Metabolic and Transcriptional Profiling of Fraxinus chinensis var. rhynchophylla Unravels Possible Constitutive Resistance against Agrilus planipennis
Abstract
1. Introduction
2. Results
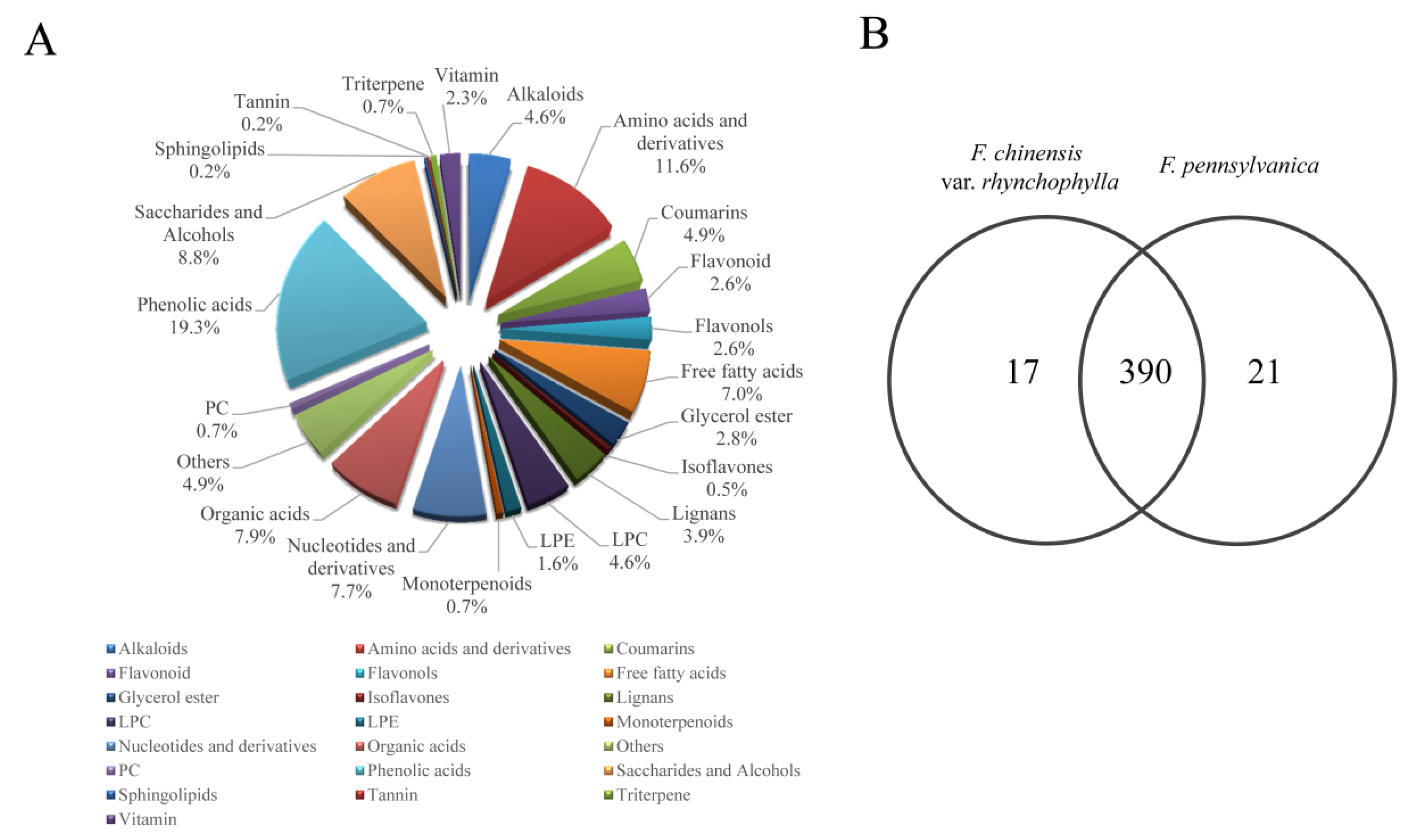
2.1. Metabolic Profiles of F. chinensis var. rhynchophylla and F. pennsylvanica
2.2. Differential Metabolites between F. chinensis var. rhynchophylla and F. pennsylvanica
2.3. Gene Expression Profiles of F. chinensis var. rhynchophylla and F. pennsylvanica
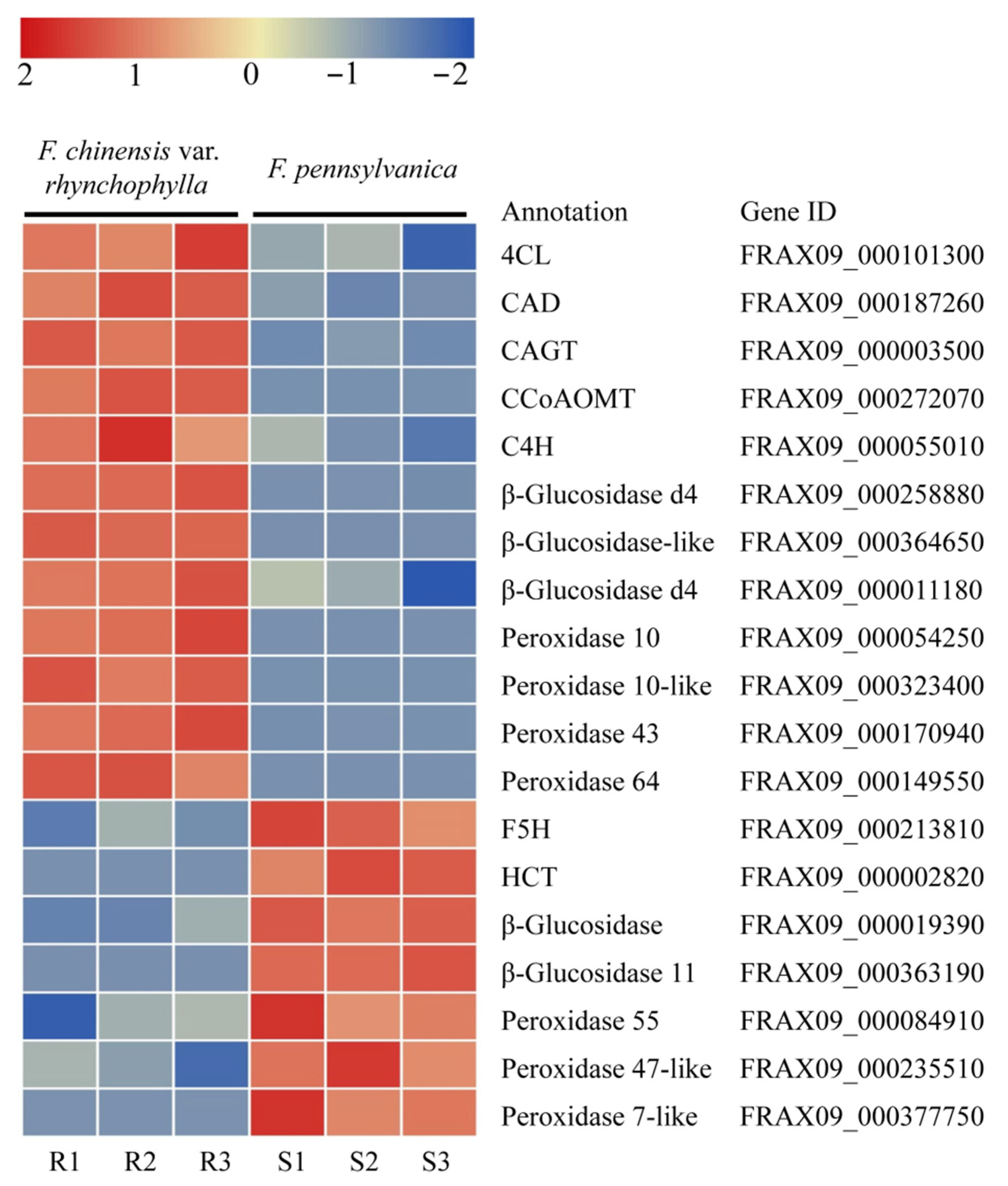
2.4. DEGs Related to Phenylpropanoids Biosynthesis
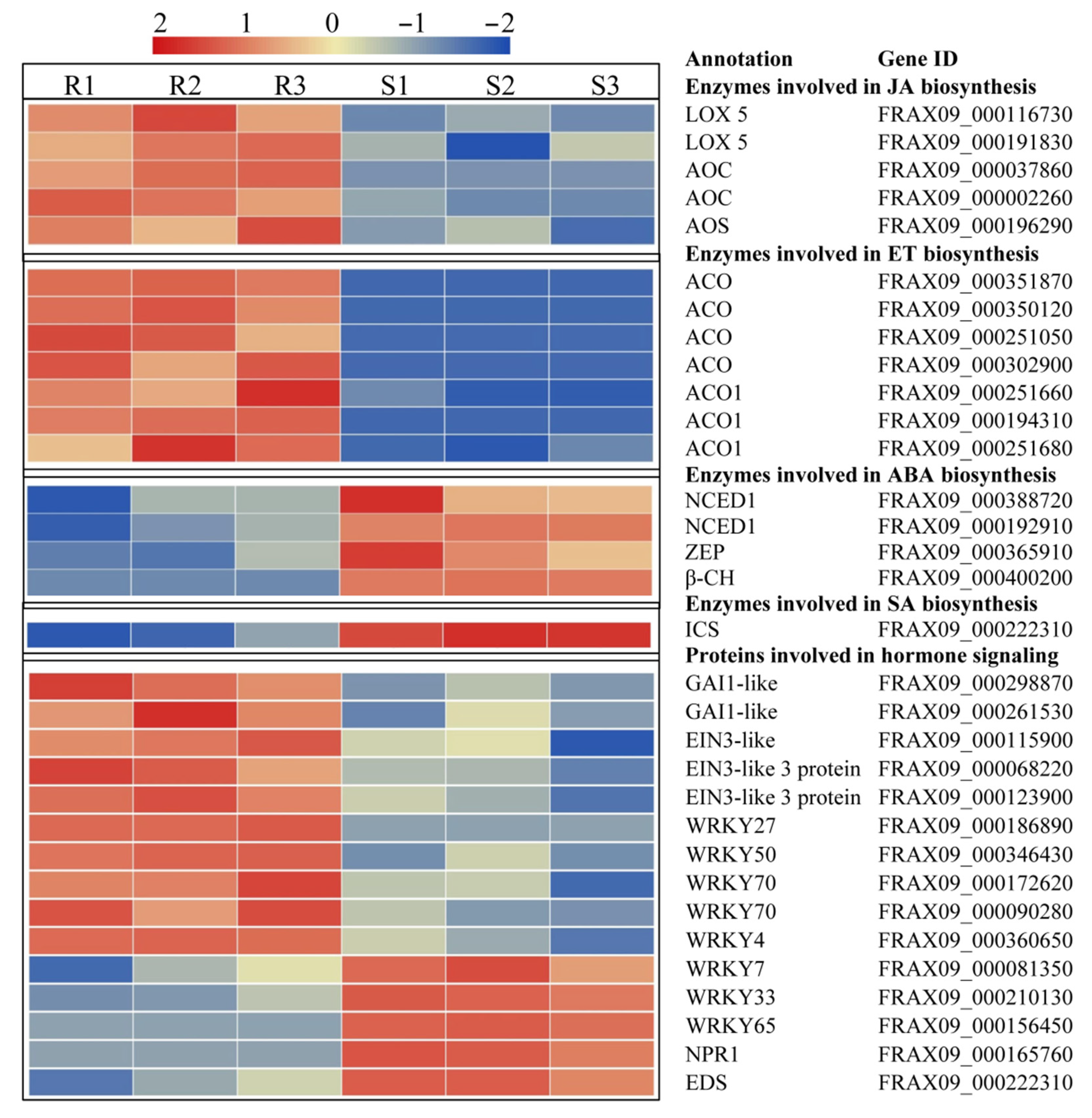
2.5. DEGs Involved in Hormone Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Metabolite Extraction and UPLC Conditions
4.3. Qualitative and Quantitative Analyses of Metabolites
4.4. Multivariate Data Analysis and Statistical Analysis
4.5. RNA Sequencing and Differential Expression Analysis
4.6. Quantitative Real-Time PCR (qRT-PCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, H.A.; Krajicek, J.E. Ash, an American Wood; American Woods Series FS-216; USDA Forest Service: Washington, DC, USA, 1973; 7p.
- MacFarlane, D.W.; Meyer, S.P. Characteristics and distribution of potential ash tree hosts for emerald ash borer. For. Ecol. Manag. 2005, 213, 15–24. [Google Scholar] [CrossRef]
- Zhao, T.; Gao, R.; Liu, H.; Bauer, S.L.; Sun, Q. Host range of emerald ash borer, Agrilus planipennis Fairmaire, its damage and the countermeasures. Acta Entomol. Sin. 2005, 48, 594–599. [Google Scholar]
- Cappaert, D.L.; McCullough, D.G.; Poland, T.M.; Siegert, N.W. Emerald ash borer in North America: A research and regulatory challenge. Am. Entomol. 2005, 51, 152–165. [Google Scholar] [CrossRef]
- Herms, D.A.; Mccullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.H. Insect resistance to crop plants. Soil Sci. 1951, 72, 481. [Google Scholar] [CrossRef]
- Kogan, M.; Ortman, E.F. Antixenosis—A New Term Proposed to Define Painter’s “Nonpreference” Modality of Resistance. Bull. Entomol. Soc. Am. 1978, 24, 175–176. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Moreira, X.; Mooney, K.A.; Rasmann, S.; Petry, W.K.; Carrillo-Gavilán, A.; Zas, R.; Sampedro, L. Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecol. Lett. 2014, 17, 537–546. [Google Scholar] [CrossRef]
- Eyles, A.; Jones, W.; Riedl, K.; Cipollini, D.; Schwartz, S.; Chan, K.; Herms, D.A.; Bonello, P. Comparative phloem chemistry of manchurian (Fraxinus mandshurica) and two north american ash species (Fraxinus americana and Fraxinus pennsylvanica). J. Chem. Ecol. 2007, 33, 1430–1448. [Google Scholar] [CrossRef]
- Cipollini, D.; Wang, Q.; Whitehill, J.G.A.; Powell, J.R.; Bonello, P.; Herms, D.A. Distinguishing defensive characteristics in the phloem of ash species resistant and susceptible to emerald ash borer. J. Chem. Ecol. 2011, 37, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Whitehill, J.; Opiyo, S.O.; Koch, J.L.; Herms, D.A.; Cipollini, D.F.; Bonello, P. Interspecific comparison of constitutive ash phloem phenolic chemistry reveals compounds unique to manchurian ash, a species resistant to emerald ash borer. J. Chem. Ecol. 2012, 38, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Rigsby, C.M.; Showalter, D.N.; Herms, D.A.; Koch, J.L.; Bonello, P.; Cipollini, D. Physiological responses of emerald ash borer larvae to feeding on different ash species reveal putative resistance mechanisms and insect counter-adaptations. J. Insect Physiol. 2015, 78, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Rigsby, C.M.; Herms, D.A.; Bonello, P.; Cipollini, D. Higher activities of defense-associated enzymes may contribute to greater resistance of manchurian ash to emerald ash borer than a closely related and susceptible congener. J. Chem. Ecol. 2016, 42, 782–792. [Google Scholar] [CrossRef]
- Bai, X.; Rivera-Vega, L.; Mamidala, P.; Bonello, P.; Herms, D.A.; Mittapalli, O. Transcriptomic signatures of ash (Fraxinus spp.) phloem. PLoS ONE 2011, 6, e16368. [Google Scholar] [CrossRef]
- Liu, H.; Bauer, L.S.; Miller, D.L.; Zhao, T.; Gao, R.; Song, L.; Luan, Q.; Jin, R.; Gao, C. Seasonal abundance of Agrilus planipennis (Coleoptera: Buprestidae) and its natural enemies Oobius agrili (hymenoptera: Encyrtidae) and Tetrastichus planipennisi (hymenoptera: Eulophidae) in china. Biol. Control 2007, 42, 61–71. [Google Scholar] [CrossRef]
- Zong, S.; Lin, J.; Wang, T.; Luo, Y. Resistance of eight species of ash trees to emerald ash borer and their mechanisms. Am. J. Agric. For. 2014, 2, 302–308. [Google Scholar] [CrossRef][Green Version]
- Duan, J.J.; Yurchenko, G.; Fuester, R. Occurrence of emerald ash borer (Coleoptera: Buprestidae) and biotic factors affecting its immature stages in the russian far east. Environ. Entomol. 2012, 41, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.; Ulyshen, M.D.; Bauer, L.S.; Gould, J.; Driesche, R.V. Measuring the impact of biotic factors on populations of immature emerald ash borers (Coleoptera: Buprestidae). Environ. Entomol. 2010, 39, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Wallander, E. Systematics of Fraxinus (Oleaceae) and evolution of dioecy. Plant Syst. Evol. 2008, 273, 25–49. [Google Scholar] [CrossRef]
- Christensen, J.H.; Bauw, G.; Welinder, K.G.; Montagu, M.V.; Boerjan, W. Purification and characterization of peroxidases correlated with lignification in poplar xylem. Plant Physiol. 1998, 118, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.P.; Terra, W.R.; Ferreia, C. Absorption of toxic beta-glucosides produced by plants and their effect on tissue trehalases from insects. Comp. Biochem. Physiol. 2006, 143, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Rodriguez, M.X.; Tohme, J.; Beeching, J.R. Accumulation of Hydroxycoumarins During Post-harvest Deterioration of Tuberous Roots of Cassava (Manihot esculenta Crantz). Ann. Bot. 2000, 86, 1153–1160. [Google Scholar] [CrossRef]
- Cabral, M.M.O.; Kelecom, A.; Garcia, E.S. Effects of the lignan, pinoresinol on the moulting cycle of the bloodsucking bug Rhodnius prolixus and of the milkweed bug Oncopeltus fasciatus. Fitoterapia 1999, 70, 561–567. [Google Scholar] [CrossRef]
- Ríos, J.L.; Giner, R.M.; Prieto, J.M. New findings on the bioactivity of lignans. Stud. Nat. Prod. Chem. 2002, 26, 183–292. [Google Scholar]
- Garcia, E.S.; Cabral, M.M.O.; Schaub, G.A.; Gottlieb, O.R.; Azambuja, P. Effects of lignoids on a hematophagous bug, Rhodnius prolixus: Feeding, ecdysis and diuresis. Phytochemistry 2002, 55, 611–616. [Google Scholar] [CrossRef]
- Schroeder, F.C.; del Campo, M.L.; Grant, J.B.; Weibel, D.B.; Smedley, S.R.; Bolton, K.L.; Meinwald, J.; Eisner, T. Pinoresinol: A lignol of plant origin serving for defense in a caterpillar. Proc. Natl. Acad. Sci. USA 2006, 103, 15497–15501. [Google Scholar] [CrossRef] [PubMed]
- Showalter, D.N. The Nature and Role of Host Defenses in Forest Pest Invasions: A Case Study Using Emerald Ash Borer (Agrilus planipennis). Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2017; p. 153. [Google Scholar]
- Umezawa, T. The cinnamate/monolignol pathway. Phytochem. Rev. 2010, 9, 1–17. [Google Scholar] [CrossRef]
- Morant, A.V.; Joergensen, K.; Joergensen, C.; Paquette, S.M.; Sánchez-Pérez, R.; Møller, B.L.; Bak, S. ChemInform Abstract: β-Glucosidases as Detonators of Plant Chemical Defense. ChemInform 2008, 69, 1795–1813. [Google Scholar] [CrossRef]
- Serdiuk, I.E.; Reszka, M.; Myszka, H.; Krzymiński, K.; Liberek, B.; Roshal, A.D. Flavonol-based fluorescent indicator for determination of β-glucosidase activity. RSC Adv. 2016, 6, 42532–42536. [Google Scholar] [CrossRef]
- Maffei, M.E.; Mithofer, A.; Boland, W. Before gene expression: Early events in plant insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef]
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.J.; Plumb, W.J.; Carey, D.W.; Mason, M.E.; Cooper, E.W.; Crowther, W.; Whittemore, A.T.; Rossiter, S.J.; Koch, J.L.; Buggs, R.J.A. Convergent molecular evolution among ash species resistant to the emerald ash borer. Nat. Ecol. Evol. 2020, 4, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Loon, L.V. NPR1: The spider in the web of induced resistance signaling pathways. Curr. Opin. Plant Biol. 2004, 7, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef]
- Verhage, A.; Vlaardingerbroek, I.; Raaymakers, C.; Van Dam, N.M.; Dicke, M.; Van Wees, S.C.M.; Pieterse, C.M.J. Rewiring of the Jasmonate Signaling Pathway in Arabidopsis during Insect Herbivory. Front. Plant Sci. 2011, 2, 47. [Google Scholar] [CrossRef]
- Nahar, K.; Kyndt, T.; Nzogela, Y.B.; Gheysen, G. Abscisic acid interacts antagonistically with classical defense pathways in rice-migratory nematode interaction. New Phytol. 2012, 196, 901–913. [Google Scholar] [CrossRef]
- Nguyen, D.; Rieu, I.; Mariani, C.; Van Dam, N.M. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef]
- Van der Meijden, E.; Marijke, W.; Henricus, J.V. Defence and regrowth, alternative plant strategies in the struggle against herbivores. Oikos 1988, 51, 355–363. [Google Scholar] [CrossRef]
- Herms, D.A.; Stone, A.K.; Chatfield, J.A. Emerald ash borer: The beginning of the end of Ash in North America? Ornam. Plants Annu. Rep. Res. Rev. 2004, 193, 62–71. [Google Scholar]
- Li, W.; Wen, L.; Chen, Z.; Zhang, Z.; Pang, X.; Deng, Z.; Liu, T.; Guo, Y. Study on metabolic variation in whole grains of four proso millet varieties reveals metabolites important for antioxidant properties and quality traits. Food Chem. 2021, 357, 129791. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, stringTie and ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. Stringtie enables improved reconstruction of a transcriptome from rna-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]

| No. | Compound Name | F. chinensis var. rhynchophylla | F. pennsylvanica a | Log2FC b | VIP |
|---|---|---|---|---|---|
| Coumarins | |||||
| 1 | 6-Hydroxy-7-methoxycoumarin | 6.86 × 105 | 1.60 × 105 | 2.10 | 1.23 |
| 2 | Daphnetin | 6.01 × 106 | 6.19 × 104 | 6.60 | 1.39 |
| 3 | Esculetin | 3.26 × 107 | 4.74 × 105 | 6.10 | 1.39 |
| 4 | Esculetin-glucoside | 1.23 × 107 | 3.81 × 105 | 5.02 | 1.39 |
| 5 | Esculin | 2.27 × 107 | 6.86 × 105 | 5.05 | 1.39 |
| 6 | Esculin hydrate | 1.52 × 107 | 6.84 × 104 | 7.79 | 1.39 |
| 7 | Fraxidin | 1.22 × 106 | 3.45 × 103 | 8.85 | 1.24 |
| 8 | Fraxetin | 3.90 × 106 | 1.27 × 105 | 6.79 | 1.22 |
| 9 | Fraxetin diglucoside | 1.53 × 107 | N.D. | - | 1.39 |
| 10 | Fraxidin 8-O-glucoside | 1.37 × 107 | 6.42 × 104 | 7.73 | 1.39 |
| 11 | Fraxin | 1.47 × 107 | N.D. | - | 1.39 |
| 12 | Isofraxetin | 1.26 × 106 | 1.16 × 104 | 6.76 | 1.38 |
| 13 | Isofraxidin | 5.36 × 105 | 8.01 × 103 | 6.06 | 1.38 |
| 14 | Licoarylcoumarin | 8.47 × 103 | 3.62 × 103 | 1.23 | 1.20 |
| 15 | Scopolin | 2.61 × 107 | 2.40 × 106 | 3.44 | 1.33 |
| Lignans | |||||
| 16 | Forsythialan B | 3.93 × 105 | N.D. | - | 1.39 |
| 17 | Pinoresinol diglucoside | 8.44 × 106 | 1.13 × 106 | 2.90 | 1.30 |
| 18 | Syringaresinol-4′-O-β-D-monoglucoside | 2.27 × 107 | 2.11 × 105 | 6.75 | 1.07 |
| Phenolic acids/Simple phenolics | |||||
| 19 | 1-O-[(E)-Caffeoyl]-β-D-glucopyranose | 3.61 × 106 | 9.19 × 105 | 1.98 | 1.33 |
| 20 | 1-O-p-Coumaroyl quinic acid | 1.91 × 105 | N.D. | - | 1.37 |
| 21 | 1′-O-β-D-(3,4-Dihydroxyphenethyl)-O-caffeoyl-glucoside | 1.98 × 107 | 3.20 × 104 | 9.28 | 1.39 |
| 22 | 3-Hydroxy-4-isopropylbenzylalcohol 3-glucoside | 2.78 × 106 | 2.86 × 105 | 3.28 | 1.34 |
| 23 | 3-O-(E)-p-Coumaroyl quinic acid | 9.23 × 105 | 4.17 × 104 | 4.47 | 1.07 |
| 24 | 3-O-Feruloyl quinic acid | 4.35 × 104 | N.D. | - | 1.36 |
| 25 | 3-O-p-Coumaroyl quinic acid O-hexoside | 1.86 × 105 | 3.14 × 103 | 5.89 | 1.13 |
| 26 | 5-(2-Hydroxyethyl)-2-O-glucosylohenol | 5.69 × 106 | 4.28 × 105 | 3.73 | 1.32 |
| 27 | 5-O-p-Coumaroyl quinic acid O-hexoside | 2.20 × 105 | 3.68 × 103 | 5.90 | 1.10 |
| 28 | 5-O-p-Coumaroyl shikimic acid O-hexoside | 3.61 × 105 | 7.89 × 104 | 2.20 | 1.32 |
| 29 | 6-O-Galloyl-β-D-glucopyranoside | 2.40 × 104 | 1.80 × 103 | 3.73 | 1.06 |
| 30 | Caffeic acid | 4.28 × 105 | 1.31 × 105 | 1.71 | 1.30 |
| 31 | Calceorioside B | 8.56 × 106 | 1.75 × 106 | 2.29 | 1.28 |
| 32 | Chlorogenic acid | 4.72 × 105 | 6.28 × 104 | 2.91 | 1.10 |
| 33 | Cimidahurinine | 5.64 × 106 | 4.57 × 105 | 3.63 | 1.32 |
| 34 | Cryptochlorogenic acid | 1.52 × 105 | 2.38 × 104 | 2.68 | 1.23 |
| 35 | Forsythiaside B | 2.06 × 105 | 1.34 × 104 | 3.94 | 1.31 |
| 36 | Glucosyloxybenzoic acid | 3.45 × 105 | N.D. | - | 1.39 |
| 37 | Isoeugenol | 4.00 × 104 | N.D. | - | 1.39 |
| 38 | Maleoyl-caffeoylquinic acid | 2.15 × 105 | 3.70 × 104 | 2.54 | 1.34 |
| 39 | Methyl ferulate | 1.38 × 105 | 5.49 × 104 | 1.33 | 1.09 |
| 40 | Methyl sinapate | 1.70 × 104 | 8.32 × 103 | 1.03 | 1.19 |
| 41 | Neochlorogenic acid | 6.39 × 105 | 8.68 × 104 | 2.88 | 1.13 |
| 42 | Petasiphenone | 2.01 × 104 | N.D. | - | 1.39 |
| 43 | Plantainoside A | 1.60 × 106 | N.D. | - | 1.39 |
| 44 | Rengyoside A | 1.41 × 105 | N.D. | - | 1.39 |
| 45 | Sinapaldehyde Glucoside | 1.62 × 107 | N.D. | - | 1.39 |
| 46 | Sinapic acid | 1.00 × 105 | 2.54 × 103 | 5.30 | 1.20 |
| 47 | Sinapoylglucuronic acid | 6.04 × 104 | N.D. | 12.71 | 1.39 |
| 48 | Sinapoyl-p-coumaroyltartaric acid | 1.96 × 104 | N.D. | 11.09 | 1.39 |
| 49 | Sorbic acid | 4.06 × 105 | 9.37 × 104 | 2.12 | 1.34 |
| Phenylethanoid | |||||
| 50 | Calceolarioside A | 1.00 × 106 | N.D. | - | 1.39 |
| Flavonoid | |||||
| 51 | Chrysoeriol O-acetylhexoside | 3.62 × 105 | 1.82 × 104 | 4.31 | 1.35 |
| Tannin | |||||
| 52 | 2-O-galloyl-β-D- glucose | 6.18 × 106 | 8.32 × 105 | 2.89 | 1.35 |
| Amino acids and derivatives | |||||
| 53 | L-Cystathionine | 3.61 × 104 | N.D. | - | 1.39 |
| 54 | Acetyltryptophan | 2.06 × 104 | N.D. | - | 1.38 |
| 55 | (5-L-Glutamyl)-L-alanine | 3.41 × 104 | 1.22 × 104 | 1.48 | 1.07 |
| 56 | L-AsparticAcid | 7.24 × 106 | 3.31 × 106 | 1.13 | 1.10 |
| Others | |||||
| 57 | Coumalic acid | 9.36 × 104 | N.D. | - | 1.39 |
| 58 | Neonuezhenide | 2.72 × 107 | 2.60 × 106 | 3.38 | 1.33 |
| 59 | D-(+)-Glucono-1,5-lactone | 1.57 × 105 | 3.85 × 104 | 2.03 | 1.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, L.; Li, J.; Wang, R.; Wang, X.; Zhao, T.; Chen, Y.; Wang, L. Metabolic and Transcriptional Profiling of Fraxinus chinensis var. rhynchophylla Unravels Possible Constitutive Resistance against Agrilus planipennis. Forests 2021, 12, 1373. https://doi.org/10.3390/f12101373
Qu L, Li J, Wang R, Wang X, Zhao T, Chen Y, Wang L. Metabolic and Transcriptional Profiling of Fraxinus chinensis var. rhynchophylla Unravels Possible Constitutive Resistance against Agrilus planipennis. Forests. 2021; 12(10):1373. https://doi.org/10.3390/f12101373
Chicago/Turabian StyleQu, Liangjian, Jifu Li, Ruizhen Wang, Xiaoyi Wang, Tonghai Zhao, Yuequ Chen, and Lijuan Wang. 2021. "Metabolic and Transcriptional Profiling of Fraxinus chinensis var. rhynchophylla Unravels Possible Constitutive Resistance against Agrilus planipennis" Forests 12, no. 10: 1373. https://doi.org/10.3390/f12101373
APA StyleQu, L., Li, J., Wang, R., Wang, X., Zhao, T., Chen, Y., & Wang, L. (2021). Metabolic and Transcriptional Profiling of Fraxinus chinensis var. rhynchophylla Unravels Possible Constitutive Resistance against Agrilus planipennis. Forests, 12(10), 1373. https://doi.org/10.3390/f12101373







