Alien Invasive Pathogens and Pests Harming Trees, Forests, and Plantations: Pathways, Global Consequences and Management
Abstract
:1. Introduction
2. IAPP Pathways
3. Factors Driving the Invasion Process
4. Invasive Alien Pathogens and Pests Harming Forests
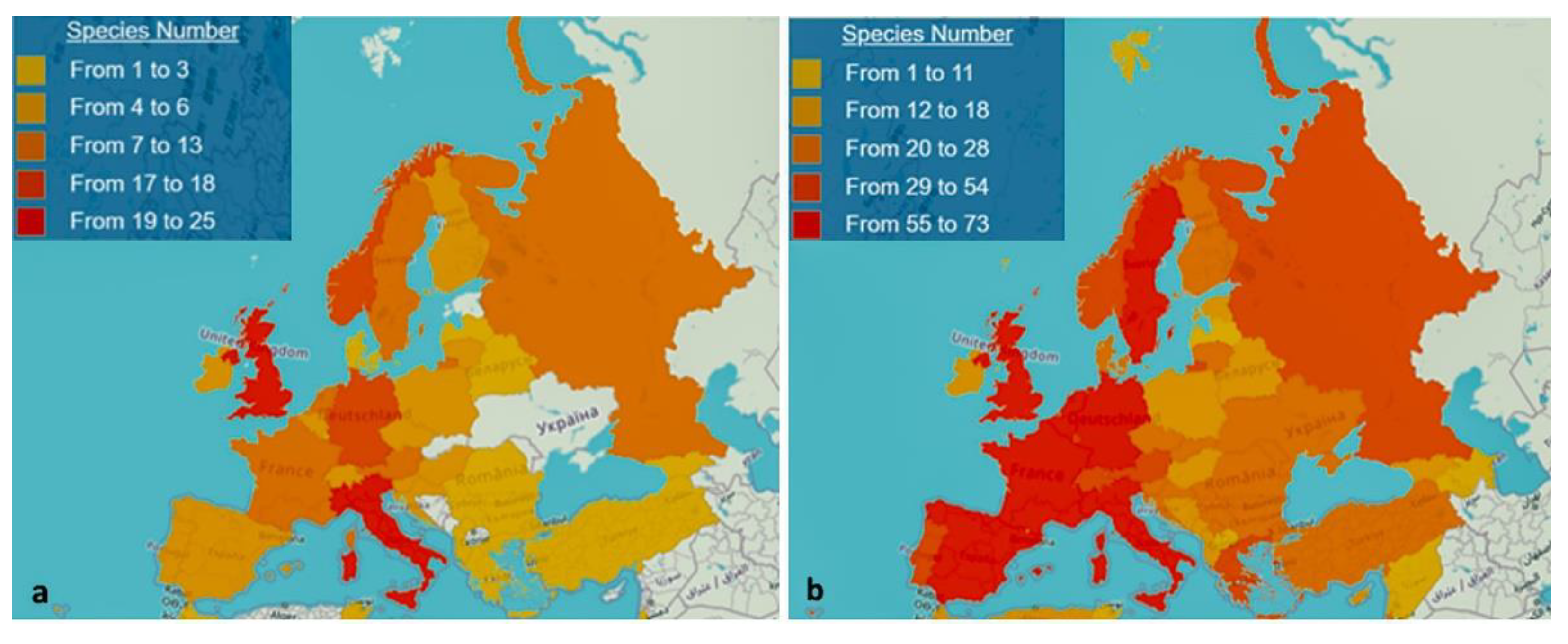
4.1. A Retrospective Look
4.2. Emerging Invaders
5. Mitigating Threats to Forest Health: Possible Solutions and Action Strategies
6. Role of Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying threats to imperiled species in the United States. BioScience 1998, 48, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.A.; Alexander, H.M.; Davidson, J.; Campbell, F.T.; Burdon, J.J.; Sniezko, R.; Brasier, C. Increasing forest loss worldwide from invasive pests requires new trade regulations. Front. Ecol. Environ. 2014, 12, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Chapman, D.; Purse, B.V.; Roy, H.E.; Bullock, J.M. Global trade networks determine the distribution of invasive non-native species. Glob. Ecol. Biogeogr. 2017, 26, 907–917. [Google Scholar] [CrossRef]
- Ascensão, F.; Capinha, C. Aliens on the move: Transportation networks and non-native species. In Railway Ecology; Borda-de-Água, L., Barrientos, R., Beja, P., Pereira, H.M., Eds.; Springer: Cham, Switzerland, 2017; pp. 293–297. ISBN 978-3-319-57496-7. [Google Scholar] [CrossRef] [Green Version]
- McNeill, M.; Phillips, C.; Young, S.; Shah, F.; Aalders, L.; Bell, N.; Gerard, E.; Littlejohn, R. Transportation of nonindigenous species via soil on international aircraft passengers’ footwear. Biol. Invasions 2011, 13, 2799–2815. [Google Scholar] [CrossRef]
- Meurisse, N.; Rassati, D.; Hurley, B.P.; Brockerhoff, E.G.; Haack, R.A. Common pathways by which non-native forest insects move internationally and domestically. J. Pest. Sci. 2019, 92, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Ginetti, B.; Moricca, S.; Squires, J.N.; Cooke, D.E.L.; Ragazzi, A.; Jung, T. Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of Acer pseudoplatanus trees in planted forests in Northern Italy. Plant Pathol. 2014, 63, 858–876. [Google Scholar] [CrossRef] [Green Version]
- Aglietti, C.; Benigno, A.; Scali, E.; Capretti, P.; Ghelardini, L.; Moricca, S. Molecular-based reappraisal of a historical record of Dothistroma needle blight in the centre of the Mediterranean region. Forests 2021, 12, 983. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Huenneke, L.F. Disturbance, diversity and invasion: Implications for conservation. Conserv. Biol. 1992, 6, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; Blackwell Publishing: Oxford, UK, 2007; 304p. [Google Scholar]
- Jeschke, J.M.; Strayer, D.L. Invasion success of vertebrates in Europe and North America. Proc. Natl. Acad. Sci. USA 2005, 102, 7198–7202. [Google Scholar] [CrossRef] [Green Version]
- Moricca, S.; Bracalini, M.; Croci, F.; Corsinovi, S.; Tiberi, R.; Ragazzi, A.; Panzavolta, T. Biotic factors affecting ecosystem services in urban and peri-urban forests in Italy: The role of introduced and impending pathogens and pests. Forests 2018, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Panzavolta, T.; Panichi, A.; Bracalini, M.; Croci, F.; Ginetti, B.; Ragazzi, A.; Tiberi, R.; Moricca, S. Dispersal and propagule pressure of Botryosphaeriaceae species in a declining oak stand is affected by insect vectors. Forests 2017, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of forest insect pests to climate change: Not so simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef]
- Freer-Smith, P.H.; Webber, J.F. Tree pests and diseases: The threat to biodiversity and the delivery of ecosystem services. Biodivers. Conserv. 2015, 26, 3167–3181. [Google Scholar] [CrossRef]
- Shine, C.; Williams, N.; Gundling, L. A Guide to Designing Legal and Institutional Frameworks on Alien Invasive Species; IUCN: Gland, Switzerland, 2000; 152p. [Google Scholar]
- Hurley, B.P.; Garnas, J.; Wingfield, M.J.; Branco, M.; Richardson, D.M.; Slippers, B. Increasing numbers and intercontinental spread of invasive insects on eucalypts. Biol. Invasions 2016, 18, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Petter, F.; Suffert, M.; McMullen, M.; Griessinger, D.; Roy, A.S. Seed-borne pests and phytosanitary issues: The role of EPPO. In Global Perspectives on the Health of Seeds and Plant Propagation Material; Gullino, M., Munkvold, G., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Banks, N.C.; Paini, D.R.; Bayliss, K.L.; Hodda, M. The role of global trade and transport network topology in the human-mediated dispersal of alien species. Ecol. Lett. 2015, 18, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Eldredge, N. Life on Earth: An Encyclopedia of Biodiversity, Ecology, and Evolution; ABC-CLIO Inc.: Santa Barbara, CA, USA, 2002; 800p. [Google Scholar]
- Liebhold, A.M.; Work, T.T.; McCullough, D.G.; Cavey, J.F. Airline baggage as a pathway for alien insect species entering the United States. Am. Entomol. 2006, 52, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Europhyt. Interceptions of Harmful Organisms in Imported Plants and Other Objects, Annual and Monthly Interception. Available online: https://ec.europa.eu/food/plants/plant-health-and-biosecurity/european-union-notification-system-plant-health-interceptions-2_en (accessed on 26 July 2021).
- McCullough, D.G.; Work, T.T.; Cavey, J.F.; Liebhold, A.M.; Marshall, D. Interceptions of nonindigenous plant pests at US ports of entry and border crossings over a 17-year period. Biol. Invasions 2006, 8, 611–630. [Google Scholar] [CrossRef]
- Boddy, L.; Griffith, G.S. Role of endophytes and latent invasion in the development of decay communities in sapwood of angiospermous trees. Sydowia 1989, 41, 41–73. [Google Scholar]
- Précigout, P.A.; Claessen, D.; Makowski, D.; Robert, C. Does the latent period of leaf fungal pathogens reflect their trophic type? A meta-analysis of biotrophs, hemibiotrophs, and necrotrophs. Phytopathology 2020, 110, 345–361. [Google Scholar] [CrossRef] [Green Version]
- Roques, A. Alien forest insects in a warmer world and a globalised economy: Impacts of changes in trade, tourism, and climate on forest biosecurity. N. Zeal. J. For. Sci. 2010, 40, 77–94. [Google Scholar]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Applic. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Williamson, M.; Fitter, A. The varying success of invaders. Ecology 1996, 77, 1661–1665. [Google Scholar] [CrossRef]
- Williamson, M. Biological Invasions; Chapman & Hall: London, UK, 1996; 244p. [Google Scholar]
- Liebhold, A.M.; MacDonald, W.L.; Bergdahl, D.; Mastro, V.C. Invasion by Exotic Forest Pests: A Threat to Forest Ecosystems. For. Sci. 1995, 41, a0001–z0001. [Google Scholar] [CrossRef] [Green Version]
- Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A.; Ragazzi, A. Endemic and emerging pathogens threatening cork oak trees: Management options for conserving a unique forest ecosystem. Plant Dis. 2016, 100, 2184–2193. [Google Scholar] [CrossRef] [Green Version]
- Jarić, I.; Cvijanović, G. The Tens Rule in invasion biology: Measure of a true impact or our lack of knowledge and understanding? Enviro. Manag. 2012, 50, 979–981. [Google Scholar] [CrossRef]
- Lovell, R.S.L.; Blackburn, T.M.; Dyer, E.E.; Pigot, A.L. Environmental resistance predicts the spread of alien species. Nat. Ecol. Evol. 2021, 5, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Philibert, A.; Desprez-Loustau, M.L.; Fabre, B.; Frey, P.; Halkett, F.; Husson, C.; Lung-Escarmant, B.; Marçais, B.; Robin, C.; Vacher, C.; et al. Predicting invasion success of forest pathogenic fungi from species traits. J. Appl. Ecol. 2011, 48, 1381–1390. [Google Scholar] [CrossRef]
- Warren, R.J.; Candeias, M.; Lafferty, A.; Chick, L.D. Regional-scale environmental resistance to non-native ant invasion. Biol. Invasions 2020, 22, 813–825. [Google Scholar] [CrossRef]
- Zenni, R.D.; Nuñez, M.A. The elephant in the room: The role of failed invasions in understanding invasion biology. Oikos 2013, 122, 801–815. [Google Scholar] [CrossRef]
- Morrison, W.E.; Hay, M.E. Herbivore preference for native vs. exotic plants: Generalist herbivores from multiple continents prefer exotic plants that are evolutionarily naïve. PLoS ONE 2011, 6, e17227. [Google Scholar] [CrossRef] [Green Version]
- Gladieux, P.; Feurtey, A.; Hood, M.E.; Snirc, A.; Clavels, J.; Dutech, C.; Roy, M.; Giraud, T. The population biology of fungal invasions. Mol. Ecol. 2015, 24, 1969–1986. [Google Scholar] [CrossRef]
- Roques, A.; Shi, J.; Auger-Rozenberg, M.-A.; Ren, L.; Augustin, S.; Luo, Y. Are invasive patterns of non-native insects related to woody plants differing between Europe and China? Front. For. Glob. Chang. 2020, 2, 91. [Google Scholar] [CrossRef] [Green Version]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2010, 17, 164–170. [Google Scholar] [CrossRef]
- Wolfe, L.M. Why alien invaders succeed: Support for the escape-from-enemy hypothesis. Am. Nat. 2002, 160, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Auger-Rozenberg, M.A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.W.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Sache, I.; Roy, A.S.; Suffert, F.; Desprez-Loustau, M.L. Invasive plant pathogens in Europe. In Biological Invasions: Economic and Environmental Costs of Alien Plant, Animal, and Microbe Species, 2nd ed.; Pimentel, D., Ed.; CRC Press-Taylor and Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.P.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenth, J.; McCulloug, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Appl. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E.; Bacher, S.; Kenis, M.; Klotz, S.; Kühn, I.; Minchin, D.; Nentwig, W.; Olenin, S.; Panov, V.; Pergl, J.; et al. Grasping at the routes of biological invasions: A framework for integrating pathways into policy. J. Appl. Ecol. 2008, 45, 323–341. [Google Scholar] [CrossRef]
- Moore, B.A. Alien Invasive Species: Impacts on Forests and Forestry. A Review; Forest Health and Biosecurity Working Paper 8. Forest Resources Development Service Working Paper FBS/8E; Forest Resources Division FAO: Rome, Italy, 2005. [Google Scholar]
- Brasier, C.M. China and the origins of Dutch elm disease: An appraisal. Plant Pathol. 1990, 39, 5–16. [Google Scholar] [CrossRef]
- Brasier, C.M.; Kirk, S.A. Designation of the EAN and NAN races of Ophiostoma novo-ulmi as subspecies. Mycol. Res. 2001, 105, 547–554. [Google Scholar] [CrossRef]
- Brasier, C.M.; Gibbs, J.N. Origin of the Dutch elm disease Epidemic in Britain. Nature 1973, 242, 607–609. [Google Scholar] [CrossRef]
- Brasier, C.M. Intercontinental spread and continuing evolution of the Dutch elm disease pathogens. In The Elms; Dunn, C.P., Ed.; Springer: Boston, MA, USA, 2000; pp. 61–72. [Google Scholar]
- Vettraino, A.M.; Morel, O.; Perlerou, C.; Robin, C.; Diamandis, S.; Vannini, A. Occurrence and distribution of Phytophthora species in European chestnut stands and their association with ink disease and crown decline. Eur. J. Plant Pathol. 2005, 111, 169–180. [Google Scholar] [CrossRef]
- Milgroom, M.G.; Wang, K.; Zhou, Y.; Lipari, S.E.; Kaneko, S. Intercontinental population structure of the chestnut blight fungus, Cryphonectria parasitica. Mycologia 1996, 88, 179–190. [Google Scholar] [CrossRef]
- Panconesi, A. Canker stain of plane trees: A serious danger to urban plantings in Europe. J. Plant Pathol. 1999, 81, 3–15. [Google Scholar]
- Moricca, S.; Panconesi, A. Canker stain of plane-trees: A serious threat to North-European urban plantations. Mitt. Biol. Bundesanst. Land- Forstwirtsch. Berl.-Dahl. 2000, 370, 97–100. [Google Scholar]
- Panconesi, A.; Moricca, S.; Dellavalle, I.; Torraca, G. The epidemiology of canker stain of Plane tree and its spread from urban plantings to spontaneous groves and natural forests. In Proceedings of the Second International Symposium on Plant Health in Urban Horticulture, Berlin, Germany, 27–29 August 2003; pp. 84–91. [Google Scholar]
- Wagener, W.W. Diseases of Cupressus. In Proceedings of the FAO-IUFRO Symposium on Internationally Dangerous Forest Diseases and Insects, Oxford, UK, 20–29 July 1964; pp. 17–24. [Google Scholar]
- Graniti, A. Cypress canker: A pandemic in progress. Annu. Rev. Phytopathol. 1998, 36, 91–118. [Google Scholar] [CrossRef] [PubMed]
- Sutton, B.C.; Gibson, I.A.S. Seiridium cardinale. CMI Description of Pathogenic Fungi and Bacteria No. 326; Commonwealth Mycological Institute: Kew, UK, 1972. [Google Scholar]
- Grasso, V.; Ponchet, J. Historique, distribution géographique et hôtes du Coryneum cardinale. Wag. See Ref. 1979, 31, 119–126. [Google Scholar]
- Graniti, A. Seiridium blight of cypress—Another ecological disaster? Plant Dis. 1993, 77, 544. [Google Scholar]
- Panzavolta, T.; Panichi, A.; Bracalini, M.; Croci, F.; Benigno, A.; Ragazzi, A.; Tiberi, R.; Moricca, S. Tree pathogens and their insect-mediated transport: Implications for oak tree die-off in a natural park area. Glob. Ecol. Conserv. 2018, 15, e00437. [Google Scholar] [CrossRef]
- Fiala, T.; Holuša, J. Occurrence of the invasive bark beetle Phloeosinus aubei on common juniper trees in the Czech Republic. Forests 2019, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Michalski, J. Revision of the Palearctic Species of the Genus Scolytus Geoffroy (Coleoptera, Scolytidae); Panstwowe Wydawnictwo Naukowe: Warsaw, Poland, 1973; 214p. [Google Scholar]
- Bloomfield, H. Elms for always. Am. For. 1979, 85, 24–26, 48, 50. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000; 12p. [Google Scholar]
- Bradshaw, C.; Leroy, B.; Bellard, C.; Roiz, D.; Albert, C.; Fournier, A.; Barbet-Massin, M.; Salles, J.M.; Simard, F.; Courchamp, F. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 2016, 7, 12986. [Google Scholar] [CrossRef]
- Montgomery, M.E.; Wallner, W.E. The gypsy moth. A westward migrant. In Dynamics of Forest Insect Populations: Patterns, Causes, Implications; Berryman, A.A., Ed.; Plenum Press: New York, NY, USA, 1988; pp. 353–376. [Google Scholar]
- Leuschner, W.A.; Young, J.A.; Waldon, S.A.; Ravlin, F.W. Potential benefits of slowing the gypsy moth’s spread. South. J. Appl. For. 1996, 20, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Bigsby, K.M.; Ambrose, M.J.; Tobin, P.C.; Sills, E.O. The cost of gypsy moth sex in the city. Urban For. Urban Green. 2014, 13, 459–468. [Google Scholar] [CrossRef]
- Morin, R.S.; Liebhold, A.M.; Gottschalk, K.W.; Acciavatti, R.; Twardus, D.; White, R.; Horsley, S.; Smith, W. Forest Health Conditions on the Allegheny National Forest (1989–1999): Analysis of Forest Health Monitoring Surveys. NA-TP-04-01; Department of Agriculture, Forest Service, Northeastern Area, State and Private Forestry: Newton Square, PA, USA, 2001. [Google Scholar]
- CABI. Available online: https://www.cabi.org/isc/datasheet/13579 (accessed on 26 July 2021).
- O’Neil, C. Cypress Aphid, Cinara cupressi; The Entomology and Forest Resources Digital Information Work Group, College of Agricultural and Environmental Sciences and Warnell School of Forest Resources, The University of Georgia: Tifton, GA, USA, 1998; Available online: www.afae.org/html/98-202.html (accessed on 27 July 2021).
- Watson, G.W.; Voegtlin, D.J.; Murphy, S.T.; Foottit, R.G. Biogeography of the Cinara cupressi complex (Hemiptera: Aphididae) on Cupressaceae, with description of a pest species introduced into Africa. Bull. Entomol. Res. 1999, 89, 271–283. [Google Scholar] [CrossRef]
- Jung, T.; Horta Jung, M.; Webber, J.F.; Kageyama, K.; Hieno, A.; Masuya, H.; Uematsu, S.; Pérez-Sierra, A.; Harris, A.R.; Forster, J.; et al. The destructive tree pathogen Phytophthora ramorum originates from the laurosilva forests of East Asia. J. Fungi 2021, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasier, C.M.; Webber, J.L. Sudden larch death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef]
- Shearer, B.; Crane, C.; Cochrane, A. A thief of time: Phytophthora cinnamomi and threatened flora. Austr. Plant Conserv. 2005, 13, 14–15. [Google Scholar] [CrossRef]
- Hansen, E.M. Phytophthora species emerging as pathogens of forest trees. Curr. For. Rep. 2015, 1, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Green, S.; Brasier, C.M.; Schlenzig, A.; McCracken, A.; MacAskill, G.A.; Wilson, M.; Webber, J.F. The destructive invasive pathogen Phytophthora lateralis found on Chamaecyparis lawsoniana across the UK. For. Pathol. 2013, 43, 19–28. [Google Scholar] [CrossRef]
- Hantula, J.; Scholler, M. NOBANIS—Invasive Alien Species Fact Sheet—Melampsoridium hiratsukanum. Database of the European Network on Invasive Alien Species. NOBANIS. 2013. Available online: https://www.nobanis.org/fact-sheets/ (accessed on 23 March 2021).
- Moricca, S.; Benigno, A.; Oliveira Longa, C.M.; Cacciola, S.O.; Maresi, G. First documentation of life cycle completion of the alien rust pathogen Melampsoridium hiratsukanum in the Eastern Alps proves its successful establishment in this mountain range. J. Fungi 2021, 7, 617. [Google Scholar] [CrossRef] [PubMed]
- McKinney, L.V.; Nielsen, L.R.; Collinge, D.B.; Thomsen, I.M.; Hansen, J.K.; Kjaer, E.D. The ash dieback crisis: Genetic variation in resistance can prove a long-term solution. Plant Pathol. 2014, 63, 485–499. [Google Scholar] [CrossRef]
- Hill, L.; Jones, G.; Atkinson, N.; Hector, A.; Hemery, G.; Brown, N. The £15 billion cost of ash dieback in Britain. Curr. Biol. 2019, 29, R315–R316. [Google Scholar] [CrossRef] [PubMed]
- Nirenberg, H.I.; O’Donnell, K. New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 1998, 90, 434–458. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Hammerbacher, A.; Ganley, R.J.; Steenkamp, E.T.; Gordon, T.R.; Wingfield, B.D. Pitch canker caused by Fusarium circinatum—A growing threat to pine plantations and forests worldwide. Australas. Plant Pathol. 2008, 37, 319–334. [Google Scholar] [CrossRef]
- Pehl, L.; Cech, T.L.; Ioos, R. Lecanosticta acicola (formerly Mycosphaerella dearnessii), Dothistroma septosporum (formerly Mycosphaerella pini) and Dothistroma pini. EPPO Bull. 2015, 45, 163–182. [Google Scholar]
- Barnes, I.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Multigene phylogenies reveal that red band needle blight of Pinus is caused by two distinct species of Dothistroma, D. septosporum and D. pini. Stud. Mycol. 2004, 50, 551–566. [Google Scholar]
- Watt, M.S.; Kriticos, D.J.; Alcaraz, S.; Brown, A.V.; Leriche, A. The hosts and potential geographic range of Dothistroma needle blight. For. Ecol. Manag. 2009, 257, 1505–1519. [Google Scholar] [CrossRef]
- Ghelardini, L.; Aglietti, C.; Loria, F.; Cerboneschi, M.; Gionni, A.; Goti, E.; Maresi, G.; Moricca, S.; Marchi, G. Dothistroma Needle Blight in protected pine forests in Italy. Manag. Biol. Invasions 2020, 11, 689–702. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Coutinho, T.A.; Roux, J.; Wingfield, B.D. The future of exotic plantation forestry in the tropics and southern hemisphere: Lessons from pitch canker. S. Afr. For. J. 2002, 195, 79–82. [Google Scholar] [CrossRef]
- Bulman, L.S.; Bradshaw, R.E.; Fraser, S.; Martn-Garca, J.; Barnes, I.; Musolin, D.L.; La Porta, N.; Woods, A.J.; Diez, J.J.; Koltay, A.; et al. A worldwide perspective on the management and control of Dothistroma needle blight. Forest Pathol. 2016, 46, 472–488. [Google Scholar] [CrossRef] [Green Version]
- Gibson, I.A.S. Impact and control of Dothistroma blight of pines. Eur. J. For. Pathol. 1974, 4, 89–100. [Google Scholar] [CrossRef]
- Villebonne, D.; Maugard, F. Rapid development of Dothistroma needle blight (Scirrhia pini) on Corsican pine (Pinus nigra subsp. laricio) in France. In La Sante des Forets, Annual Report 1998; Les Cahiers du Département de la santé des forêts (DSF); DERF: Paris, France, 1999. [Google Scholar]
- Newton, L.P.; Fowler, G.; Neeley, A.D.; Schall, R.A.; Takeuchi, Y. Pathway Assessment: Geosmithia sp. and Pityophthorus juglandis Blackman Movement from the Western into the Eastern United States; U.S. Department of Agriculture Animal and Plant Health Inspection Service: Raleigh, NC, USA, 2009; 50p.
- Montecchio, L.; Faccoli, M. First record of thousand cankers disease Geosmithia morbida and walnut twig beetle Pityophthorus juglandis on Juglans nigra in Europe. Plant Dis. 2014, 98, 696. [Google Scholar] [CrossRef]
- Moricca, S.; Bracalini, M.; Benigno, A.; Ginetti, B.; Pelleri, F.; Panzavolta, T. Disease Note. Thousand cankers disease caused by Geosmithia morbida and its insect vector Pityophthorus juglandis first reported on Juglans nigra in Tuscany, Central Italy. Plant Dis. 2019, 103, 369. [Google Scholar] [CrossRef]
- Umeda, C.; Eskalen, A.; Paine, T.D. Polyphagous Shot Hole Borer and Fusarium Dieback in California. In Insects and Diseases of Mediterranean Forest Systems; Paine, T., Lieutier, F., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Haack, R.A.; Cavey, J.F.; Hoebeke, E.R.; Law, K. Anoplophora glabripennis: A new tree-infesting exotic cerambycid invades New York. Mich. Entomol. Soc. Newsl. 1996, 41, 1–3. [Google Scholar]
- EPPO. Anoplophora chinensis Found under Glasshouse in Georgia (US). Available online: https://gd.eppo.int/reporting/article-3421 (accessed on 26 July 2021).
- Hérard, F.; Maspero, M. History of discoveries and management of the citrus longhorned beetle, Anoplophora chinensis, in Europe. J. Pest Sci. 2019, 92, 117–130. [Google Scholar] [CrossRef]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 2010, 55, 521–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegert, N.W.; Mccullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- EPPO. Agrilus Planipennis. EPPO Datasheets on Pests Recommended for Regulation. Available online: https://gd.eppo.int (accessed on 26 July 2021).
- Orlova-Bienkowskaja, M.J.; Drogvalenko, A.N.; Zabaluev, I.A.; Sazhnev, A.S.; Peregudova, E.Y.; Mazurov, S.G.; Komarov, E.V.; Struchaev, V.V.; Martynov, V.V.; Nikulina, T.V.; et al. Current range of Agrilus planipennis Fairmaire, an alien pest of ash trees, in European Russia and Ukraine. Ann. For. Sci. 2020, 77, 29. [Google Scholar] [CrossRef]
- Drogvalenko, A.N.; Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Record of the emerald ash borer (Agrilus planipennis) in Ukraine is confirmed. Insects 2019, 10, 338. [Google Scholar] [CrossRef] [Green Version]
- Marzano, M.; Hall, C.; Dandy, N.; Fisher, C.L.; Diss-Torrance, A.; Haight, R.G. Lessons from the frontline: Exploring how stakeholders may respond to emerald ash borer management in Europe. Forests 2020, 11, 617. [Google Scholar] [CrossRef]
- Thompson, I.; Mackey, B.; McNulty, S.; Mosseler, A. Forest Resilience, Biodiversity, and Climate Change: A Synthesis of the Biodiversity/Resilience/Stability Relationship in Forest Ecosystems; Technical Series no. 43; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2009; 67p. [Google Scholar]
- Rytter, L.; Johansson, K.; Karlsson, B.; Stener, L.-G. Tree species, genetics and regeneration for bioenergy feedstock in Northern Europe. In Forest Bioenergy Production, Management, Carbon Sequestration and Adaptation; Kellomäki, S., Kilpeläinen, A., Alam, A., Eds.; Springer: New York, NY, USA, 2013; pp. 7–37. [Google Scholar]
- Pérez, G.; Hunter, G.C.; Slippers, B.; Pérez, C.; Wingfield, B.D.; Wingfield, M.J. Teratosphaeria (Mycosphaerella) nubilosa, the causal agent of Mycosphaerella leaf disease (MLD), recently introduced into Uruguay. Eur. J. Plant Pathol. 2009, 125, 109–118. [Google Scholar] [CrossRef]
- European Commission. The Insect Killing Our Palm Trees. EU Efforts to Stop the Red Palm Weevil; Office for Official Publications of the European Communities: Luxembourg, 2011. [Google Scholar]
- Burks, R.A.; Mottern, J.L.; Waterworth, R.; Paine, T.D. First report of the Eucalyptus gall wasp, Ophelimus maskelli (Hymenoptera: Eulophidae), an invasive pest on Eucalyptus, from the Western Hemisphere. Zootaxa 2015, 3926, 448–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittenberg, R.; Cock, M.J.W. Best practices for the prevention and management of invasive alien species. In Invasive Alien Species: A New Synthesis; Mooney, H.A., Mack, R.N., McNeely, J.A., Neville, L.E., Scheiand, P.J., Waage, J.K., Eds.; Island Press: Washington, DC, USA, 2005; pp. 209–232. [Google Scholar]
- Rizzo, D.; Moricca, S.; Bracalini, M.; Benigno, A.; Bernardo, U.; Luchi, N.; Da Lio, D.; Nugnes, F.; Cappellini, G.; Salemi, C.; et al. Rapid detection of Pityophthorus juglandis (Blackman) (Coleoptera, Curculionidae) with the Loop-Mediated Isothermal Amplification (LAMP) method. Plants 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.; Gilbert, M.; De Canniere, C.; Gregoire, J.C. Coniferous round wood imports from Russia and Baltic countries to Belgium. A pathway analysis for assessing risks of exotic pest insect introductions. Divers. Distrib. 2008, 14, 318–328. [Google Scholar] [CrossRef]
- Matthews, J.; Beringen, R.; Creemers, R.; Hollander, H.; Van Kessel, N.; Van der Kleef, H.; van der Velde, G.; Verbrugge, L.N.H.; Leuven, R.S.E.W. Horizon Scanning for New Invasive Non-Native Species in the Netherlands; Department of Environmental Science, Institute for Water and Wetland Research, Faculty of Science, Radboud University Nijmegen: Nijmegen, The Netherlands, 2014; 117p. [Google Scholar]
- Mack, R.N.; Barrett, S.C.H.; deFur, P.L.; MacDonald, W.L.; Madden, L.V.; Marshall, D.S.; McCullough, D.G.; McEvoy, P.B.; Nyrop, J.P.; Reichard, S.E.H.; et al. Predicting Invasions of Nonindigenous Plants and Plant Pests; National Academy of Sciences: Washington DC, USA, 2002; 194p. [Google Scholar]
- Shine, C.; Kettunen, M.; Genovesi, P.; Essl, F.; Gollasch, S.; Rabitsch, W.; Scalera, R.; Starfinger, U.; Ten Brink, P. Assessment to Support Continued Development of the EU Strategy to Combat Invasive Alien Species; Institute for European Environmental Policy (IEEP): Brussels, Belgium, 2010; 298p. [Google Scholar]
- Kenis, M.; Li, H.; Fan, J.T.; Courtial, B.; Auger-Rozenberg, M.A.; Yart, A.; Eschen, R.; Roques, A. Sentinel nurseries to assess the phytosanitary risks from insect pests on importations of live plants. Sci. Rep. 2018, 8, 11217. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Li, H.-M.; Eschen, R.; Morales-Rodriguez, C.; Vannini, A. The sentinel tree nursery as an early warning system for pathway risk assessment: Fungal pathogens associated with Chinese woody plants commonly shipped to Europe. PLoS ONE 2017, 12, e0188800. [Google Scholar] [CrossRef] [Green Version]
- McCartney, H.A.; Foster, S.J.; Fraaije, B.A.; Ward, E. Molecular diagnostics for fungal plant pathogens. Pest. Manag. Sci. 2003, 59, 129–142. [Google Scholar] [CrossRef]
- Rizzo, D.; Da Lio, D.; Bartolini, L.; Cappellini, G.; Bruscoli, T.; Bracalini, M.; Benigno, A.; Salemi, C.; Del Nista, D.; Aronadio, A.; et al. A duplex real-time PCR with probe for simultaneous detection of Geosmithia morbida and its vector Pityophthorus juglandis. PLoS ONE 2020, 15, e0241109. [Google Scholar] [CrossRef]
- Fekrat, L.; Aghl, M.Z.; Tahan, V. Application of the LAMP assay as a diagnostic technique for rapid identification of Thrips tabaci (Thysanoptera: Thripidae). J. Econ. Entomol. 2015, 108, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Blaser, S.; Diem, H.; Von Felten, A.; Gueuning, M.; Andreou, M.; Boonham, N.; Tomlinson, J.; Müller, P.; Utzinger, J.; E Frey, J.; et al. From laboratory to point of entry: Development and implementation of a loop-mediated isothermal amplification (LAMP)-based genetic identification system to prevent introduction of quarantine insect species. Pest Manag. Sci. 2018, 74, 1504–1512. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, D.; Luchi, N.; Da Lio, D.; Bartolini, L.; Nugnes, F.; Cappellini, G.; Bruscoli, T.; Salemi, C.; Griffo, R.V.; Garonna, A.P.; et al. Development of a loop-mediated isothermal amplification (LAMP) assay for the identification of the invasive wood borer Aromia bungii (Coleoptera: Cerambycidae) from frass. Biotech 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Tordoni, E.; Ametrano, C.G.; Banchi, E.; Ongaro, S.; Pallavicini, A.; Bacaro, G.; Muggia, L. Integrated eDNA metabarcoding and morphological analyses assess spatio-temporal patterns of airborne fungal spores. Ecol. Indic. 2021, 121, 107032. [Google Scholar] [CrossRef]
- Martin, R.R.; James, D.; Lévesque, C.A. Impacts of molecular diagnostic technologies on plant disease management. Annu. Rev. Phytopathol. 2000, 38, 207–239. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, M.; Liu, M.; Ju, Y. Monitoring exotic forest pest based on high-resolution remote sensing image and CART model. In Proceedings of the 3rd International Congress on Image and Signal Processing, Yantai, China, 16–18 October 2010; Tan, Z.H., Wan, Y., Xiang, T., Song, Y., Eds.; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA; pp. 2203–2206. [Google Scholar] [CrossRef]
- Hall, R.; Castilla, G.; White, J.; Cooke, B.; Skakun, R. Remote sensing of forest pest damage: A review and lessons learned from a Canadian perspective. Can. Entomol. 2016, 148, S296–S356. [Google Scholar] [CrossRef]
- Hoyer-Tomiczek, U.; Sauseng, G.; Hoch, G. Scent detection dogs for the Asian longhorn beetle, Anoplophora glabripennis. EPPO Bull. 2016, 46, 148–155. [Google Scholar] [CrossRef]
- Mosconi, F.; Campanaro, A.; Carpaneto, G.M.; Chiari, S.; Hardersen, S.; Mancini, E.; Maurizi, E.; Sabatelli, S.; Zauli, A.; Mason, F.; et al. Training of a dog for the monitoring of Osmoderma eremita. Nat. Conserv. 2017, 20, 237–264. [Google Scholar] [CrossRef] [Green Version]
- Brown, N.; van den Bosch, F.; Parnell, S.; Denman, S. Integrating regulatory surveys and citizen science to map outbreaks of forest diseases: Acute oak decline in England and Wales. Proc. Royal Soc. B 2017, 284, 20170547. [Google Scholar] [CrossRef] [Green Version]
- Panzavolta, T.; Bracalini, M.; Tiberi, R. Impatto delle invasioni biologiche sul paesaggio urbano. In Paesaggi Abitati: Dalla Percezione al Sistema Complesso; Bolletti, S., Puma, P., Eds.; Edifir Edizioni Firenze s.r.l.: Florence, Italy, 2021; pp. 73–79. [Google Scholar]
- Bonney, R.; Shirk, J.L.; Phillips, T.B.; Wiggins, A.; Ballard, H.L.; Miller-Rushing, A.J.; Parrish, J.K. Next steps for citizen science. Science 2014, 343, 1436–1437. [Google Scholar] [CrossRef]
- Bonney, R.; Phillips, T.B.; Ballard, H.L.; Enck, J.W. Can citizen science enhance public understanding of science? Public Underst. Sci. 2016, 25, 2–16. [Google Scholar] [CrossRef]
- Suckling, D.M.; Stringer, L.D.; Stephens, A.E.A.; Woods, B.; Williams, D.G.; Baker, G.; El-Sayed, A.M. From integrated pest management to integrated pest eradication: Technologies and future needs. Pest Manag. Sci. 2013, 70, 179–189. [Google Scholar] [CrossRef]
- Pluess, T.; Jarosik, V.; Pysek, P.; Cannon, R.; Pergl, J.; Breukers, A.; Bacher, S. Which factors affect the success or failure of eradication campaigns against alien species? PLoS ONE 2012, 7, e48157. [Google Scholar] [CrossRef]
- Moricca, S.; Bracalini, M.; Benigno, A.; Panzavolta, T. Observations on the non-native thousand cankers disease of walnut in Europe’s southernmost outbreak. Global Ecol. Conserv. 2020, 23, e01159. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Kean, J.M. Eradication and containment of non-native forest insects: Successes and failures. J. Pest Sci. 2019, 92, 83–91. [Google Scholar] [CrossRef]
- Vainio, E.J.; Bezos, D.; Bragança, H.; Cleary, M.; Fourie, G.; Georgieva, M.; Ghelardini, L.; Hannunen, S.; Ioos, R.; Martín-García, J.; et al. Sampling and detection strategies for the pine pitch canker (PPC) disease pathogen Fusarium circinatum in Europe. Forests 2019, 10, 723. [Google Scholar] [CrossRef] [Green Version]
- Ramsfield, T.D.; Bentz, B.J.; Faccoli, M.; Jactel, H.; Brockerhoff, E.G. Forest health in a changing world: Effects of globalization and climate change on forest insect and pathogen impacts. Forestry 2016, 89, 245–252. [Google Scholar] [CrossRef]
- Caltagirone, L.E.; Doutt, R.L. The history of the vedalia beetle importation to California and its impact on the development of biological control. Annu. Rev. Entomol. 1989, 34, 1–16. [Google Scholar] [CrossRef]
- McEvoy, P.; Cox, C.; Coombs, E. Successful biological control of ragwort, Senecio jacobaea, by introduced insects in Oregon. Ecol. Appl. 1991, 1, 430–442. [Google Scholar] [CrossRef]
- Radcliffe, E.B.; Flanders, K.L. Biological control of alfalfa weevil in North America. Integr. Pest Manag. Rev. 1998, 3, 225–242. [Google Scholar] [CrossRef]
- Ferracini, C.; Ferrari, E.; Saladini, M.A.; Pontini, M.; Corradetti, M.; Alma, A. Non-target host risk assessment for the parasitoid Torymus sinensis. BioControl 2015, 60, 583–594. [Google Scholar] [CrossRef]
- Vasiliauskas, R.; Larsson, E.; Larsson, K.-H.; Stenlid, J. Persistence and long-term impact of Rotstop biological control agent on mycodiversity in Picea abies stumps. Biol. Control. 2005, 32, 295–304. [Google Scholar] [CrossRef]
- Tubby, K.V.; Scott, D.; Webber, J.F. Relationship between stump treatment coverage using the biological control product PG Suspension, and control of Heterobasidion annosum on Corsican pine, Pinus nigra ssp. Laricio. For. Path. 2008, 38, 37–46. [Google Scholar] [CrossRef]
- Korhonen, K.; Stenlid, J. Biology of Heterobasidion annosum. In Heterobasidion annosum. Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 43–70. [Google Scholar]
- Slippers, B.; Hurley, B.P.; Wingfield, M.J. Sirex woodwasp: A model for evolving management paradigms of invasive forest pests. Annu. Rev. Entomol. 2015, 60, 601–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moricca, S.; Ragazzi, A. Biological and integrated means to control rust diseases. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Ciancio, A., Mukerji, K.J., Eds.; Springer: Berlin, Germany, 2008; pp. 303–329. [Google Scholar]
- Van Lenteren, J.C.; Bale, J.; Bigler, F.; Hokkanen, H.M.T.; Loomans, A.J.M. Assessing risks of releasing exotic biological control agents of arthropod pests. Annu. Rev. Entomol. 2006, 51, 609–634. [Google Scholar] [CrossRef] [Green Version]
- Yara, K.; Sasawaki, T.; Kunimi, Y. Hybridization between introduced Torymus sinensis (Hymenoptera: Torymidae) and indigenous T. beneficus (late-spring strain), parasitoids of the asian chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae). Biol. Control. 2010, 54, 14–18. [Google Scholar] [CrossRef]
- Messing, R.; Brodeur, J. Current challenges to the implementation of classical biological control. BioControl 2018, 63, 1–9. [Google Scholar] [CrossRef]
- ec.europa.eu. Available online: https://ec.europa.eu/commission/presscorner/detail/en/QANDA_19_6710 (accessed on 6 September 2021).
- IPPC. Establishing a National Plant Protection Organization; A guide to understand the principal requirements for establishing an organization to protect national plant resources from pests; FAO: Rome, Italy, 2015. [Google Scholar]
- Scalera, R. How much is Europe spending on invasive alien species? Biol. Invasions 2010, 12, 173–177. [Google Scholar] [CrossRef]
- Holdenreider, O.; Pautasso, M.; Weisberg, P.J.; Lonsdale, D. Tree diseases and landscape processes: The challenge of landscape pathology. Trends. Ecol. Evol. 2004, 19, 446–452. [Google Scholar] [CrossRef]
- Douglas, H.; Dang, B.T.; Gill, B.D.; Huber, J.; Mason, P.F.; Parker, D.J.; Sinclair, B.J. The importance of taxonomy in responses to invasive alien species. Biodiversity 2009, 10, 92–99. [Google Scholar] [CrossRef]
- Gluck-Thaler, E.; Jason, C.; Slot, J.C. Dimensions of horizontal gene transfer in eukaryotic microbial pathogens. PLoS Pathog. 2015, 11, e1005156. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.M. The rise of the hybrid fungi. Nature 2000, 405, 134–135. [Google Scholar] [CrossRef] [PubMed]
- McMullan, M.; Rafiqi, M.; Kaithakottil, G.; Clavijo, B.J.; Bilham, L.; Orton, E.; Percival-Alwyn, L.; Ward, B.J.; Edwards, A.; Saunders, D.G.O.; et al. The ash dieback invasion of Europe was founded by two genetically divergent individuals. Nat. Ecol. Evol. 2018, 2, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Albanese, G.; Saponari, M.; Faggioli, F. Phytosanitary certification. In Olive Germplasm—The Olive Cultivation, Table and Olive Oil Industry in Italy; Muzzalupo, I., Ed.; InTech Publisher: Rijeka, Croatia, 2012; pp. 107–132. ISBN 9789535108849. [Google Scholar]
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward understanding the ecological impacts of non-native species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzavolta, T.; Bracalini, M.; Benigno, A.; Moricca, S. Alien Invasive Pathogens and Pests Harming Trees, Forests, and Plantations: Pathways, Global Consequences and Management. Forests 2021, 12, 1364. https://doi.org/10.3390/f12101364
Panzavolta T, Bracalini M, Benigno A, Moricca S. Alien Invasive Pathogens and Pests Harming Trees, Forests, and Plantations: Pathways, Global Consequences and Management. Forests. 2021; 12(10):1364. https://doi.org/10.3390/f12101364
Chicago/Turabian StylePanzavolta, Tiziana, Matteo Bracalini, Alessandra Benigno, and Salvatore Moricca. 2021. "Alien Invasive Pathogens and Pests Harming Trees, Forests, and Plantations: Pathways, Global Consequences and Management" Forests 12, no. 10: 1364. https://doi.org/10.3390/f12101364
APA StylePanzavolta, T., Bracalini, M., Benigno, A., & Moricca, S. (2021). Alien Invasive Pathogens and Pests Harming Trees, Forests, and Plantations: Pathways, Global Consequences and Management. Forests, 12(10), 1364. https://doi.org/10.3390/f12101364






