Variability in Carbon Stocks across a Chronosequence of Masson Pine Plantations and the Trade-Off between Plant and Soil Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Analyses and Calculations
2.4. Statistical Analysis
3. Results
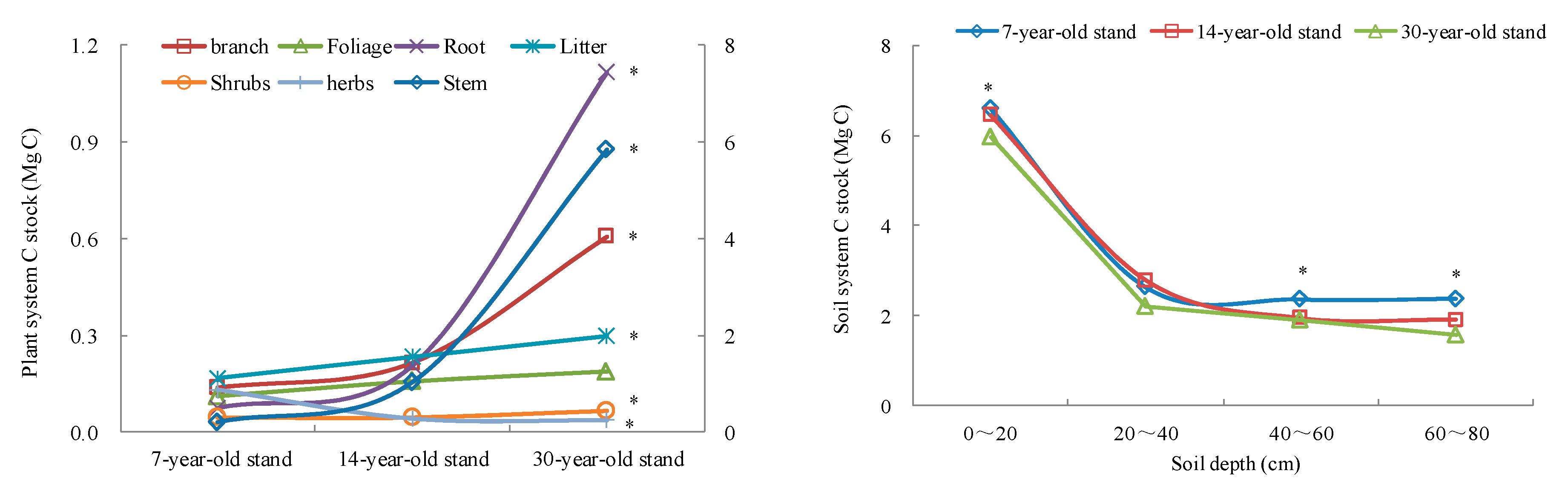
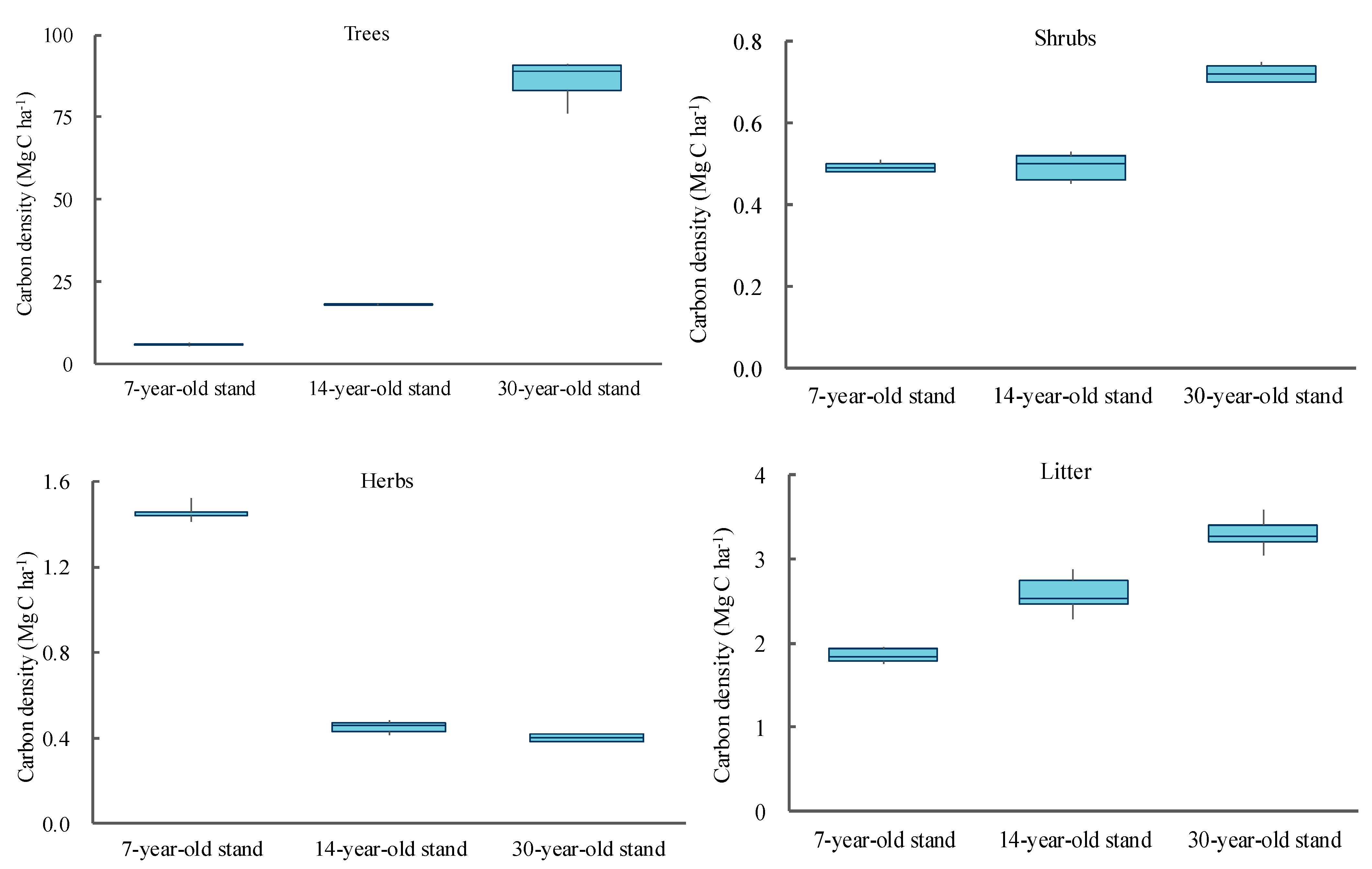
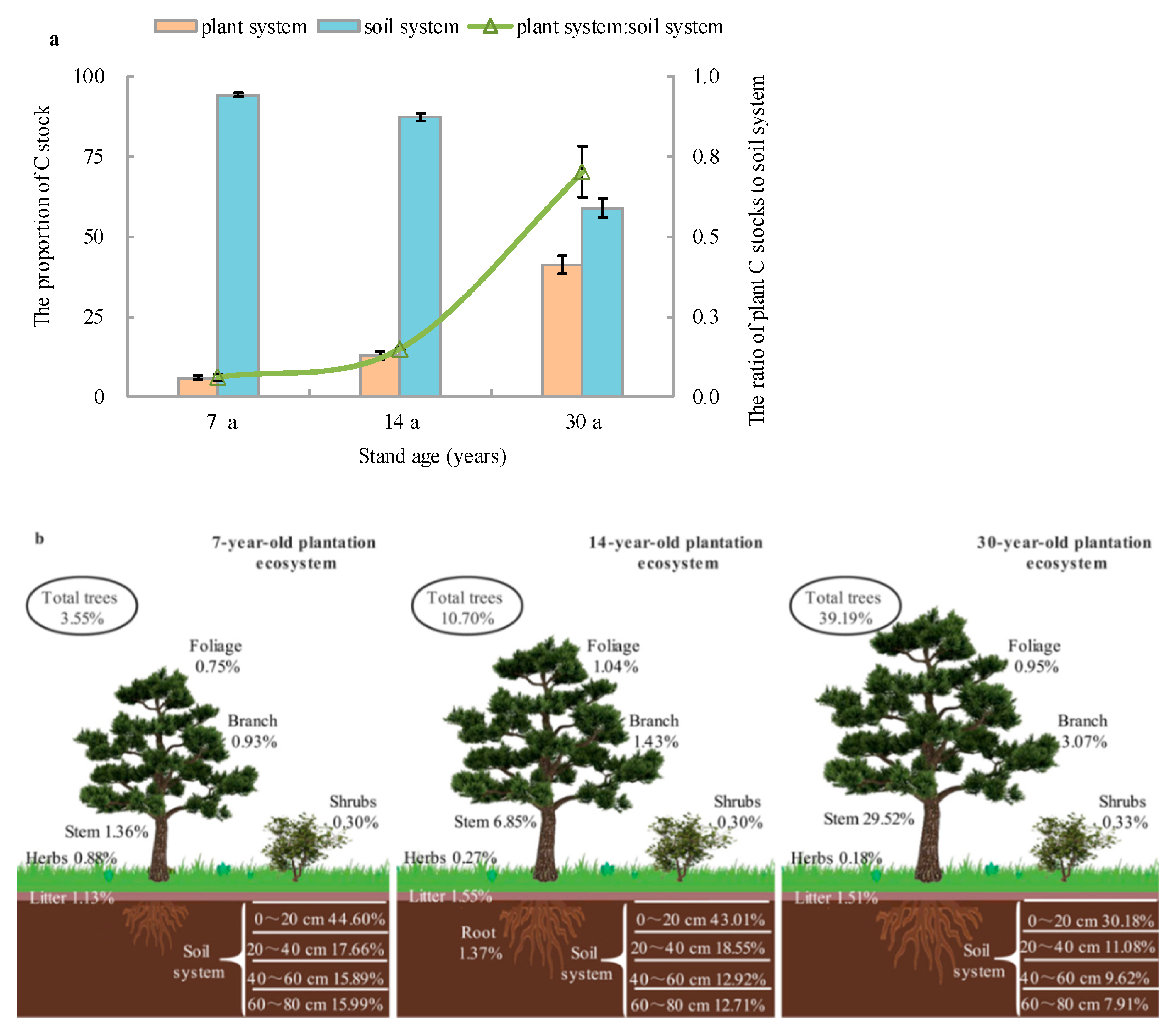
3.1. Carbon Stocks and Allocation Characteristics
3.2. The Variation of C Density across a Chronosequence
3.3. Allocation Proportion of Ecosystem C Stocks
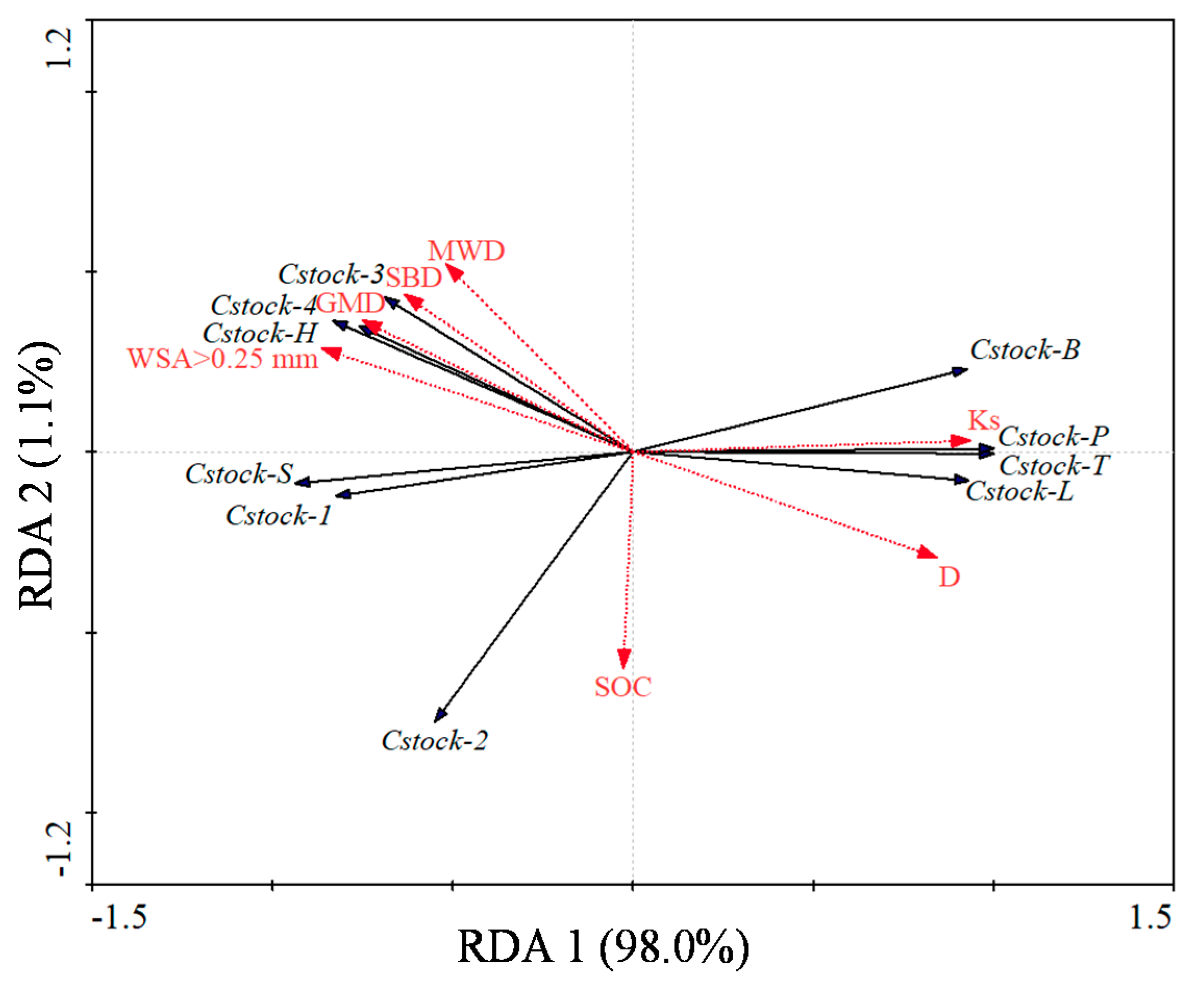
3.4. The Contribution of Soil Properties to C Stocks
4. Discussion
4.1. Carbon Partitioning in the Plant-Soil System
4.2. Correlation between Plant and Soil Systems
4.3. Controls of C Stocks in Plant-Soil Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Nayak, A.K.; Das, B.S.; Panigrahi, N.; Dasgupta, P.; Mohanty, S.; Kumar, U.; Panneerselvam, P.; Pathak, H. Effects of water deficit stress on agronomic and physiological responses of rice and greenhouse gas emission from rice soil under elevated atmospheric CO2. Sci. Total Environ. 2019, 650, 2032–2050. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Palmer, P.I.; Liu, Y.; Fang, S.X.; Bösch, H.; O’Dell, C.W.; Tang, X.P.; Yang, D.X.; Liu, L.X.; et al. Large Chinese land carbon sink estimated from atmospheric carbon dioxide data. Nature 2020, 586, 720–723. [Google Scholar] [CrossRef]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Fargione, J. Natural climate solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef] [Green Version]
- FAO. Global Forest Resources Assessment. Key Findings 2020. Available online: www.fao.org/forest-resources-assessment (accessed on 29 September 2021).
- Brown, H.C.A.; Berninger, F.A.; Larjavaara, M.; Appiah, M. Above-ground carbon stocks and timber value of old timber plantations, secondary and primary forests in southern Ghana. For. Ecol. Manag. 2020, 472, 118236. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, H.R.; Liu, S.R.; Zhou, G.Y.; Fang, J.Y.; Yu, G.R.; Tang, X.L.; Wang, W.T.; Yan, J.H.; Wang, G.X.; et al. Mapping forest type and age in China’s plantations. Sci. Total Environ. 2020, 744, 140790. [Google Scholar] [CrossRef]
- Kiew, F.; Hirata, R.; Hirano, T.; Xhuan, W.G.; Aries, E.B.; Kemudang, K.; Wenceslaus, J.; San, L.K.; Melling, L. Carbon dioxide balance of an oil palm plantation established on tropical peat. Agric. For. Meteorol. 2020, 295, 108189. [Google Scholar] [CrossRef]
- Na, M.; Sun, X.Y.; Zhang, Y.D.; Sun, Z.H.; Rousk, J. Higher stand densities can promote soil carbon storage after conversion of temperate mixed natural forests to larch plantations. Eur. J. For. Res. 2021, 140, 373–386. [Google Scholar] [CrossRef]
- Tamang, M.; Chettri, R.; Vineeta; Shukla, G.; Bhat, J.A.; Kumar, A.; Kumar, M.; Suryawanshi, A.; CabralPinto, M.; Chakravarty, S. Stand Structure, Biomass and Carbon Storage in Gmelina arborea Plantation at Agricultural Landscape in Foothills of Eastern Himalayas. Land 2021, 10, 387. [Google Scholar] [CrossRef]
- Gaurav, M.; Avishek, S.; Krishna, G.; Jyoti, N.A.; Rattan, L.; Rosa, F. Changes in soil carbon stocks under plantation systems and natural forests in Northeast India. Ecol. Model. 2021, 446, 109500. [Google Scholar] [CrossRef]
- Ma, Q.L.; Wang, X.Y.; Chen, F.; Wei, L.Y.; Zhang, D.; Jin, H.J. Carbon Sequestration of Sand-fixing Plantation of Haloxylon ammodendron in Shiyang River Basin: Storage, Rate and Potential. Glob. Ecol. Conserv. 2021, 28, e01607. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, Z.; Liu, Y.; Wu, G.L. Carbon accumulation by Pinus sylvestris forest plantations after different periods of afforestation in a semiarid sandy ecosystem. Land Degrad. Dev. 2021, 32, 2094–2104. [Google Scholar] [CrossRef]
- Cao, J.X.; Pan, H.; Chen, Z.; Shang, H. Dynamics in Stoichiometric Traits and Carbon, Nitrogen, and Phosphorus Pools across Three Different-Aged Picea asperata Mast. Plantations on the Eastern Tibet Plateau. Forests 2020, 11, 1346. [Google Scholar] [CrossRef]
- Jones, I.L.; DeWalt, S.J.; Lopez, O.R.; Bunnefeld, L.; Pattison, Z.; Dent, D.H. Above- and belowground carbon stocks are decoupled in secondary tropical forests and are positively related to forest age and soil nutrients respectively. Sci. Total Environ. 2019, 697, 133987. [Google Scholar] [CrossRef] [PubMed]
- Justine, M.F.; Yang, W.; Wu, F.; Khan, M.N. Dynamics of biomass and carbon sequestration across a chronosequence of masson pine plantations. J. Geophys. Res. Biogeosciences 2017, 122, 578–591. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; Lützow, M.v.; Marin-Spiotta, E.; Wesemael, B.v.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Waring, B.G.; Sulman, B.N.; Reed, S.; Smith, A.P.; Averill, C.; Creamer, C.A.; Cusack, D.F.; Hall, S.J.; Jastrow, J.D.; Jilling, A.; et al. From pools to flow: The PROMISE framework for new insights on soil carbon cycling in a changing world. Glob. Chang. Biol. 2020, 26, 6631–6643. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.L.; Guo, L.M.; Gao, Y.X.; Qi, L.; Cao, B.H. Effects of Seed Size and Sand Burial on Germination and Early Growth of Seedlings for Coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China. Forests 2019, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, Z.Y.; Chen, T.; Zhao, T.Y.; Song, L.H.; Mei, L. Fertilization and clear-cutting effect on greenhouse gases emission of pinewood nematode damaged Masson pine plantation. Ecosyst. Health Sustain. 2021, 7, 1868271. [Google Scholar] [CrossRef]
- Ge, J.L.; Xu, W.T.; Liu, Q.; Tang, Z.Y.; Xie, Z.Q. Patterns and environmental controls of soil organic carbon density in Chinese shrublands. Geoderma 2020, 363, 114161. [Google Scholar] [CrossRef]
- Sever, M.Z.O.; Alberti, G.; Vedove, G.D.; Marjanović, H. Temporal Evolution of Carbon Stocks, Fluxes and Carbon Balance in Pedunculate Oak Chronosequence under Close-To-Nature Forest Management. Forests 2019, 10, 814. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.F.; Chen, W.; Zhang, Y. Bulk density of mineral and organic soils in the Canada’s arctic and sub-arctic. Inf. Process. Agric. 2015, 2, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Kool, D.; Tong, B.; Tian, Z.; Heitman, J.L.; Sauer, T.J.; Horton, R. Soil water retention and hydraulic conductivity dynamics following tillage. Soil Tillage Res. 2019, 193, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.X.; Zhou, Y.C.; He, H.Z. Effects of rehabilitation through afforestation on soil aggregate stability and aggregate-associated carbon after forest fires in subtropical China. Geoderma 2020, 376, 114548. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Huang, X.F.; Zhou, Y.C.; Zhang, Z.M. Carbon Sequestration Anticipation Response to land use change in a mountainous karst basin in China. J. Environ. Manag. 2018, 228, 40–46. [Google Scholar] [CrossRef]
- Fan, H.B.; Liu, W.F.; Wu, J.P.; Li, Y.Y.; Yuan, Y.H.; Liao, Y.C.; Huang, R.Z.; Su, X.Q. Ecosystem carbon pools in mixed stands of hardwood species and masson pine. J. Trop. For. Sci. 2013, 25, 154–165. Available online: https://www.jstor.org/stable/23617029 (accessed on 29 September 2021).
- Nagler, M.; Fontana, V.; Lair, G.J.; Radtke, A.; Tasser, E.; Zerbe, S.; Tappeiner, U. Different management of larch grasslands in the European Alps shows low impact on above- and belowground carbon stocks. Agric. Ecosyst. Environ. 2015, 213, 186–193. [Google Scholar] [CrossRef]
- Terrer, C.; Phillips, R.P.; Hungate, B.A.; Rosende, J.; Ridge, J.P.; Craig, M.E.; Groenigen, K.J.v.; Keenan, T.F.; Sulman, B.N.; Stocker, B.D.; et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature 2021, 591, 599–603. [Google Scholar] [CrossRef]
- Ming, A.G.; Jia, H.Y.; Zhao, J.L.; Tao, Y.; Li, Y.F. Above- and below-ground carbon stocks in an indigenous tree (Mytilaria laosensis) plantation chronosequence in subtropical China. PLoS ONE 2014, 9, e109730. [Google Scholar] [CrossRef]
- Wellock, M.L.; Rashad, R.; Laperle, C.M.; Matthias, P.; Gerard, K. Changes in ecosystem carbon stocks in a grassland ash (Fraxinus excelsior) afforestation chronosequence in Ireland. J. Plant. Ecol. 2014, 7, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.H.; Li, P.; Ding, J.; Zhao, X.; Ma, W.H.; Ji, C.J.; Fang, J.Y. Increased topsoil carbon stock across China’s forests. Glob. Chang. Biol. 2014, 20, 2687–2696. [Google Scholar] [CrossRef]
- Ziche, D.; Grüneberg, E.; Hilbrig, L.; Höhle, J.; Kompa, T.; Liski, J.; Repo, A.; Wellbrock, N. Comparing soil inventory with modelling: Carbon balance in central European forest soils varies among forest types. Sci. Total Environ. 2019, 647, 1573–1585. [Google Scholar] [CrossRef]
- Makita, N.; Fujii, S. Tree species effects on microbial respiration from decomposing leaf and fine root litter. Soil Biol. Biochem. 2015, 88, 39–47. [Google Scholar] [CrossRef]
- Cao, J.B.; He, X.X.; Chen, Y.Q.; Chen, Y.P.; Zhang, Y.J.; Yu, S.Q.; Zhou, L.X.; Liu, Z.F.; Zhang, C.L.; Fu, S.L. Leaf litter contributes more to soil organic carbon than fine roots in two 10-year-old subtropical plantations. Sci. Total Environ. 2020, 704, 135341. [Google Scholar] [CrossRef]
- Fang, J.Y.; Guo, Z.D.; Piao, S.L.; Chen, A.P. Estimation of carbon sink in China land from 1981 to 2000. Sci. China Ser. D Earth Sci. 2007, 37, 804–812. [Google Scholar]
- Wei, Y.M.; Liao, Z.W.; Mao, X.Y. Organic Carbon: A New Concept for Development of Modern Fertilizers and Plant Nutrition Theory. Asian Agric. Res. 2020, 12, 50–53. [Google Scholar] [CrossRef]
- Henry, R.J.; Furtado, A.; Rangan, P. Pathways of Photosynthesis in Non-Leaf Tissues. Biology 2020, 9, 438. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kumar, A.; Kuzyakov, Y.; Börjesson, G.; Blagodatskaya, E. Interactive priming effect of labile carbon and crop residues on SOM depends on residue decomposition stage: Three-source partitioning to evaluate mechanisms. Soil Biol. Biochem. 2018, 126, 179–190. [Google Scholar] [CrossRef]
- Vannoppen, W.; Vanmaercke, M.; De Baets, S.; Poesen, J. A review of the mechanical effects of plant roots on concentrated flow erosion rates. Earth Sci. Rev. 2015, 150, 666–678. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.J.; Dai, Q.H.; Hu, G.; Jiao, Q.; Fu, W.B. Effects of vegetation type on the microbial characteristics of the fissure soil-plant systems in karst rocky desertification regions of SW China. Sci. Total Environ. 2020, 712, 136543. [Google Scholar] [CrossRef]
- Feudis, M.D.; Car De Lli, V.; Massaccesi, L.; Hofmann, D.; Berns, A.; Bol, R.; Cocco, S.; Corti, G.; Agnelli, A. Altitude affects the quality of the water-extractable organic matter (WEOM) from rhizosphere and bulk soil in European beech forests. Geoderma 2017, 302, 6–13. [Google Scholar] [CrossRef]
- Wang, H.X.; Wu, C.Y.; Chen, D.S.; Liu, H.Y.; Sun, X.M.; Zhang, S.G. Changes in soil carbon and nutrients and related extracellular enzymes in successive rotations of Japanese larch plantations. Catena 2021, 204, 105386. [Google Scholar] [CrossRef]
- Li, M.M.; Zhang, X.C.; Pang, G.W.; Han, F.P. The estimation of soil organic carbon distribution and storage in a small catchment area of the Loess Plateau. Catena 2013, 101, 11–16. [Google Scholar] [CrossRef]
- Gao, X.; Ding, G.J.; Zhai, S.S.; Chen, M.F.; Du, H.D. Spatial distribution of root biomass of Pinus massoniana plantations under different planting densities. J. Cent. South. Univ. For. Technol. 2014, 34, 71–75. [Google Scholar]
- Li, M.; Han, X.Z.; Du, S.L.; Li, L.J. Profile stock of soil organic carbon and distribution in croplands of Northeast China. Catena 2019, 174, 285–292. [Google Scholar] [CrossRef]
- Martínez, G.L.B.; Korthals, G.W.; Brussaard, L.; Mainardi, G.; De, D.G.B. Litter quality drives nitrogen release, and agricultural management (organic vs. conventional) drives carbon loss during litter decomposition in agro-ecosystems. Soil Biol. Biochem. 2021, 153, 108115. [Google Scholar] [CrossRef]
- Bader, N.E.; Cheng, W. Rhizosphere priming effect of Populus fremontii obscures the temperature sensitivity of soil organic carbon respiration. Soil Biol. Biochem. 2006, 39, 600–606. [Google Scholar] [CrossRef]
- Feng, W.T.; Liang, J.Y.; Hale, L.E.; Jung, C.G.; Chen, J.; Zhou, J.Z.; Xu, M.G.; Yuan, M.T.; Wu, L.Y.; Bracho, R.; et al. Enhanced decomposition of stable soil organic carbon and microbial catabolic potentials by long-term field warming. Glob. Chang. Biol. 2017, 23, 4765–4776. [Google Scholar] [CrossRef]
- Howard, P.; Loveland, P.J.; Bradley, R.I.; Dry, F.T.; Howard, D.C. The carbon content of soil and its geographical distribution in Great Britain. Soil Use Manag. 1995, 11, 9–15. [Google Scholar] [CrossRef]
- An, S.; Mentler, A.; Mayer, H.; Blum, W.E.H. Soil aggregation, aggregate stability, organic carbon and nitrogen in different soil aggregate fractions under forest and shrub vegetation on the Loess Plateau, China. Catena 2010, 81, 226–233. [Google Scholar] [CrossRef]
- Akpa, S.I.C.; Odeh, I.O.A.; Bishop, T.F.A.; Hartemink, A.E.; Amapu, I.Y. Total soil organic carbon and carbon sequestration potential in Nigeria. Geoderma 2016, 271, 202–215. [Google Scholar] [CrossRef]
- Alidoust, E.; Afyuni, M.; Hajabbasi, M.A.; Mosaddeghi, M.R. Soil carbon sequestration potential as affected by soil physical and climatic factors under different land uses in a semiarid region. Catena 2018, 171, 62–71. [Google Scholar] [CrossRef]
- Kabir, E.B.; Bashari, H.; Mosaddeghi, M.; Bassiri, M. Soil aggregate stability and organic matter as affected by land-use change in central Iran. Arch. Agron. Soil Sci. 2017, 63, 1823–1837. [Google Scholar] [CrossRef]
- Zhao, P.P.; Shao, M.G.; Wang, T.J. Spatial Distributions of Soil Surface-Layer Saturated Hydraulic Conductivity and Controlling Factors on Dam Farmlands. Water Resour. Manag. 2010, 24, 2247–2266. [Google Scholar] [CrossRef]
- Ye, G.P.; Lin, Y.X.; Kuzyakov, Y.; Liu, D.Y.; Luo, J.F.; Lindsey, S.; Wang, W.J.; Fan, J.B.; Ding, W.X. Manure over crop residues increases soil organic matter but decreases microbial necromass relative contribution in upland Ultisols: Results of a 27-year field experiment. Soil Biol. Biochem. 2019, 134, 15–24. [Google Scholar] [CrossRef]




| Stand Age (a) | Slope Direction | Slope (°) | Elevation (m) | Soil Type | Bulk Density (g/cm−3) | Carbon (g/kg) | Nitrogen (g/kg) | Phosphorus (g/kg) | Canopy Density | Average Diameter at Breast High (cm) | Average Tree Height (m) | Tree Density (Individual/hm2) | Main Species |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | NW 35° | 28 | 1072–1076 | yellow soil | 1.28 | 20.46 | 0.93 | 0.17 | 0.5 | 3.25 | 2.78 | 1187 | Dicranopteris linearis, Imperata cylindrica (L.) Beauv., Miscanthus floridulus (Lab.) Warb. ex Schum. et Laut., Nandina domestica |
| 14 | NE 13° | 35 | 1063–1066 | yellow soil | 1.12 | 23.19 | 1.54 | 0.24 | 0.7 | 11.03 | 10.67 | 1103 | |
| 30 | NE 45° | 30 | 1067–1068 | yellow soil | 1.08 | 22.80 | 1.00 | 0.16 | 0.9 | 25.10 | 15.43 | 403 |
| Soil Depth (cm) | Term | Nonstandard Coefficient | Standard Coefficient | Adjusted R2 | p Value |
|---|---|---|---|---|---|
| 0–20 | (Constant) | 3.608 | 0.802 | 0.001 | |
| SBD | 2.514 | 0.909 | |||
| 20–40 | (Constant) | 4.216 | 0.644 | 0.019 | |
| MWD | 1.087 | 1.039 | |||
| SBD | −2.203 | −0.653 | |||
| 40–60 | (Constant) | 0.827 | 0.723 | 0.002 | |
| WSA > 0.25 mm | 0.820 | 0.870 | |||
| 60–80 | (Constant) | 0.533 | 0.684 | 0.004 | |
| GMD | 4.632 | 0.851 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Dai, Q.; Xu, F.; Peng, X.; Yan, Y. Variability in Carbon Stocks across a Chronosequence of Masson Pine Plantations and the Trade-Off between Plant and Soil Systems. Forests 2021, 12, 1342. https://doi.org/10.3390/f12101342
He J, Dai Q, Xu F, Peng X, Yan Y. Variability in Carbon Stocks across a Chronosequence of Masson Pine Plantations and the Trade-Off between Plant and Soil Systems. Forests. 2021; 12(10):1342. https://doi.org/10.3390/f12101342
Chicago/Turabian StyleHe, Jie, Quanhou Dai, Fengwei Xu, Xudong Peng, and Youjin Yan. 2021. "Variability in Carbon Stocks across a Chronosequence of Masson Pine Plantations and the Trade-Off between Plant and Soil Systems" Forests 12, no. 10: 1342. https://doi.org/10.3390/f12101342





