Explant, Medium, and Plant Growth Regulator (PGR) Affect Induction and Proliferation of Callus in Abies koreana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Explant Materials and Sterilization
2.2. Experimental Design of Media and PGR Combinations for Callogenesis
2.3. Measurements of Callus Growth Parameters and Statistical Analysis of the Data
2.4. Orthogonal Design Test of Callus Proliferation
2.5. Measurements of Callus Proliferation and Statistical Analysis of the Data
3. Results
3.1. Induction of Calli
3.2. Browning of Calli
3.3. Fresh Weight of Calli
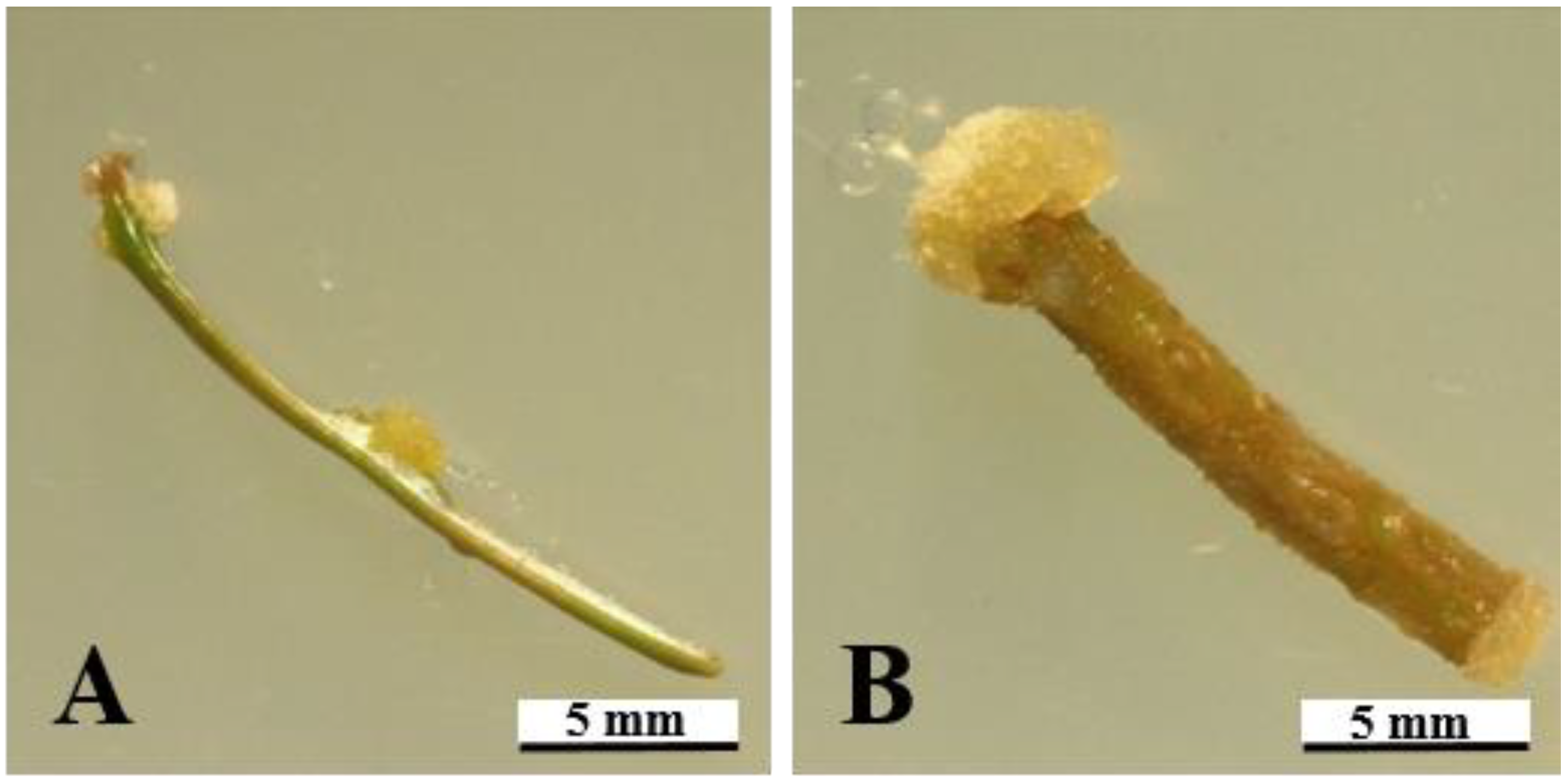
3.4. Morphology of Calli
3.5. Proliferation and Browning of Callus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farjon, A. A Handbook of the World’s Conifers (2 Vols.); Brill: Boston, MA, USA, 2010. [Google Scholar]
- Seo, M.; Sowndhararajan, K.; Kim, S. Influence of binasal and uninasal inhalations of essential oil of Abies koreana twigs on electroencephalographic activity of human. Behav. Neurol. 2016, 2016, 9250935. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.J.; Kim, S.S.; Oh, T.H.; Lee, N.H.; Hyun, C.G. Abies koreana essential oil inhibits drug resistant skin pathogen growth and LPS-induced inflammatory effects of murine macrophage. Lipids 2009, 44, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.B. Abies koreana and its new forms discovered. J. Korean For. Soc. 1970, 10, 5–6. [Google Scholar]
- Lee, Y.W.; Hong, S.C. Ecological studies on the vegetational characteristics of the Abies koreana forest. J. Korean Soc. For. Sci. 1995, 84, 247–257. [Google Scholar]
- Chang, C.S.; Jeon, J.I.; Hyun, J.O. An analysis of morphological variation in Abies koreana Wilson and A. nephrolepis (Traut.) Maxim. of Korea (Pinaceae) and their phylogenetic problems. J. Korean Soc. For. Sci. 1997, 86, 378–390. [Google Scholar]
- Koo, K.A.; Kong, W.S.; Park, S.U.; Lee, J.H.; Kim, J.; Jung, H. Sensitivity of Korean fir (Abies koreana Wils.), a threatened climate relict species, to increasing temperature at an island subalpine area. Ecol. Modell. 2017, 353, 5–16. [Google Scholar] [CrossRef]
- Woo, S.Y. Forest decline of the world: A linkage with air pollution and global warming. Afr. J. Biotechnol. 2009, 8, 7409–7414. [Google Scholar]
- Kim, Y.S.; Chang, C.S.; Kim, C.S.; Gardner, M. Abies koreana. The IUCN Red List of Threatened Species 2011: E.T31244A9618913. 2011. Available online: https://www.iucnredlist.org/search?query=Abies%20koreana&searchType=species (accessed on 22 August 2021). [CrossRef]
- Kwak, M.; Hong, J.K.; Park, J.H.; Lee, B.Y.; Suh, M.H.; Kim, C.S. Genetic assessment of Abies koreana (Pinaceae), the endangered Korean fir, and conservation implications. Conserv. Genet. 2017, 18, 1165–1176. [Google Scholar] [CrossRef]
- Noland, T.L.; Parker, W.C.; Morneault, A.E. Natural variation in seed characteristics of eastern white pine (Pinus strobus L.). New For. 2006, 32, 87–103. [Google Scholar] [CrossRef]
- Song, J.H.; Jang, K.H.; Hur, S.-D. Variation of seed and germination characteristics of natural populations of Abies koreana Wilson, a Korean endemic species. J. Korean Soc. For. Sci. 2010, 99, 849–854. [Google Scholar]
- Nunes, S.; Santos, C.; Moutinho-Pereira, J.; Correia, C.; Oliveira, H.; de Oliveira, J.M.F.; Pereira, V.T.; Almeida, T.; Marum, L.; Dias, M.C. Physiological characterization and true-to-typeness evaluation of in vitro and ex vitro seedlings of Pinus elliottii: A contribution to breeding programs. Plant Physiol. Biochem. 2016, 107, 222–227. [Google Scholar] [CrossRef]
- Nunes, S.S.C. Characterization, Micropropagation and Preservation of Pinus Genotypes. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 2016. [Google Scholar]
- Gupta, P.K.; Durzan, D.J. Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep. 1985, 4, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Lelu-Walter, M.A.; Bernier-Cardou, M.; Klimaszewska, K. Clonal plant production from self-and cross-pollinated seed families of Pinus sylvestris (L.) through somatic embryogenesis. Plant Cell Tissue Organ Cult. 2008, 92, 31–45. [Google Scholar] [CrossRef]
- Salaj, T.; Klubicová, K.; Matusova, R.; Salaj, J. Somatic embryogenesis in selected conifer trees Pinus nigra Arn. and Abies hybrids. Front. Plant Sci. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murasnige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Quoirin, M.; Lepoivre, P. Improved media for in vitro culture of Prunus sp. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
- Huang, J. Efficiency of Callus and Regeneration Bud Induction from Different Explants of Cunninghamia lanceolata. Master’s Thesis, Fujian Agriculture and Forestry University, Fujian, China, 2017. [Google Scholar]
- Bonga, J.M. Adventitious shoot formation in cultures of immature female strobili of Larix decidua. Physiol. Plant 1984, 62, 416–421. [Google Scholar] [CrossRef]
- Li, X.; Huang, F.; Gbur, E., Jr. Effect of basal medium, growth regulators and Phytagel concentration on initiation of embryogenic cultures from immature zygotic embryos of loblolly pine (Pinus taeda L.). Plant Cell Rep. 1998, 17, 298–301. [Google Scholar] [CrossRef]
- Szczygiel, K.; Hazubska-Przybyl, T.; Bojarczuk, K. Somatic embryogenesis of selected coniferous tree species of the genera Picea, Abies and Larix. Acta Soc. Bot. Pol. 2007, 76, 7–15. [Google Scholar]
- Nawrot-Chorabik, K. Embryogenic callus induction and differentiation in silver fir (Abies alba Mill.) tissue cultures. Dendrobiology 2008, 59, 31–40. [Google Scholar]
- De Diego, N.; Montalbán, I.; Moncaleán, P. In vitro regeneration of adult Pinus sylvestris L. trees. S. Afr. J. Bot. 2010, 76, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Fett-Neto, A.G.; DiCosmo, F.; Reynolds, W.; Sakata, K. Cell culture of Taxus as a source of the antineoplastic drug taxol and related taxanes. Bio/technology 1992, 10, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Qarshi, I.A.; Nazir, H.; Ullah, I.; Rashid, M.; Shinwari, Z.K. In vitro callogenesis and organogenesis in Taxus wallichiana ZUCC, The Himalayan Yew. Pak. J. Bot. 2013, 45, 1755–1759. [Google Scholar]
- Bhat, S.; Gangoo, S.; Geelani, S.; Qasba, S.; Parray, A. Callus culture and organogenesis in fir (Abies pindrow Royle). J. Cell Tissue Res. 2014, 14, 4653–4658. [Google Scholar]
- Hohtola, A. Seasonal changes in explant viability and contamination of tissue cultures from mature Scots pine. Plant Cell Tissue Organ Cult. 1988, 15, 211–222. [Google Scholar] [CrossRef]
- Shanjani, P.S. Nitrogen effect on callus induction and plant regeneration of Juniperus excelsa. Int. J. Agric. Biol. 2003, 5, 419–422. [Google Scholar]
- Rout, G.; Mohapatra, A.; Jain, S.M. Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol. Adv. 2006, 24, 531–560. [Google Scholar] [CrossRef]
- Gao, F.; Peng, C.; Wang, H.; Shen, H.; Yang, L. Selection of culture conditions for callus induction and proliferation by somatic embryogenesis of Pinus koraiensis. J. For. Res. 2021, 32, 483–491. [Google Scholar] [CrossRef]
- Santana, M.G.; Velásquez, S.R.; Mata, J. Carbon source effect on cacao organogenesis and somatic embryogenesis. Cytogenetic analysis. Agron. Trop. 2010, 60, 193–202. [Google Scholar]
- Sivachandran, R.; Gnanam, R.; Sudhakar, D.; Suresh, J.; Ram, S.G.; Selvi, R.G. Development of in vitro gynogenesis system in cocoa (Theobroma cacao L.). Chem. Sci. Rev. Lett. 2017, 6, 2169–2177. [Google Scholar]






| Treatment Code | Auxin (mg·L−1) | Cytokinin (mg·L−1) | |||
|---|---|---|---|---|---|
| 2,4-D | NAA | KT | TDZ | 6-BA | |
| T1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| T2 | 0.0 | 2.0 | 0.5 | 0.0 | 2.0 |
| T3 | 0.0 | 2.0 | 1.0 | 0.0 | 2.0 |
| T4 | 0.0 | 2.0 | 0.0 | 0.1 | 2.0 |
| T5 | 0.0 | 2.0 | 0.0 | 0.2 | 2.0 |
| T6 | 1.0 | 2.0 | 0.5 | 0.0 | 0.0 |
| T7 | 1.0 | 2.0 | 1.0 | 0.0 | 0.0 |
| T8 | 1.0 | 2.0 | 0.0 | 0.1 | 0.0 |
| T9 | 1.0 | 2.0 | 0.0 | 0.2 | 0.0 |
| T10 | 1.0 | 0.0 | 0.5 | 0.0 | 2.0 |
| T11 | 1.0 | 0.0 | 1.0 | 0.0 | 2.0 |
| T12 | 1.0 | 0.0 | 0.0 | 0.1 | 2.0 |
| T13 | 1.0 | 0.0 | 0.0 | 0.2 | 2.0 |
| Treatment Code | Auxin (mg·L−1) | Cytokinin (mg·L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2,4-D (A) | NAA (B) | 6-BA (C) | KT (D) | |||||
| P1 | 0.0 | A1 | 0.0 | B1 | 0.5 | C1 | 0.0 | D1 |
| P2 | 0.0 | A1 | 0.5 | B2 | 1.0 | C2 | 0.5 | D2 |
| P3 | 0.0 | A1 | 1.0 | B3 | 1.5 | C3 | 1.0 | D3 |
| P4 | 0.0 | A1 | 1.5 | B4 | 2.0 | C4 | 1.5 | D4 |
| P5 | 1.0 | A2 | 1.0 | B3 | 0.5 | C1 | 0.5 | D2 |
| P6 | 1.0 | A2 | 1.5 | B4 | 1.0 | C2 | 0.0 | D1 |
| P7 | 1.0 | A2 | 0.0 | B1 | 1.5 | C3 | 1.5 | D4 |
| P8 | 1.0 | A2 | 0.5 | B2 | 2.0 | C4 | 1.0 | D3 |
| P9 | 2.0 | A3 | 1.5 | B4 | 0.5 | C1 | 1.0 | D3 |
| P10 | 2.0 | A3 | 1.0 | B3 | 1.0 | C2 | 1.5 | D4 |
| P11 | 2.0 | A3 | 0.5 | B2 | 1.5 | C3 | 0.0 | D1 |
| P12 | 2.0 | A3 | 0.0 | B1 | 2.0 | C4 | 0.5 | D2 |
| P13 | 3.0 | A4 | 0.5 | B2 | 0.5 | C1 | 1.5 | D4 |
| P14 | 3.0 | A4 | 0.0 | B1 | 1.0 | C2 | 1.0 | D3 |
| P15 | 3.0 | A4 | 1.5 | B4 | 1.5 | C3 | 0.5 | D2 |
| P16 | 3.0 | A4 | 1.0 | B3 | 2.0 | C4 | 0.0 | D1 |
| Explant (A) | Medium (B) | PGR (C, mg·L−1) | Induction (%) | Browning (%) | FW (mg) |
|---|---|---|---|---|---|
| F-test z | A | *** | *** | *** | |
| B | *** | *** | *** | ||
| C | *** | ** | *** | ||
| A × B | *** | *** | *** | ||
| A × C | *** | *** | *** | ||
| B × C | *** | ** | NS | ||
| A × B × C | *** | *** | NS | ||
| Variable | Proliferation (%) * | Browning (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |
| K1 | 252.3 | 722.0 | 617.5 | 682.3 | 90.0 | 53.8 | 46.3 | 30.8 |
| K2 | 853.7 | 722.2 | 788.3 | 758.8 | 33.8 | 24.6 | 30.0 | 36.3 |
| K3 | 895.2 | 709.0 | 811.5 | 786.3 | 9.6 | 36.3 | 34.6 | 38.8 |
| K4 | 954.7 | 802.6 | 738.6 | 728.5 | 8.8 | 27.5 | 31.3 | 36.3 |
| R | 702.4 | 93.6 | 194.0 | 103.9 | 81.3 | 29.2 | 16.3 | 7.9 |
| Optimal level | A4 | B4 | C3 | D3 | A4 | B2 | C2 | D1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Jeong, B.R. Explant, Medium, and Plant Growth Regulator (PGR) Affect Induction and Proliferation of Callus in Abies koreana. Forests 2021, 12, 1388. https://doi.org/10.3390/f12101388
Guo G, Jeong BR. Explant, Medium, and Plant Growth Regulator (PGR) Affect Induction and Proliferation of Callus in Abies koreana. Forests. 2021; 12(10):1388. https://doi.org/10.3390/f12101388
Chicago/Turabian StyleGuo, Ge, and Byoung Ryong Jeong. 2021. "Explant, Medium, and Plant Growth Regulator (PGR) Affect Induction and Proliferation of Callus in Abies koreana" Forests 12, no. 10: 1388. https://doi.org/10.3390/f12101388







