A.; Callesen, I.; Pedersen, N.B.; Hansen, J.K. Projecting tree-growth responses into future climate: A study case from a Danish-wide common garden. Agric. For. Meteorol. 2017, 247, 240–251. [Google Scholar] [CrossRef]
- Vitali, V.; Büntgen, U.; Jürgen, B. Seasonality matters—The effects of past and projected seasonal climate change on the growth of native and exotic conifer species in Central Europe. Dendrochronologia 2018, 48, 1–9. [Google Scholar] [CrossRef]
- Buras, A.; Menzel, A. Projecting tree species composition changes of European forests for 2061–2090 under RCP 4.5 and RCP 8.5 scenarios. Front. Plant Sci. 2019, 9, 1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Sun, J.; Chen, X. Projection and uncertainty analysis of global precipitation-related extremes using CMIP5 models. Int. J. Climatol. 2014, 34, 2730–2748. [Google Scholar] [CrossRef]
- Lindholm, M.; Lehtonen, H.; Kolström, T.; Meriläinen, J.; Eronen, M.; Timonen, M. Climatic signals extracted from ring-width chronologies of Scots pines from the northern, middle and southern parts of the boreal forest belt in Finland. Silva Fenn. 2000, 34, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Andreassen, K.; Solberg, S.; Tveito, O.E.; Lystad, S.L. Regional differences in climatic responses of Norway spruce (Picea abies L. Karst) growth in Norway. For. Ecol. Manag. 2006, 222, 211–221. [Google Scholar] [CrossRef]
- Lapointe-Garant, M.-P.; Huang, J.-G.; Gea-Izquierdo, G.; Raulier, F.; Bernier, P.; Berninger, F. Use of tree rings to study the effect of climate change on trembling aspen in Québec. Glob. Chang. Biol. 2010, 16, 2039–2051. [Google Scholar] [CrossRef]
- Nehrbass-Ahles, C.; Babst, F.; Klesse, S.; Nötzli, M.; Bouriaud, O.; Neukom, R.; Dobbertin, M.; Frank, D. The influence of sampling design on tree-ring-based quantification of forest growth. Glob. Chang. Biol. 2014, 20, 2867–2885. [Google Scholar] [CrossRef] [PubMed]
- Primicia, I.; Camarero, J.J.; Janda, P.; Čada, V.; Morrissey, R.C.; Trotsiuk, V.; Bače, R.; Teodosiu, M.; Svoboda, M. Age, competition, disturbance and elevation effects on tree and stand growth response of primary Picea abies forest to climate. For. Ecol. Manag. 2015, 354, 77–86. [Google Scholar] [CrossRef]
- Rabbel, I.; Neuwirth, B.; Bogena, H.; Diekkrüger, B. Exploring the growth response of Norway spruce (Picea abies) along a small-scale gradient of soil water supply. Dendrochronologia 2018, 52, 123–130. [Google Scholar] [CrossRef]
- Häusser, M.; Szymczak, S.; Garel, E.; Santoni, S.; Huneau, F.; Bräuning, A. Growth variability of two native pine species on Corsica as a function of elevation. Dendrochronologia 2019, 54, 49–55. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 1990, 21, 167–196. [Google Scholar] [CrossRef]
- Cole, C.T.; Anderson, J.E.; Lindroth, R.L.; Waller, D.M. Rising concentrations of atmospheric CO2 have increased growth in natural stands of quaking aspen (Populus tremuloides). Glob. Chang. Biol. 2010, 16, 2186–2197. [Google Scholar] [CrossRef]
- Savva, Y.; Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Vaganov, E.A.; Modrzynski, J. Interannual growth response of Norway spruce to climate along an altitudinal gradient in the Tatra Mountain, Poland. Trees Struct. Funct. 2006, 20, 735–766. [Google Scholar] [CrossRef]
- Caudullo, G.; Tinner, W.; de Rigo, D. Picea abies in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 114–116. [Google Scholar]
- Nord-Larsen, T.; Johannsen, V.K.; Riis-Nielsen, T.; Thomsen, I.M.; Bentsen, N.S.; Gundersen, P.; Jørgensen, B.B. Skove og Plantager 2017: Forest Statistics 2017; Institut for Geovidenskab og Naturforvaltning, Københavns Universitet: Frederiksberg, Denmark, 2018. [Google Scholar]
- Nord-Larsen, T.; Pretzch, H. Biomass production dynamics for common forest tree species in Denmark–Evaluation of a common garden experiment after 50 yrs of measurements. For. Ecol. Manag. 2017, 400, 645–654. [Google Scholar] [CrossRef]
- Bouriaud, O.; Popa, I. Comparative dendroclimatic study of Scots pine, Norway spruce, and silver fir in the Vrancea Range, Eastern Carpathian Mountains. Trees Struct. Funct. 2009, 23, 95–106. [Google Scholar] [CrossRef]
- Boden, S.; Kahle, H.; Von Wilpert, K.; Spiecker, H. Resilience of Norway spruce (Picea abies (L.) Karst) growth to changing climatic conditions in Southwest Germany. For. Ecol. Manag. 2014, 315, 12–21. [Google Scholar] [CrossRef]
- Hentschel, R.; Rosner, S.; Kayler, Z.E.; Andreassen, K.; Børja, I.; Solberg, S.; Tveito, O.E.; Priesack, E.; Gessler, A. Norway spruce physiological and anatomical predisposition to dieback. For. Ecol. Manag. 2014, 322, 27–36. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Rathgeber, C.B.K.; Ulrich, E. Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba, Picea abies and Pinus sylvestris). J. Veg. Sci. 2010, 21, 364–376. [Google Scholar] [CrossRef]
- Solberg, S. Summer drought: A driver for crown condition and mortality of Norway spruce in Norway. For. Pathol. 2004, 34, 93–104. [Google Scholar] [CrossRef]
- Bouriaud, O.; Leban, J.-M.; Bert, D.; Deleuze, C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005, 25, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef]
- Panayotov, M.; Kulakowski, D.; Tsvetanov, N.; Krumm, F.; Barbeito, I.; Bebi, P. Climate extremes during high competition contribute to mortality in unmanaged self-thinning Norway spruce stands in Bulgaria. For. Ecol. Manag. 2016, 369, 74–88. [Google Scholar] [CrossRef]
- Rosner, S.; Světlík, J.; Andreassen, K.; Børja, I.; Dalsgaard, L.; Evans, R.; Luss, S.; Tveito, O.E.; Solberg, S. Novel hydraulic vulnerability proxies for a boreal conifer species reveal that opportunists may have lower survival prospects under extreme climatic events. Front. Plant Sci. 2016, 7, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Büntgen, U.; Frank, D.C.; Schmidhalter, M.; Neuwirth, B.; Seifert, M.; Esper, J. Growth/climate response shift in a long subalpine spruce chronology. Trees Struct. Funct. 2006, 20, 99–110. [Google Scholar] [CrossRef] [Green Version]
- Carrer, M.; Motta, R.; Nola, P. Significant mean and extreme climate sensitivity of Norway spruce and Silver fir at mid-elevation mesic sites in the Alps. PLoS ONE 2012, 7, e50755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castagneri, D.; Petit, G.; Carrer, M. Divergent climate response on hydraulic-related xylem anatomical traits of Picea abies along a 900-m altitudinal gradient. Tree Physiol. 2015, 35, 1378–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haylock, M.R.; Hofstra, N.; Klein Tank, A.M.G.; Klok, E.J.; Jones, P.D.; New, M. A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. 2008, 113, D20119. [Google Scholar] [CrossRef] [Green Version]
- Regent Instruments Canada Inc. WINDENDRO for Tree-Ring Analysis; Regent Instruments Canada Inc.: Québec City, QC, Canada, 2012. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Cook, E.R.; Peters, K. The smoothing spline: A new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree-Ring Bull. 1981, 41, 45–53. [Google Scholar]
- Cook, E.R.; Kairiukstis, L.A. (Eds.) Methods of Dendrochronology: Applications in the Environmental Sciences; Springer: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- De Martonne, E. Une nouvelle fonction climatologique: L’indice d’aridité. Meteorologie 1926, 2, 449–458. [Google Scholar]
- Zang, C.; Biondi, F. Dendroclimatic calibration in R: The bootRes package for response and correlation function analysis. Dendrochronologia 2013, 31, 68–74. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach; Springer: Heidelberg, Germany, 2002; pp. 60–74. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 14.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Chatterjee, S.; Hadi, A.S. Analysis of Collinear Data. In Regression Analysis by Example, 4nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- IPCC. Climate Change 2013: The Physical Science Basis; Part of Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]
- Hamann, A.; Wang, T.; Spittlehouse, D.L.; Murdock, T.Q. A comprehensive, high-resolution database of historical and projected climate surfaces for western North America. Bull. Am. Meteorol. Soc. 2013, 94, 1307–1309. [Google Scholar] [CrossRef]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Schmitt, U.; Jalkanen, R.; Eckstein, D. Cambium dynamics of Pinus sylvestris and Betula spp. in the northern boreal forest in Finland. Silva Fenn. 2004, 38, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Partanen, J.; Koski, V.; Hänninen, H. Effects of photoperiod and temperature on the timing of bud burst in Norway spruce (Picea abies). Tree Physiol. 1998, 18, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Carneros, E.; Yakovlev, I.; Viejo, M.; Olsen, J.E.; Fossda, C.G. The epigenetic memory of temperature during embryogenesis modifies the expression of bud burst-related genes in Norway spruce epitypes. Planta 2017, 246, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, H.; Nöjd, P.; Kahle, H.-P.; Neumann, U.; Tveite, B.; Mielikäinen, K.; Röhle, H.; Spiecker, H. Large-scale climatic variability and radial increment variation of Picea abies (L.) Karst. in central and northern Europe. Trees Struct. Funct. 2003, 17, 173–184. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Bayramzadeh, V.; Oribe, Y.; Kubo, T.; Funada, R. Temperature responses of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii x P. grandidentata) under natural conditions. Tree Physiol. 2008, 28, 1813–1819. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Rossi, S.; Anfodillo, T.; Saracino, A. Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern Italy. Tree Physiol. 2008, 28, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Lupi, C.; Morin, H.; Deslauriers, A.; Rossi, S. Xylem phenology and wood production: Resolving the chicken-or-egg dilemma. Plant Cell Environ. 2010, 33, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.; Hoch, G.; Körner, C. Early season temperature controls cambial activity and total tree ring width at the alpine treeline. Plant Ecol. Divers. 2013, 6, 365–375. [Google Scholar] [CrossRef]
- Zang, C.; Pretzsch, H.; Rothe, A. Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees Struct. Funct. 2012, 26, 557–569. [Google Scholar] [CrossRef]
- Zang, C.; Hartl-Meier, C.; Dittmar, C.; Rothe, A.; Menzel, A. Patterns of drought tolerance in major European temperate forest trees: Climatic drivers and levels of variability. Glob. Chang. Biol. 2014, 20, 3767–3779. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Rigling, A.; Bugmann, H.; Weber, P.; Brang, P. Growth response of five co-occurring conifers to drought across a wide climatic gradient in Central Europe. Agric. For. Meteorol. 2014, 197, 1–12. [Google Scholar] [CrossRef]
- Schmid, I.; Kazda, M. Root distribution of Norway spruce in monospecific and mixed stands on different soils. For. Ecol. Manag. 2002, 159, 37–47. [Google Scholar] [CrossRef]
- Zweifel, R.; Rigling, A.; Dobbertin, M. Species-specific stomatal response of trees to drought—A link to vegetation dynamics? J. Veg. Sci. 2009, 20, 442–454. [Google Scholar] [CrossRef]
- Stinziano, J.R.; Hüner, N.P.A.; Way, D.A. Warming delays autumn declines in photosynthetic capacity in a boreal conifer, Norway spruce (Picea abies). Tree Physiol. 2015, 35, 1303–1313. [Google Scholar] [CrossRef] [Green Version]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Atkin, O.K.; Macherel, D. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 2009, 103, 581–597. [Google Scholar] [CrossRef] [Green Version]
- Gričar, J.; Prislan, P.; De Luis, M.; Gryc, V.; Hacurová, J.; Vavrčík, H.; Čufar, K. Plasticity in variation of xylem and phloem cell characteristics of Norway spruce under different local conditions. Front. Plant Sci. 2015, 6, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigurdsson, B.D.; Roberntz, P.; Freeman, M.; Næss, M.; Saxe, H.; Thorgeirsson, H.; Linder, S. Impact studies on Nordic forests: Effects of elevated CO2 and fertilization on gas exchange. Can. J. For. Res. 2002, 32, 779–788. [Google Scholar] [CrossRef]
- Handa, I.T.; Körner, C.; Hättenschwiler, S. Conifer stem growth at the altitudinal treeline in response to four years of CO2 enrichment. Glob. Chang. Biol. 2006, 12, 2417–2430. [Google Scholar] [CrossRef]
- Bellassen, V.; Viovy, N.; Luyssaert, S.; Le Maire, G.; Schelhaas, M.-J.; Ciais, P. Reconstruction and attribution of the carbon sink of European forests between 1950 and 2000. Glob. Chang. Biol. 2011, 17, 3274–3292. [Google Scholar] [CrossRef]
- Sigurdsson, B.D.; Medhurst, J.L.; Wallin, G.; Eggertsson, O.; Linder, S. Growth of mature boreal Norway spruce was not affected by elevated [CO2] and/or air temperature unless nutrient availability was improved. Tree Physiol. 2013, 33, 1192–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallin, G.; Hall, M.; Slaney, M.; Räntfors, M.; Medhurst, J.; Linder, S. Spring photosynthetic recovery of boreal Norway spruce under conditions of elevated [CO2] and air temperature. Tree Physiol. 2013, 33, 1177–1191. [Google Scholar] [CrossRef] [Green Version]
- Reyer, C.; Lasch-Born, P.; Suckow, F.; Gutsch, M.; Murawski, A.; Pilz, T. Projections of regional changes in forest net primary productivity for different tree species in Europe driven by climate change and carbon dioxide. Ann. For. Sci. 2014, 71, 211–225. [Google Scholar] [CrossRef]
- Baig, S.; Medlyn, B.E.; Mercado, L.M.; Zaehle, S. Does the growth response of woody plants to elevated CO2 increase with temperature? A model-oriented meta-analysis. Glob. Chang. Biol. 2015, 21, 4303–4319. [Google Scholar] [CrossRef] [Green Version]
- Schaphoff, S.; Reyer, C.P.O.; Schepaschenko, D.; Gerten, D.; Shvidenko, A. Tamm Review: Observed and projected climate change impacts on Russia’s forests and its carbon balance. For. Ecol. Manag. 2016, 361, 432–444. [Google Scholar] [CrossRef] [Green Version]
- Asshoff, R.; Zotz, G.; Körner, C. Growth and phenology of mature temperate forest trees in elevated CO2. Glob. Chang. Biol. 2006, 12, 848–861. [Google Scholar] [CrossRef]
- Leuzinger, S.; Bader, M.K.F. Experimental vs. modeled water use in mature Norway spruce (Picea abies) exposed to elevated CO2. Front. Plant Sci. 2012, 3, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavergne, A.; Graven, H.; De Kauwe, M.G.; Keenan, T.F.; Medlyn, B.E.; Prentice, I.C. Observed and modelled historical trends in the water-use efficiency of plants and ecosystems. Glob. Chang. Biol. 2019, 25, 2242–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, M.; Madsen, K.S.; Ludwigsen, C.A.; Boberg, F.; Christensen, T.; Cappelen, J.; Christensen, O.B.; Andersen, K.K.; Christensen, J.H. Fremtidige Klimaforandringer i Danmark [Future Climate Changes in Denmark]; Danmarks Klimacenter, Rapport No. 6; Danmarks Meteorologiske Institut: Copenhagen, Denmark, 2014. [Google Scholar]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Holuša, J.; Lubojacký, J.; Čurn, V.; Tonka, T.; Lukášová, K.; Horák, J. Combined effects of drought stress and Armillaria infection on tree mortality in Norway spruce plantations. For. Ecol. Manag. 2018, 427, 434–445. [Google Scholar] [CrossRef]
- Neto, O.C.; Aguiar, A.V.; Twyford, A.D.; Neaves, L.E.; Pennington, R.T.; Lopes, A.V. Genetic and ecological outcomes of Inga vera Subsp. affinis (Leguminosae) tree plantations in a fragmented tropical landscape. PLoS ONE 2014, 9, e99903. [Google Scholar] [CrossRef] [Green Version]
- Ivetić, V.; Devetaković, J.; Nonić, M.; Stanković, D.; Šijačić-Nikolić, M. Genetic diversity and forest reproductive material—From seed source selection to planting. iForest 2016, 9, 801–812. [Google Scholar] [CrossRef] [Green Version]
- Iverson, L.R.; Prasad, A.M.; Matthews, S.N.; Peters, M. Estimating potential habitat for 134 eastern US tree species under six climate scenarios. For. Ecol. Manag. 2008, 254, 390–406. [Google Scholar] [CrossRef]
- Lasch, P.; Badeck, F.W.; Lindner, M.; Suckow, F. Sensitivity of simulated forest growth to changes in climate and atmospheric CO2. Forstwiss. Centralbl. 2002, 121, 155–171. [Google Scholar]
- Buisson, L.; Thuiller, W.; Casajus, N.; Lek, S.; Grenouillet, G. Uncertainty in ensemble forecasting of species distribution. Glob. Chang. Biol. 2010, 16, 1145–1157. [Google Scholar] [CrossRef]
- Monastersky, R. Global carbon dioxide levels near worrisome milestone. Nature 2013, 497, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Depardieu, C.; Girardin, M.P.; Nadeau, S.; Lenz, P.; Bousquet, J.; Isable, N. Adaptive genetic variation to drought in a widely distributed conifer suggests a potential for increasing forest resilience in a drying climate. New Phytol. 2020, 227, 427–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trujillo-Moya, C.; George, J.-P.; Fluch, S.; Geburek, T.; Grabner, M.; Karanitsch-Ackerl, S.; Konrad, H.; Mayer, K.; Sehr, E.M.; Wischnitzki, E.; et al. Drought sensitivity of Norway spruce at the species’ warmest fringe: Quantitative and molecular analysis reveals high genetic variation among and within provenances. G3 Genes Genom. Genet. 2018, 8, 1225–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonesson, J.; Eriksson, G. Genetic variation in drought tolerance in Picea abies seedlings and its relationship to growth in controlled and field environments. Scand. J. For. Res. 2003, 18, 7–18. [Google Scholar] [CrossRef]
- Jamnická, G.; Fleischer, P., Jr.; Konôpková, A.; Pšidová, E.; Kučerová, J.; Kurjak, D.; Živčák, M.; Ditmarová, L’. Norway spruce (Picea abies L.) provenances use different physiological strategies to cope with water deficit. Forests 2019, 10, 651. [Google Scholar] [CrossRef] [Green Version]
- Yakovlev, I.; Fossdal, C.G.; Johnsen, Ø. MicroRNAs, the epigenetic memory and climatic adaptation in Norway spruce. New Phytol. 2010, 187, 1154–1169. [Google Scholar] [CrossRef]
- Yakovlev, I.; Asantea, D.K.A.; Fossdal, C.G.; Junttila, O.; Johnsen, Ø. Differential gene expression related to an epigenetic memory affecting climatic adaptation in Norway spruce. Plant Sci. 2011, 180, 132–139. [Google Scholar] [CrossRef]
- Kijowska-Oberc, J.; Staszak, A.M.; Kamiński, J.; Ratajczak, E. Adaptation of forest trees to rapidly changing climate. Forests 2020, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Batista, R.A.; Köhler, C.; Hennig, L. Polycomb Repressive Complex 2-mediated histone modification H3K27me3 is associated with embryogenic potential in Norway spruce. J. Exp. Bot. 2020, 71, 6366–6378. [Google Scholar] [CrossRef]

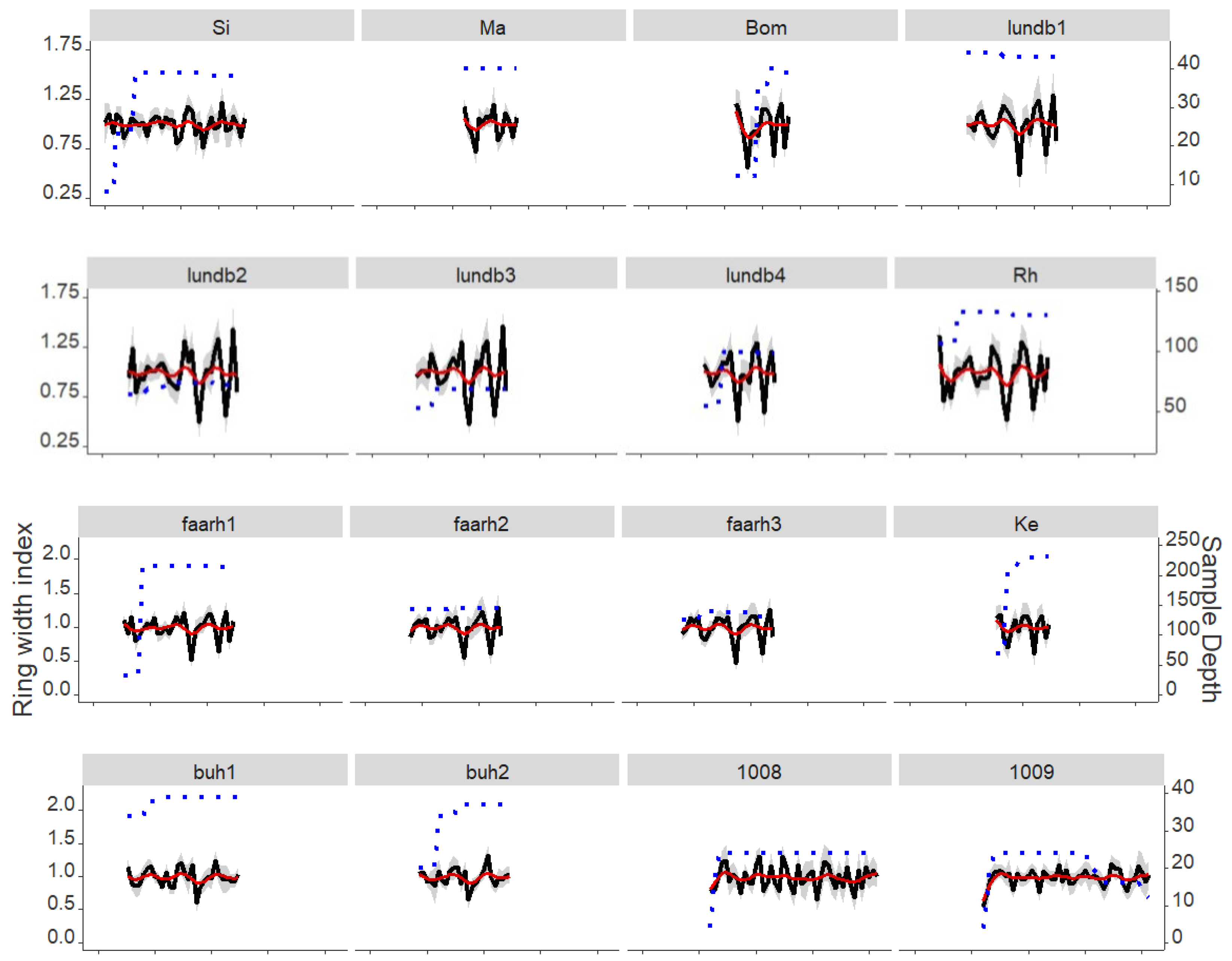
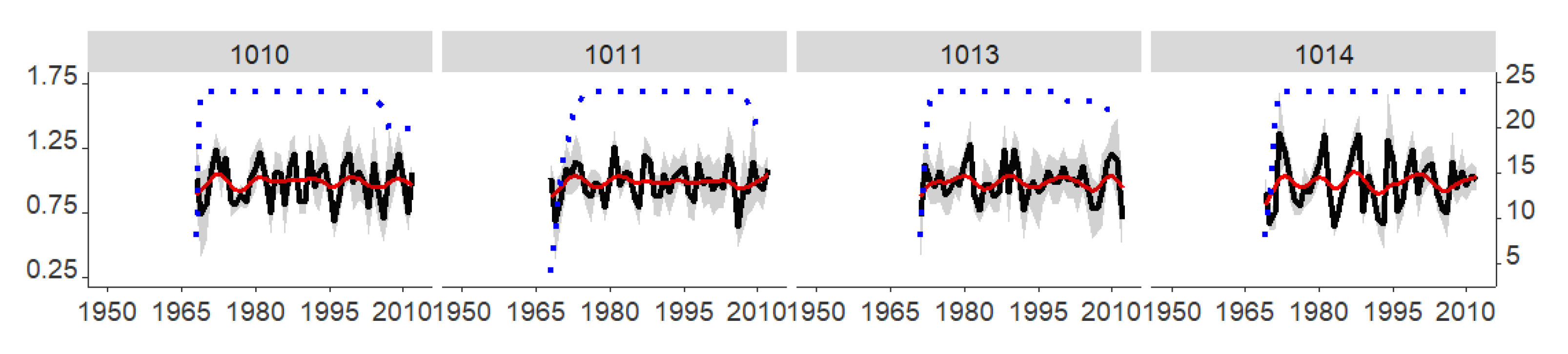
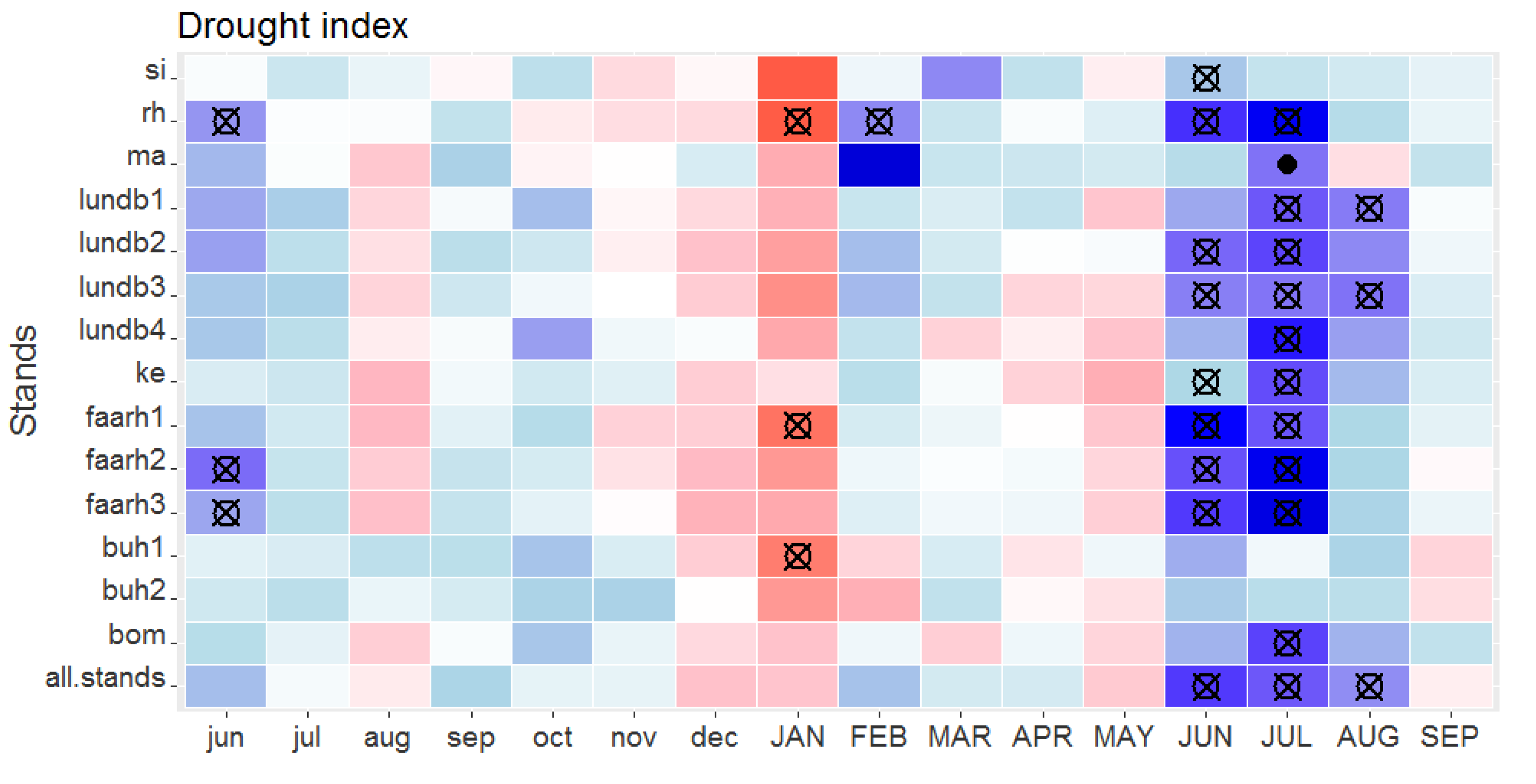

| Sites (Forests) | Abbreviation | Number of Stands | Year of Establishment | Latitude (N) | Longitude (E) | Mean Annual Temperature a (°C) | Annual Precipitation a (mm) | Trees/Radii | Period | Average Ring Width (mm) | Rbar b | EPS b | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fourteen Norway spruce stands (1. Plantation) | Buderupholm Hollandshus | Buh | 2 | 1942–1943 | 56.8 | 9.9 | 7.4 | 651 | 20/76 | 1958–1987 | 2.90 ± 1.28 | 0.67 | 0.97 |
| Fårhus plantage | Faarh | 3 | 1951 | 54.9 | 9.3 | 7.8 | 814 | 127/505 | 1958–1987 | 3.44 ± 1.30 | 0.68 | 1.00 | |
| Lundbæk plantage | Lundb | 4 | 1942–1952 | 54.9 | 9.2 | 7.8 | 823 | 72/286 | 1957–1986 | 3.10 ± 1.32 | 0.64 | 0.99 | |
| Kelstrup plantage | Ke | 1 | 1951 | 54.9 | 9.4 | 7.8 | 814 | 58/232 | 1973–1987 | 3.48 ± 1.60 | 0.63 | 0.99 | |
| Silkeborg | Si | 1 | 1932 | 56.1 | 9.5 | 7.7 | 679 | 10/40 | 1950–1987 | 2.98 ± 1.13 | 0.55 | 0.91 | |
| Bommerlund plantage | Bom | 1 | 1959 | 54.9 | 9.4 | 7.8 | 814 | 10/40 | 1973–1987 | 4.08 ± 1.60 | 0.69 | 0.94 | |
| Mattrup | Ma | 1 | 1950 | 55.9 | 9.5 | 7.8 | 673 | 10/40 | 1973–1987 | 4.29 ± 1.48 | 0.74 | 0.97 | |
| Rens Hedegård | Rh | 1 | 1934 | 54.9 | 9.1 | 7.8 | 823 | 33/132 | 1958–1987 | 3.03 ± 1.29 | 0.73 | 0.99 | |
| Six trials from a Danish common garden experiment. For further information, please see Huang et al. [9]. (2. Common garden) | 1008 | - | 1 | 1964–1965 | 56.5 | 10.5 | 8.1 | 635 | 6/24 | 1969–2012 | 3.34 ± 1.76 | 0.42 | 0.94 |
| 1009 | - | 1 | 1964–1965 | 55.9 | 9.2 | 8.2 | 748 | 6/24 | 1970–2012 | 3.35 ± 1.59 | 0.32 | 0.90 | |
| 1010 | - | 1 | 1964–1965 | 55.5 | 11.8 | 8.7 | 536 | 6/24 | 1969–2012 | 3.04 ± 1.84 | 0.46 | 0.95 | |
| 1011 | - | 1 | 1964–1965 | 56.0 | 12.4 | 8.6 | 574 | 6/24 | 1970–2012 | 3.05 ± 1.47 | 0.38 | 0.93 | |
| 1013 | - | 1 | 1964–1965 | 57.2 | 9.5 | 8.0 | 644 | 6/24 | 1972–2012 | 2.05 ± 1.49 | 0.33 | 0.91 | |
| 1014 | - | 1 | 1964–1965 | 56.3 | 8.4 | 8.5 | 740 | 6/24 | 1971–2012 | 2.17 ± 1.12 | 0.59 | 0.97 |
| Stand/Trial | Model a | AIC b | ΔiAIC c | R2 |
|---|---|---|---|---|
| Si | (+) DI38 | −62.0 | 0 | 0.25 |
| (−) lag1tg89 | −60.1 | 1.9 | 0.15 | |
| Rh | (+) DI58 (−) lag1tg89 | −17.9 | 0 | 0.58 |
| (+) DI58 | −16.0 | 1.9 | 0.47 | |
| Ma | (+) lag1DI56 (+) lag1tg612 (−) lag1tg6122 | −19.3 | 0 | 0.84 |
| (+) lag1DI56 (−) lag1DI562 (+) lag1tg612 | −19.3 | 0 | 0.52 | |
| lundb1 | (+) DI78 (+) Tg35 | −26.7 | 0 | 0.69 |
| lundb2 | (+) DI58 | −17.0 | 0 | 0.48 |
| (+) DI58 (+) Tg5 | −16.4 | 0.6 | 0.56 | |
| (+) DI58 (+) lag1tg7_1 (−) lag1tg7_12 | −15.9 | 1.1 | 0.62 | |
| (+) DI58 (−) DI582 (−) lag1tg7_1 | −15.9 | 1.1 | 0.49 | |
| lundb3 | (+) DI78 (+) Tg5 | −14.7 | 0 | 0.70 |
| lundb4 | (+) DI78 (+) Tg35 | −19.7 | 0.0 | 0.78 |
| Ke | (+) DI78 | −15.1 | 0.0 | 0.74 |
| (+) DI78 (−) Tg45 (+) Tg452 | −13.8 | 1.3 | 0.83 | |
| (+) DI78 (−) DI782 (+) Tg45 | −13.8 | 1.3 | 0.88 | |
| (+) DI78 (+) lag1DI46 | −13.3 | 1.8 | 0.83 | |
| faarh1 | (+) DI67 | −31.6 | 0.0 | 0.53 |
| faarh2 | (+) DI67 | −24.4 | 0.0 | 0.59 |
| (+) DI67 (−) lag1tg6_1 | −23.9 | 0.5 | 0.66 | |
| (+) DI67 (+) lag1tg6_1 (−) lag1tg6_12 | −23.2 | 1.2 | 0.72 | |
| (+) DI67 (−) DI672 (−) lag1tg6_1 | −23.2 | 1.2 | 0.74 | |
| faarh3 | (+) DI67 (−) DI672 | −29.2 | 0.0 | 0.80 |
| (+) DI67 | −28.0 | 1.2 | 0.66 | |
| buh1 | (+) lag1DI811 (−) lag1tg710 | −35.2 | 0.0 | 0.51 |
| buh2 | (+) DI67 | −28.8 | 0.0 | 0.33 |
| (+) Tg45 | −27.9 | 0.9 | 0.21 | |
| (+) lag1DI611 | −27.0 | 1.8 | 0.31 | |
| Bom | (+) DI78 | −12.0 | 0.0 | 0.73 |
| (+) DI78 (−) Tg45 (+) Tg452 | −10.4 | 1.6 | 0.81 | |
| (+) DI78 (−) DI782 (+) Tg45 | −10.4 | 1.6 | 0.78 | |
| 1008 | (+) DI67 | −42.6 | 0 | 0.46 |
| (+) DI67 (−) lag1tg910 | −42.4 | 0.2 | 0.54 | |
| 1009 | (+) DI67 | −58.9 | 0 | 0.15 |
| 1010 | (−) lag1tg810 | −28.9 | 0 | 0.12 |
| (−) Tg6 | −28.7 | 0.2 | 0.13 | |
| 1011 | (−) Tg6 | −46.5 | 0 | 0.19 |
| 1013 | (−) DI45 | −34.0 | 0 | 0.09 |
| (−) lag1tg612 | −33.5 | 0.5 | 0.05 | |
| (+) Tg45 | −33.3 | 0.7 | 0.04 | |
| (+) lag1DI11 | −32.8 | 1.2 | 0.06 | |
| 1014 | (+) DI67 | −27.8 | 0 | 0.42 |
| (+) DI67 (−) lag1tg69 | −26.9 | 0.9 | 0.48 | |
| (+) DI67 (−) lag1tg69 (+) lag1tg692 | −25.9 | 1.9 | 0.55 | |
| (+) DI67 (−) DI672 (−) lag1tg69 | −25.9 | 1.9 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Fonti, P.; Ræbild, A.; Larsen, J.B.; Wellendorf, H.; Hansen, J.K. Variability among Sites and Climate Models Contribute to Uncertain Spruce Growth Projections in Denmark. Forests 2021, 12, 36. https://doi.org/10.3390/f12010036
Huang W, Fonti P, Ræbild A, Larsen JB, Wellendorf H, Hansen JK. Variability among Sites and Climate Models Contribute to Uncertain Spruce Growth Projections in Denmark. Forests. 2021; 12(1):36. https://doi.org/10.3390/f12010036
Chicago/Turabian StyleHuang, Weiwei, Patrick Fonti, Anders Ræbild, Jørgen Bo Larsen, Hubert Wellendorf, and Jon Kehlet Hansen. 2021. "Variability among Sites and Climate Models Contribute to Uncertain Spruce Growth Projections in Denmark" Forests 12, no. 1: 36. https://doi.org/10.3390/f12010036





