Abstract
Favolaschia calocera was originally described from Madagascar, and reported to have a worldwide distribution. In the current study, samples of the Favolaschia calocera from Central America, Australia, China, Kenya, Italy, New Zealand, and Thailand were analyzed by using both morphological and molecular methods. Phylogenetic analyses were based on the internal transcribed spacer (ITS) dataset, and the combined five-locus dataset of ITS, large subunit nuclear ribosomal RNA gene (nLSU), the small subunit mitochondrial rRNA gene (mt-SSU), the small subunit of nuclear ribosomal RNA gene (nu-SSU), and the translation elongation factor 1α (TEF1). Our study proves that Favolaschia calocera is a species complex, and six species are recognized in the complex including four new species. Three new species F. brevibasidiata, F. brevistipitata, and F. longistipitata from China; and one new species F. minutissima from Asia. In addition, Favolaschia claudopus (Singer) Q.Y. Zhang & C. Dai, earlier treated as a variety of Favolaschia calocera R. Heim, were raised to species rank. Illustrated descriptions of these five new taxa are given. An identification key and a comparison of the characteristics of species in the Favolaschia calocera complex are provided.
1. Introduction
Favolaschia (Pat.) Pat. (Mycenaceae, Agaricales) was established based on F. gaillardii (Pat.) Pat. [1]. It is characterized by poroid hymenophore, a monomitic hyphal system, gelatinous hyphal structure, the presence of gloeocystidia and acanthocystida, and amyloid basidiospores [1,2,3,4,5]. Favolaschia produces basidiomes on dead plant material, but they may have a biotrophic phase as found in Mycena [6,7], since many are known to be host specific. More than 100 taxa have been recorded in Favolaschia, although it is suspected that these taxa represent only around 50 species [8,9,10]. The genus has a worldwide distribution (excluding Antarctica), with a high diversity in South America [5].
Favolaschia calocera R. Heim was first described from Madagascar [11]. It differs from other species in the genus by its bright orange or yellow basidiocarps with a distinct laterally stipe and numerous gloeocystidia and acanthocysts. In addition, Favolaschia calocera is a conspicuous fungus, and it is known to occur in different environments, such as forests or shrub-lands, non-forested rural areas, home or public gardens etc., and occurs on over 50 different plant species, such as decaying dicotyledonous tree trunks, logs, and branches [12,13].
Favolaschia calocera has always been considered to be an invasive fungus by several countries [14,15]. Outside its type locality in Madagascar, it was found in New Zealand and Italy in 1969 and 1999 [13,16]. Johntson et al. [12,13] mentioned that Favolaschia calocera spread to Australia and New Zealand presumably by shipped timber. Vizzini and Zotti [16] believed that Favolaschia calocera in Norfolk Island was introduced from New Zealand. Over the past couple of decades, it has spread into Europe. In the process of the migration, the morphology and molecules of Favolaschia calocera gradually diverged. As early as in 1974, Singer [8] reported the morphological differences of Favolaschia calocera collections from Madagascar and New Zealand. Based on molecular phylogeographic analysis, Vizzini et al. [14] and Ainsworth et al. [17] indicated Favolaschia calocera is divided into two main groups: the European and Oceania samples clustered in one group, and the American and Asian materials formed another group. Until recently, there has been no systematic study on the distribution of this species in China. Our initial investigation found that Favolaschia calocera is common in tropical China, and displays a higher genetic variability.
The aim of this study was to investigate the diversity and phylogeny within the Favolaschia calocera species complex, and five new taxa were described. The key characteristics for distinguishing species are provided.
2. Materials and Methods
2.1. Morphological Studies
Studied specimens collected by authors or new collections from China and Australia are deposited at the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Sections of basidiocarps were studied microscopically according to Cui et al. [18] using a Nikon Eclipse 80i microscope with phase contrast illumination. Color terms are cited from Petersen [19]. In presenting spore size variation, 5% of measurements were excluded from each end of the range and this value is given in parentheses. The following abbreviations are used in the description: KOH = 5% potassium hydroxide; IKI = Melzer’s reagent; IKI+ = amyloid; CB = Cotton Blue; CB− = acyanophilous; L = arithmetic average of all spores; W = arithmetic average of all spores; Q = the L/W ratio; n = number of spores measured from the given number of specimens.
2.2. DNA Extraction and Sequencing
A CTAB rapid plant genome extraction kit (Aidlab Biotechnologies, Co., Ltd., Beijing, China) was used to obtain PCR products from dried specimens, following the manufacturer’s instructions with some modifications [20]. To generate PCR amplicons, the following primer pairs were used: ITS5 and ITS4 for ITS, and LR0R and LR7 for nLSU, MS1 and MS2 for mt-SSU, NS1 and NS4 for nu-SSU, and 983F and 1567R for TEF1 [21,22].
The PCR cycling schedules for different DNA sequences used in this study followed those used in Zhou et al. and Liu et al. [23,24] with some modifications. The PCR procedure for ITS, mt-SSU, and TEF1 was as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles at 94 °C for 40 s, 54 °C for 45 s, and 72 °C for 1 min, and a final extension of 72 °C for 10 min. The PCR procedure for nLSU was as follows: initial denaturation at 94 °C for 1 min, followed by 35 cycles at 94 °C for 30 s, 50 °C for 1 min and 72 °C for 1.5 min, and a final extension of 72 °C for 10 min. The PCR procedure for the nu-SSU was as follows: initial denaturation at 94 °C for 1 min, followed by 34 cycles at 94 °C for 30 s, 53 °C for 1 min, and 72 °C for 1.5 min, and a final extension of 72 °C for 10 min. The PCR products were purified and sequenced at the Beijing Genomics Institute (BGI), China with the same primers and the sequences were deposited in GenBank and are listed in Table 1.

Table 1.
A list of species, specimens, and GenBank accession numbers of the sequences used in this study.
2.3. Phylogenetic Analyses
Besides the newly generated sequences for this study, other related sequences from GenBank were also included in the phylogenetic analysis. Panellus stipticus (Bull.) P. Karst. is used as the outgroup in Figure 1 [26], and Favolaschia varariotecta Singer is used as the outgroup in Figure 2 [14]. The sequences used ClustalX [27] and were manually adjusted in BioEdit [28]. Trees were shown in TreeView 1.6.6 [29].
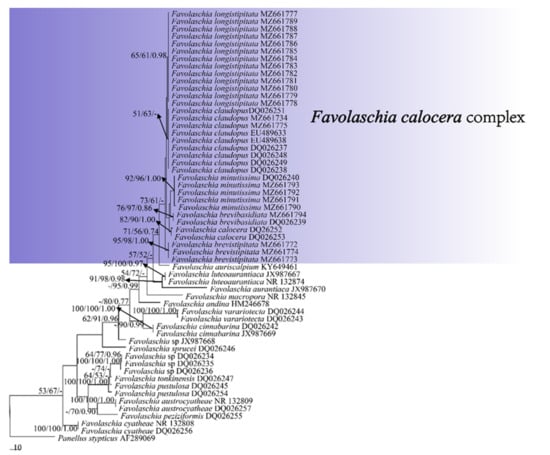
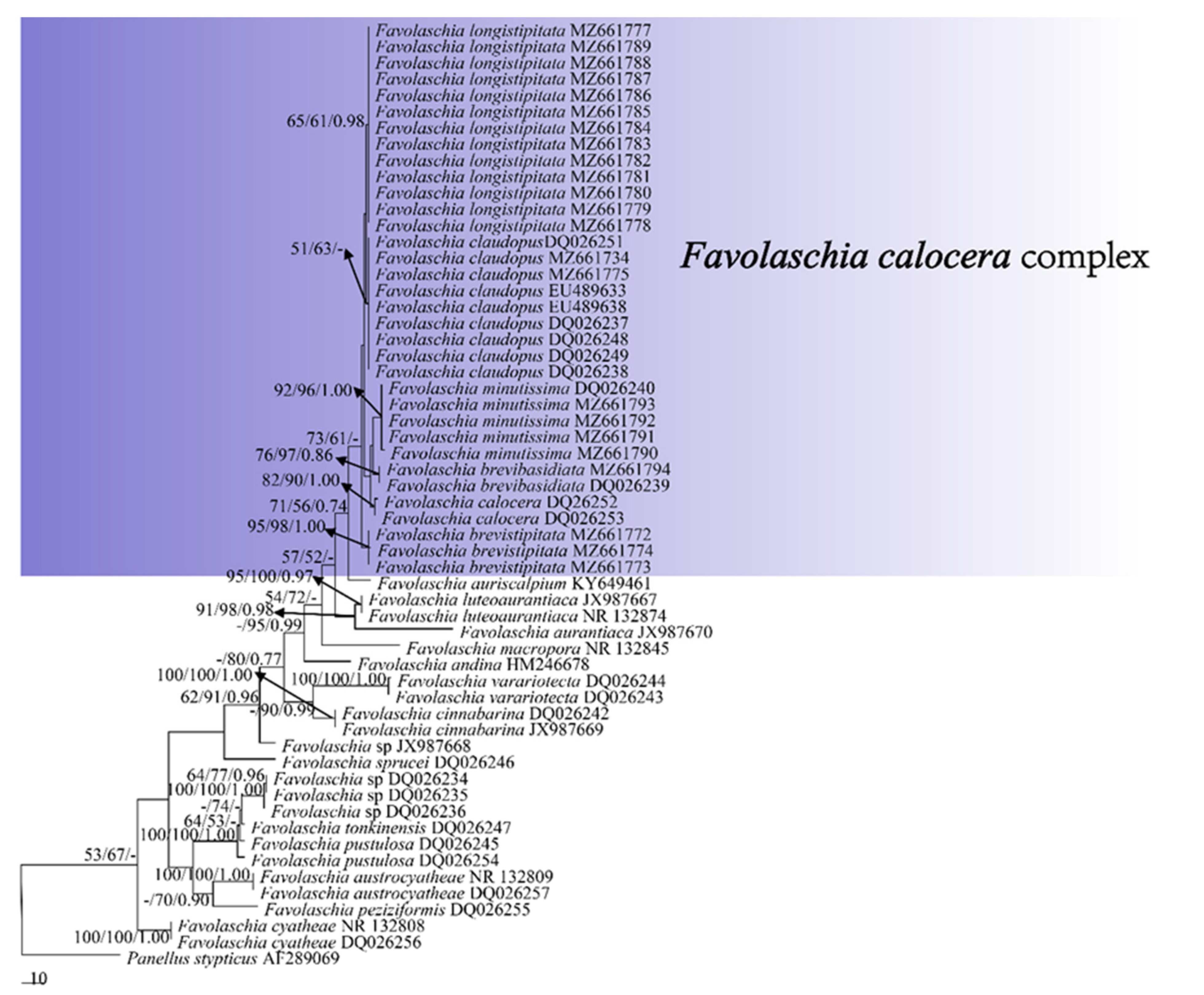
Figure 1.
Maximum parsimony tree illustrating the phylogeny of the Favolaschia calocera complex and related group based on the combined sequences dataset of ITS. Branches are labelled with parsimony bootstrap values higher than 50%, and Bayesian posterior probabilities more than 0.70.
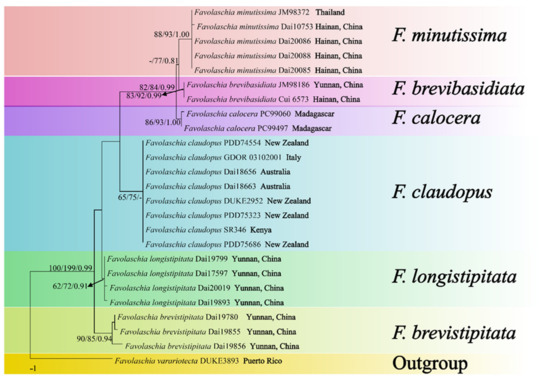
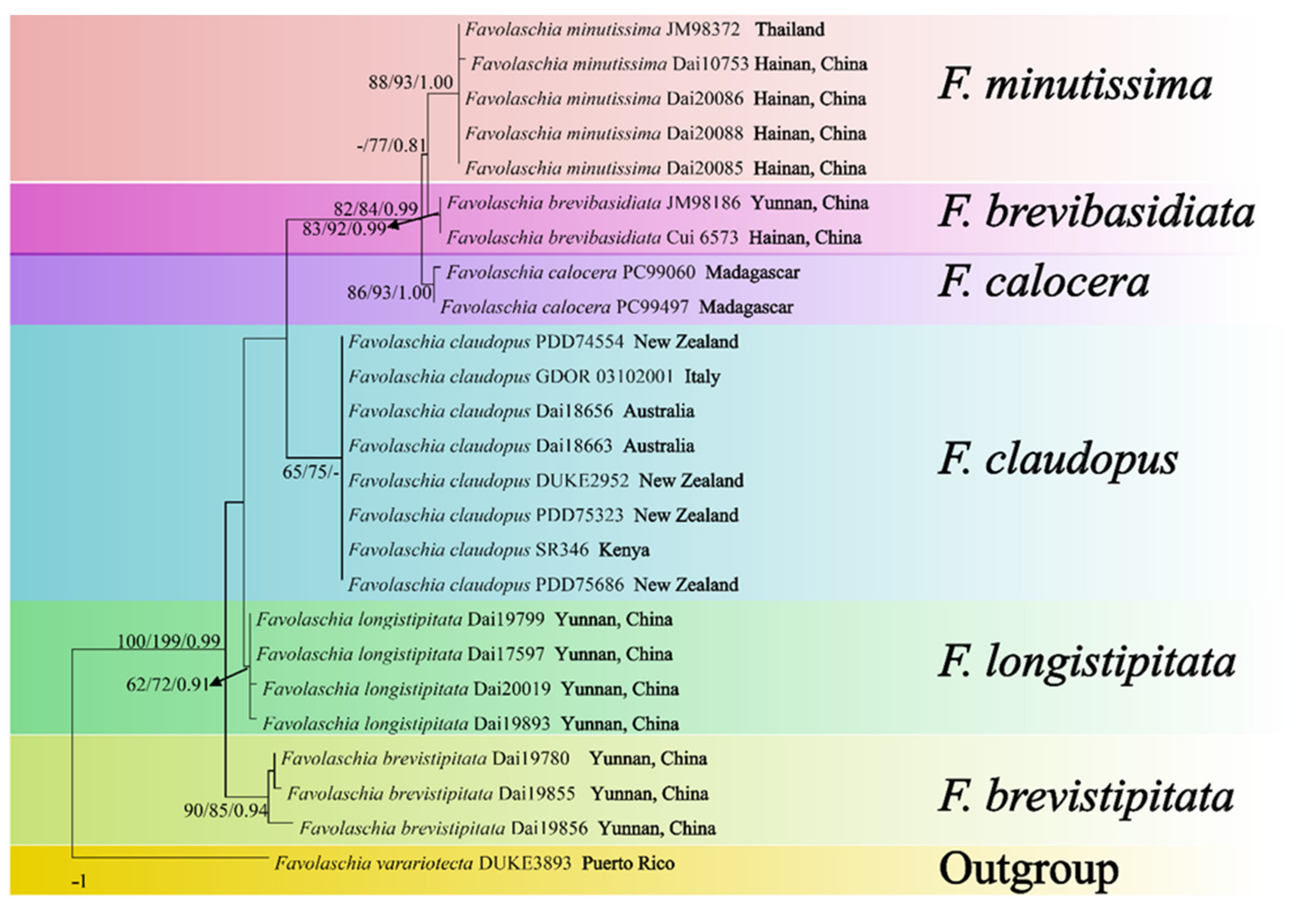
Figure 2.
Maximum parsimony tree illustrating the phylogeny of the Favolaschia calocera complex based on ITS + nLSU + mt-SSU + nu-SSU + TEF1 sequences. Branches are labelled with parsimony bootstrap values higher than 50%, and Bayesian posterior probabilities more than 0.70.
Maximum parsimony (MP) analysis was used for the ITS and the ITS + nLSU + mt-SSU + nu-SSU + TEF1 datasets in PAUP* 4.0b10 [30]. Trees were generated using 100 replicates of random stepwise addition of sequence and tree-bisection reconnection (TBR) branch-swapping algorithm, with all characters given equal weight. Branch supports for all parsimony analyses were estimated by performing 1000 bootstrap (BT) replicates [31]. Descriptive tree statistics, tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for each maximum parsimonious tree generated.
RAxML 7.2.8 (GitHub, San Francisco, CA, USA) was used for maximum likelihood (ML) analysis. The default settings of the GTR+I+G model were used for all parameters in the ML analysis [32]. The branch support values were obtained using nonparametric bootstrapping with 1000 replicates [33].
Bayesian interference (BI) analyses were calculated with MrBayes 3.1.2 [34]. Four Markov chains were run for 5,000,000 generations until the split deviation frequency value was less than 0.01 and trees were sampled every 100 generations. Branches that received bootstrap support for BS (bootstrap support for MP and ML) values and BPPs (Bayesian posterior probabilities for BI) simultaneously not less than 50% and 0.70, respectively, were considered as significantly supported.
3. Results
3.1. Molecular Phylogeny
The ITS base contained 57 sequences representing 23 taxa. The ITS dataset had an aligned length of 816 characters, of which 522 characters are constant, 122 characters are variable but parsimony uninformative, and 172 characters are parsimony informative. MP analysis yielded two equally parsimonious trees (TL = 542, CI = 0.675, RI = 0.847, RC = 0.572, HI = 0.325). BI and ML analyses produced consensus trees similar to MP analysis, and only the MP tree is shown. BI showed an average standard deviation of split frequencies = 0.006573.
The combined five-gene (ITS + nLSU + mt-SSU + nu-SSU + TEF1) dataset of 25 samples represented 7 taxa. It had an aligned length of 3470 characters, of which 3358 characters are constant, 65 characters are variable but parsimony uninformative, and 87 characters are parsimony informative. MP analysis yielded one tree (TL = 126, CI = 0.913, RI = 0.941, RC = 0.859, HI = 0.087). BI and ML analyses produced consensus trees similar to MP analysis, and only the MP tree is shown. BI showed an average standard deviation of split frequencies = 0.009683.
The phylogeny (Figure 1), based on ITS, shows that the five new taxa and Favolaschia calocera from Madagascar form six distinct lineages with robust support and cluster in the F. calocera complex clade. The phylogeny (Figure 2) based on ITS + nLSU + mt-SSU + nu-SSU + TEF1 results in a similar topology to the phylogeny based on ITS sequences, and is nested within the Favolaschia calocera complex, forming six distinct lineages.
3.2. Taxonomy

Figure 3.
Dried basidiocarps of Favolaschia brevibasidiata (Holotype). Scale bar = 5 mm.
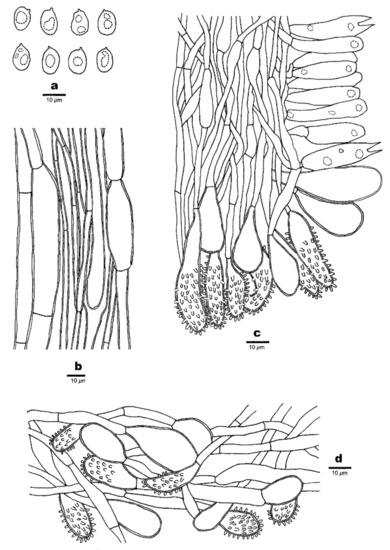
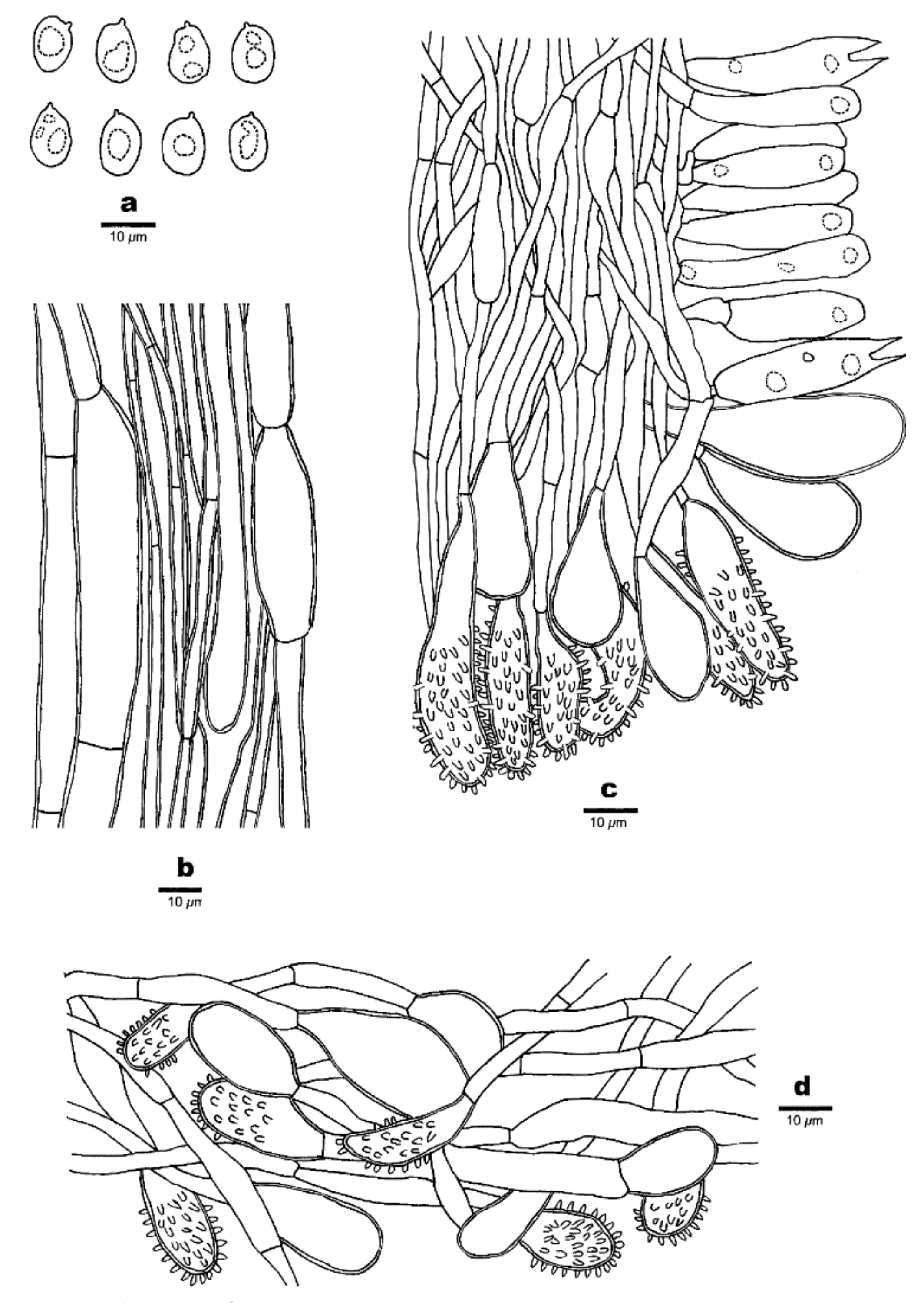
Figure 4.
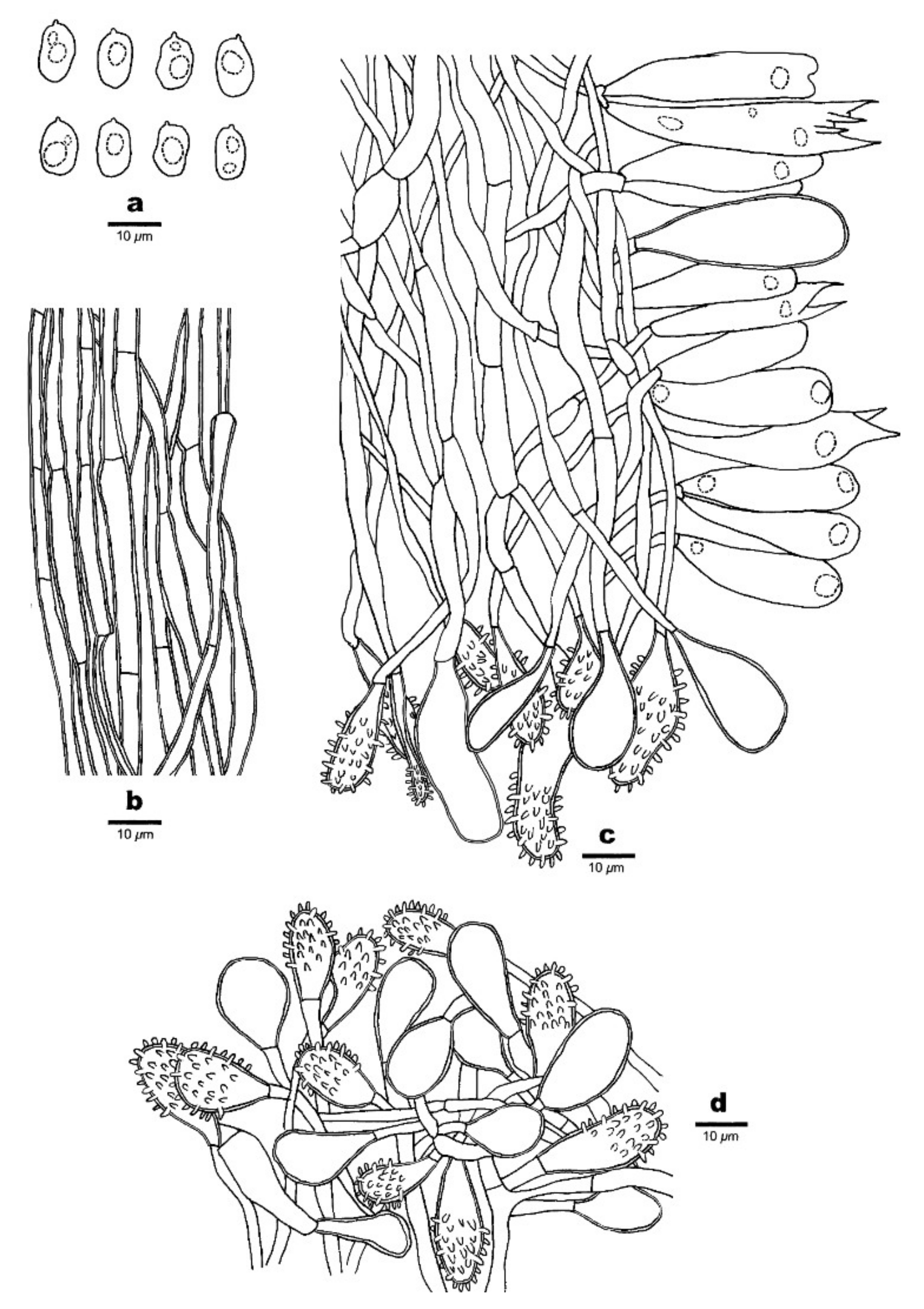
Microscopic structures of Favolaschia brevibasidiata. (Cui 6573, holotype). (a) Basidiospores. (b) Medullary hyphae of the stipe. (c) Hyphae at edge of pores showing gloeocystidia, acanthocystida, basidia and basidioles. (d) Hyphae of pileus cortex, composed of a hymeniform layer of acanthocysts and gloeocystidia.
MycoBank: MB 841445.
Diagnosis. Differs from F. brevistipitata, F. claudopus, and F. longistipitata in color. Differs from F. calocera and F. minutissima in smaller basidia.
Type. CHINA, Hainan Province, Ledong County, Jianfengling Nature Reserve, 11 May 2009, Cui 6573 (holotype, BJFC004426).
Etymology. Brevibasidiata (Lat.): referring to the species having short basidia.
Basidiocarps annual, gregarious, gelatinous. Pileus 2–7 × 1.5–4 mm, orbicular, apricot-orange when fresh, cream buff when dry; pileal surface slightly undulated in a reticulate pattern matching the pores below, sometimes faintly pruinose when dry. Hymenophore concolorous with pileal surface, poroid, 50–120 pores per basidiocarp; pores 0.4–1 mm in diam, pentagonal to somewhat irregular or polygonal in shape, larger near the base and smaller near the edge, the marginal pores often incomplete, pore edges pruinose when dry. Stipe obvious, laterally attached, concolorous with pileus, cylindrical or tapered to a slightly wider base, sometimes curved, very finely velutinate under a lens, 2–8 mm long.
Basidiospores 10–12(–13.5) × 6–7.8(–8) μm, L = 11.33 μm, W = 6.9 μm, Q = 1.64 (n = 30/1), ellipsoid to broadly ellipsoid, hyaline, thin-walled, smooth, with some guttules, faintly IKI+, CB−. Basidia 23–30 × 8–10 μm, cylindric or clavate, few fusoid, contain some guttules, 2–spored, sterigmata 1.5–4 μm long; basidioles in shape similar to basidia, but slightly smaller. Gloeocystidia present at the edges of pores, in hymenium and pileipellis, slightly thick-walled, smooth, contents dense and yellow-orange, those in hymenium clavate to ventricose, 25–36 × 10–18 μm; those at the pore edges more or less the same size as those in pileipellis, clavate, cylindrical or subglobose, 13–36 × 10–22 μm. Acanthocysts at the edges of pores and in pileipellis, slightly thick-walled, contents dense and yellow-orange, clavate, short vesiculose to pyriform-elongated, 15–47 × 9–12 μm. Tramal hyphae subparallel along tubes, strongly gelatinized, partly branched, hyaline, thin-walled, some slightly inflated, 2–4(–6) μm in diameter. Pileipellis comprising a palisade of acanthocysts and gloeocystidia. Hyphae in stipe parallel along stipe, slightly thick-walled, some inflated, 3–6(–11) µm in diameter. Clamp connections absent.

Figure 5.
Fresh basidiocarps of Favolaschia brevistipitata (Holotype). Scale bar = 10 mm.

Figure 6.
Dried basidiocarps of Favolaschia brevistipitata (Holotype). Scale bar = 5 mm.
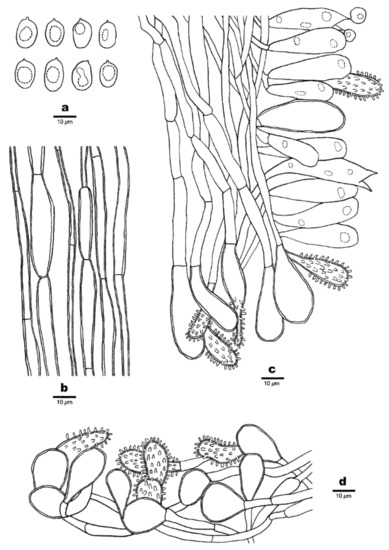
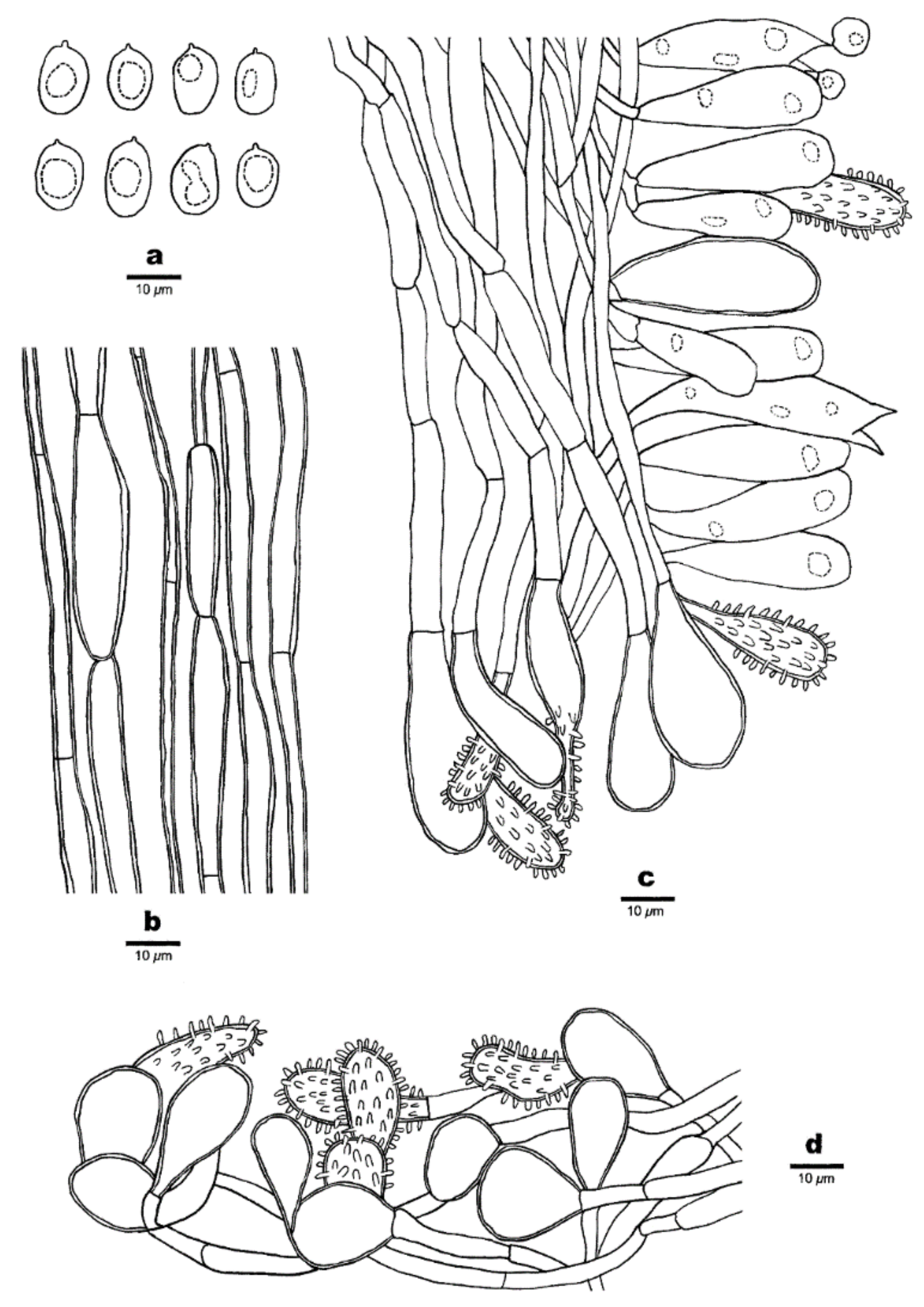
Figure 7.
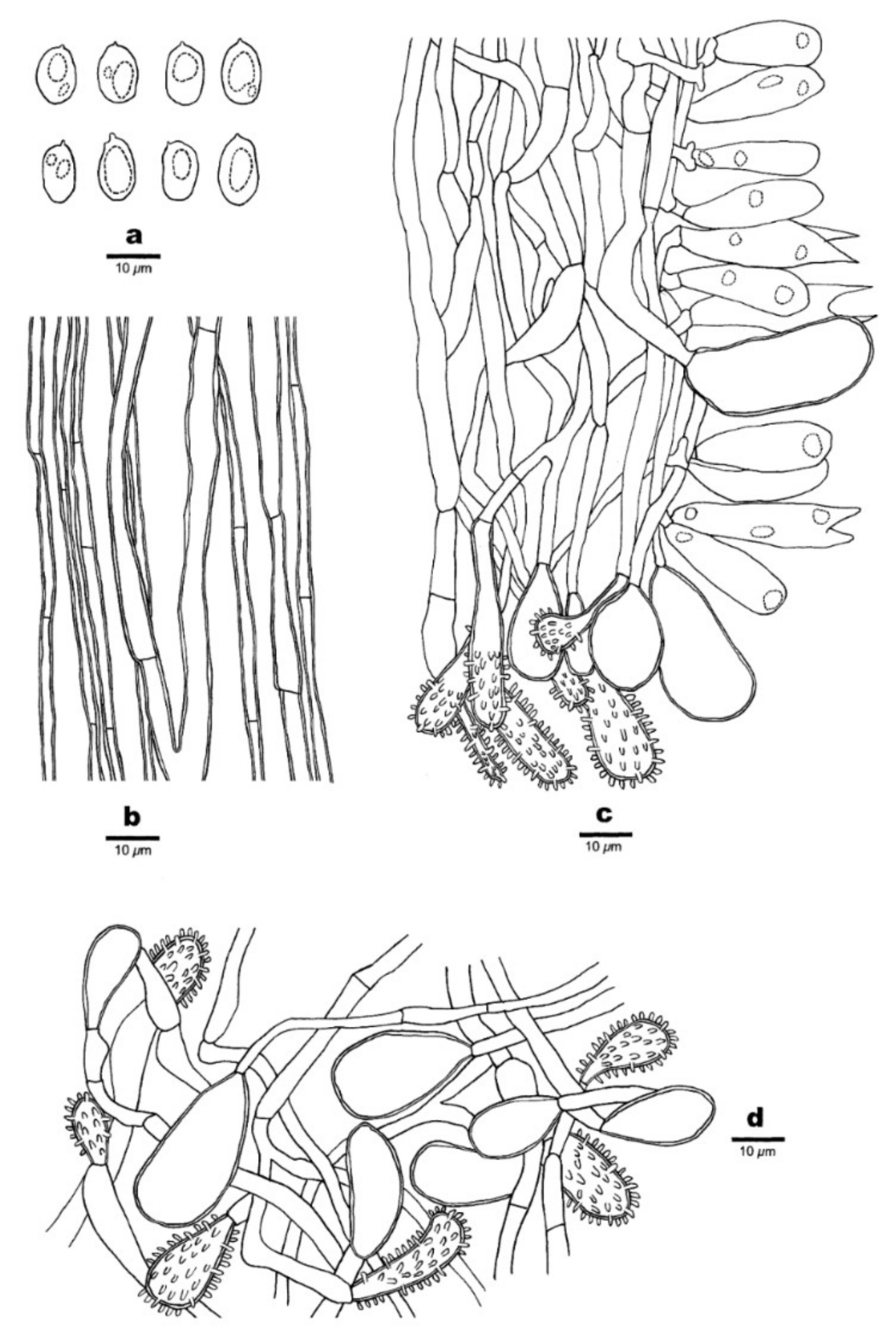
Microscopic structures of Favolaschia brevistipitata (Dai 19780, holotype). (a) Basidiospores. (b) Medullary hyphae of the stipe. (c) Hyphae at edge of pores showing gloeocystidia, acanthocystida, basidia and basidioles. (d) Hyphae of pileus cortex, composed of a hymeniform layer of acanthocysts and gloeocystidia.
MycoBank: MB 841446.
Diagnosis. Differs from F. brevibasidiata, F. calocera and F. minutissima in color. Differs from F. claudopus and F. longistipitata in shorter stipes.
Type. CHINA, Yunnan Province, Pingbian County, Daweishan National Forest Park, fallen angiosperm branch, 26 June 2019, Dai 19780 (holotype, BJFC031455).
Etymology. Brevistipitatus (Lat.): referring to the species having a short stipe.
Basidiocarps annual, gregarious, gelatinous. Pileus 2–10 × 1–6 mm, reniform to subcircular, lemon-chrome when fresh, becoming curry yellow when dry; pileal surface slightly undulate in a reticulate pattern matching the pores below, sometimes faintly pruinose when dry. Hymenophore concolorous with pileal surface poroid, 50–180 pores per basidiocarp; pores 0.4–1.5 mm in diameter, pentagonal to hexagonal and sometimes irregularly elongated, larger near the base and smaller near the edge, the marginal pores often incomplete, pore edges pruinose when dry. Stipe often scarcely developed, but mostly present, laterally attached, concolorous with pileus, short and curved, cylindric, very finely velutinate under a lens, 1–4 mm long.
Basidiospores (8–)9.5–13(–14) × 6–8.5(–9.6) μm, L = 11.38 μm, W = 7.26 μm, Q = 1.54–1.61 (n = 90/3), ellipsoid to broadly ellipsoid, hyaline, thin-walled, smooth, with some guttules, faintly IKI+, CB−. Basidia 28–46 × 9–12 μm, cylindric or clavate, contain some guttules, 2–spored, sterigmata 5–7 μm long; basidioles in shape similar to basidia, but slightly smaller. Gloeocystidia present at edges of pores, in hymenium and pileipellis, slightly thick-walled, smooth, contents dense and yellow-orange, those in hymenium clavate, 35–43 × 9–16 μm; those at pore edges more or less the same size as those in pileipellis, clavate, subglobose or ventricose, 18–45 × 10–25 μm. Acanthocysts at edges of pores, in hymenium and pileipellis, slightly thick-walled, contents dense and yellow-orange, clavate to oblong, few pyriform-elongated to irregular shaped, 15–45 × 6–11 μm. Tramal hyphae subparallel along tubes, strongly gelatinized, partly branched, hyaline, thin-walled, some slightly inflated, 3–5(–8) μm in diameter. Pileipellis comprising a palisade of acanthocysts and gloeocystidia. Hyphae in stipe parallel along stipe, slightly thick-walled, some inflated, 3–5(–10) µm in diameter. Clamp connections absent.
Additional specimens (paratypes) examined. CHINA, Yunnan Province, Pingbian County, Daweishan National Forest Park, fallen angiosperm branch, 26 June 2019, Dai 19855 (BJFC031530), Dai 19856 (BJFC031531).

Figure 8.
Dried basidiocarps of Favolaschia claudopus (Epitype). Scale bar = 5 mm.
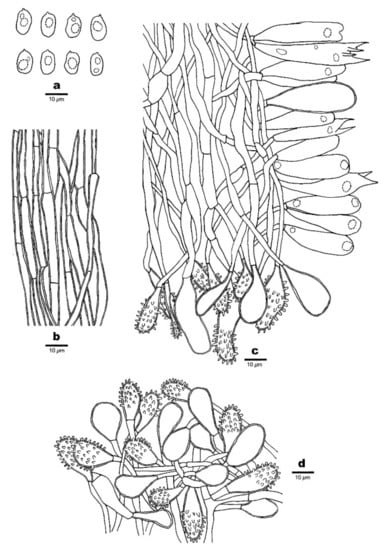
Figure 9.
Microscopic structures of Favolaschia claudopus (Dai 18656, holotype). (a) Basidiospores. (b) Medullary hyphae of the stipe. (c) Hyphae at edge of pores showing gloeocystidia, acanthocystida, basidia and basidioles. (d) Hyphae of pleus cortex, composed of a hymeniform layer of acanthocysts and gloeocystidia.
Basionym: Favolaschia calocera R. Heim var. claudopus Singer, Nova Hedwigia 50: 101, 1974—Holotype: New Zealand, Auckland, Waitakere Ranges, Waiatarua, on Elaeagnus pungens, July 1973, PDD 31006.
Epitype (designated here): Australia, Melbourne, Dandenong Ranges Botanical Garden, fallen trunk of Eucalyptus, 12 May 2018, Dai 18656 (BJFC027124, MEL).
MycoBank no.: MB 841449
Diagnosis. Differs from F. brevibasidiata, F. brevistipitata, F. longistipitata, and F. minutissima in larger pores. Differs from F. calocera in color.
Basidiocarps annual, gregarious, gelatinous. Pileus 4–14 × 3–10 mm, conchoid or reniform, lemon-chrome when fresh, becoming curry yellow when dry; pileal surface slightly undulate in a reticulate pattern matching the pores below, sometimes faintly pruinose when dry. Hymenophore concolorous with pileal surface, poroid, 30–170 pores per basidiocarp; pores 0.5–2.5 mm in diameter, pentagonal to hexagonal and sometimes irregularly elongated, larger near the base and smaller near the edge, the marginal pores often incomplete, pore edges pruinose when dry. Stipe obvious, laterally attached, concolorous with pileus, straight, cylindrical, or tapered to a slightly wider base, very finely velutinate under a lens, 3–10 mm long.
Basidiospores (8–)9.8–15(–15.5) × 6–8.8(–9) μm, L = 12.11 μm, W = 7.12 μm, Q = 2.09–2.14 (n = 60/2), mostly ellipsoid or ovoid, few oblong, hyaline, thin-walled, smooth, with some guttules, faintly IKI+, CB−. Basidia 25–49 × 6–11 μm, cylindric or clavate, contain some guttules, 2(–4)–spored, sterigmata 6–9 μm long; basidioles in shape similar to basidia, but slightly smaller. Gloeocystidia present at the edges of pores, in hymenium and pileipellis, slightly thick-walled, smooth, contents dense and yellow-orange, those in hymenium ellipsoid or clavate, 35–50 × 10–14 μm; those at pore edges more or less the same size as those in pileipellis, clavate, subglobose to oblong, 17–52 × 8–26 μm. Acanthocysts at the edges of pores and in pileipellis, slightly thick-walled, contents dense and yellow-orange, clavate, subclavate with a constriction, sometimes elongated to irregular shaped, 15–47 × 6–12 μm. Tramal hyphae subparallel along tubes, strongly gelatinized, partly branched, hyaline, thin-walled, some slightly inflated, 2–6(–8) μm in diameter. Pileipellis comprising a palisade of acanthocysts and gloeocystidia. Hyphae in stipe parallel along stipe, slightly thick-walled, some slightly inflated, 2–4(–6) µm in diameter. Clamp connections absent.
Additional specimen examined. Australia, Melbourne, Dandenong Ranges Botanical Garden, fallen trunk of Eucalyptus, 12 May 2018, Dai 18663 (BJFC027131).

Figure 10.
Fresh basidiocarps of Favolaschia longistipitata (Holotype). Scale bar = 10 mm.

Figure 11.
Dried basidiocarps of Favolaschia longistipitata (Holotype). Scale bar = 5 mm.
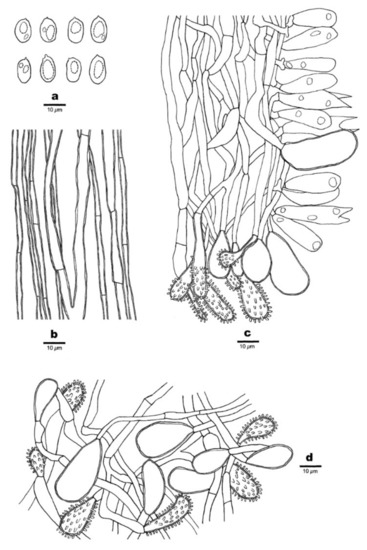
Figure 12.
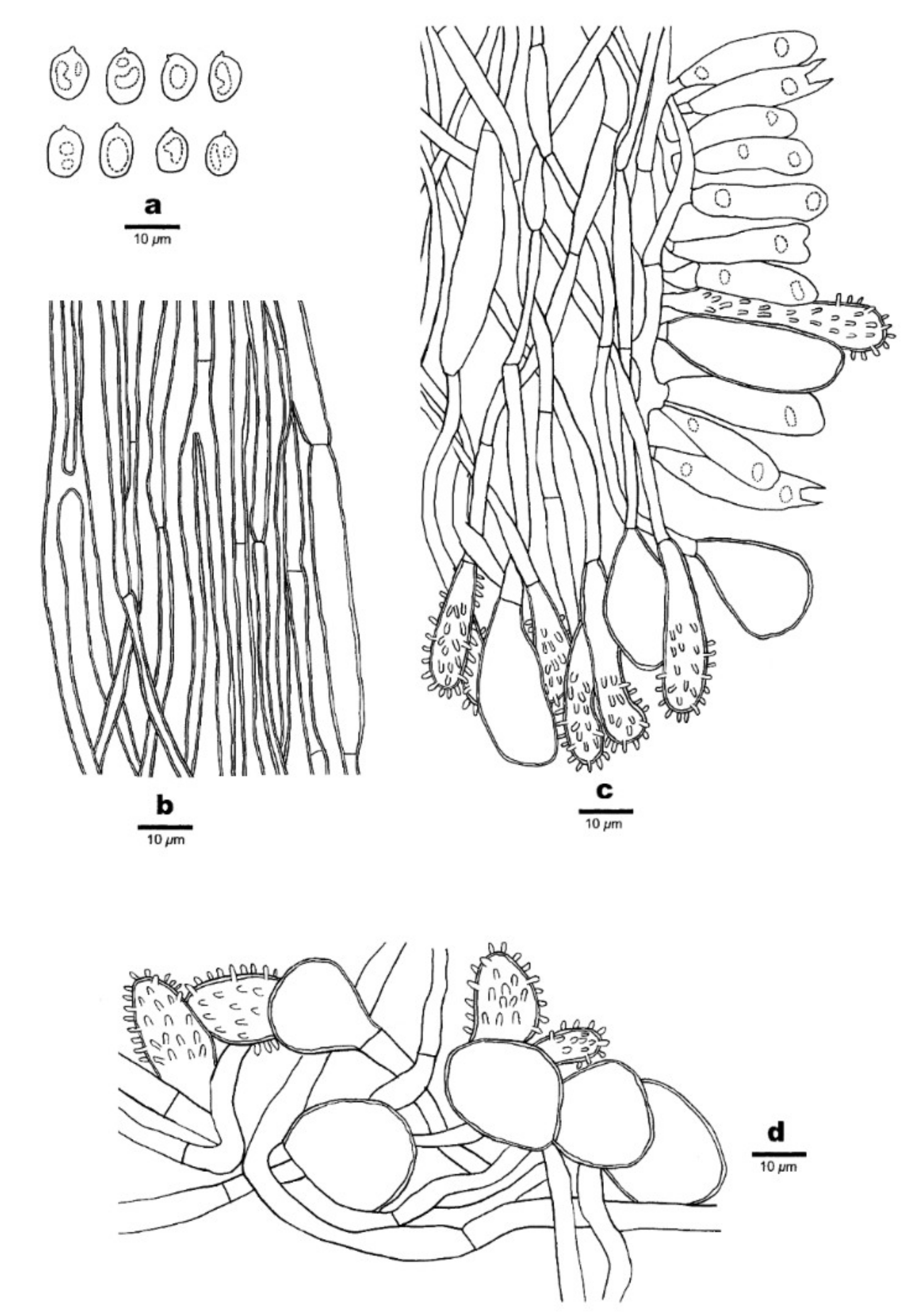
Microscopic structures of Favolaschia longistipitata (Dai 19799, holotype). (a) Basidiospores. (b) Medullary hyphae of the stipe. (c) Hyphae at the edge of pores showing gloeocystidia, acanthocystida, basidia, and basidioles. (d) Hyphae of the pileus cortex, composed of a hymeniform layer of acanthocysts and gloeocystidia.
MycoBank no.: MB 841447.
Diagnosis. Differs from F. brevibasidiata, F. calocera, and F. minutissima in color. Differs from F. brevistipitata in longer stipes. Differs from F. claudopus in smaller pores.
Type. CHINA, Yunnan Province, Pingbian County, Daweishan National Forest Park, fallen angiosperm branch, 26 June 2019, Dai 19799 (holotype, BJFC031474).
Etymology. Longistipitata (Lat.): referring to the species having a long stipe.
Basidiocarps annual, gregarious, gelatinous. Pileus 2–12 × 1.5–8 mm, flabelliform to subcircular, lemon-chrome when fresh, becoming curry yellow when dry; pileal surface slightly undulated in a reticulate pattern matching the pores below, sometimes faintly pruinose when dry. Hymenophore concolorous with pileal surface, poroid, 40–240 pores per basidiocarp; pores 0.5–1.5 mm in diameter, pentagonal to hexagonal and sometimes irregularly elongated, larger near the base and smaller near the edge, the marginal pores often incomplete, pore edges pruinose when dry. Stipe obvious, laterally attached, concolorous with pileus, cylindrical or tapered to a slightly wider base, sometimes curved, very finely velutinate under a lens, 4–12 mm long.
Basidiospores (9–)9.8–13 × 6–8 μm, L = 11.31 μm, W = 6.72 μm, Q = 1.62–1.74 (n = 120/4), ellipsoid to oblong, hyaline, thin-walled, smooth, with some guttules, faintly IKI+, CB−. Basidia 32–47 × 9–13 μm, cylindric or clavate, few fusoid, contain some guttules, 2–spored, sterigmata 4–6 μm long; basidioles in shape similar to basidia, but slightly smaller. Gloeocystidia present at the edges of pores, in hymenium and pileipellis, slightly thick-walled, smooth, contents dense and yellow-orange, those in hymenium pyriform, 25–45 × 10–12 μm; those at pore edges more or less the same size as those in pileipellis, subglobose or ventricose, 20–40 × 6–21 μm. Acanthocysts at the edges of pores and in pileipellis, slightly thick-walled, contents dense and yellow-orange, elongated to irregular shaped, 16–42 × 5–13 μm. Tramal hyphae subparallel along tubes, strongly gelatinized, partly branched, hyaline, thin-walled, some slightly inflated, 2–4(–6) μm in diameter. Pileipellis comprising a palisade of acanthocysts and gloeocystidia. Hyphae in stipe parallel along stipe, slightly thick-walled, some slightly inflated, 2–4(–7) µm in diameter. Clamp connections absent.
Additional specimens (paratypes) examined. CHINA, Yunnan Province, Gengma County, Nangunhe Nature Reserve, on a fallen branch of Castanopsis, 11 July 2013, Dai 13226 (BJFC014716); Kunming, Xiaoshao Forest Farm, on a fallen angiosperm branch, 1 July 2019, Dai 20019 (BJFC031693); Lincang, Linxiang District, Xiaodaohe Forest Farm, on fallen branch of Castanopsis, 10 July 2013, Dai 13221(BJFC014711); Nanhua County, Dazhongshan Nature Reserve, on a fallen branch of Quercus, Cui 11128 (BJFC015243); Pingbian County, Daweishan National Forest Park, on a fallen angiosperm branch, 26 June 2019, Dai 19781 (BJFC031456), on a fallen trunk, 27 June 2019, Dai 19893 (BJFC031567); Wuding County, Shizishan Nature Reserve, on a fallen angiosperm trunk, 15 August 2019, Dai 20328 (BJFC031996), Dai 20355 (BJFC032023), on a fallen branch of Alnus, Dai 20341 (BJFC032009); Xinping County, Mopanshan Forest Park, on a fallen angiosperm branch, 15 May 2017, Dai 17597 (BJFC025129), Dai 17598 (BJFC025130), Dai 17601 (BJFC025133).

Figure 13.
Fresh basidiocarps of Favolaschia minutissima (Holotype). Scale bar = 10 mm.

Figure 14.
Dried basidiocarps of Favolaschia minutissima (Holotype). Scale bar = 5 mm.
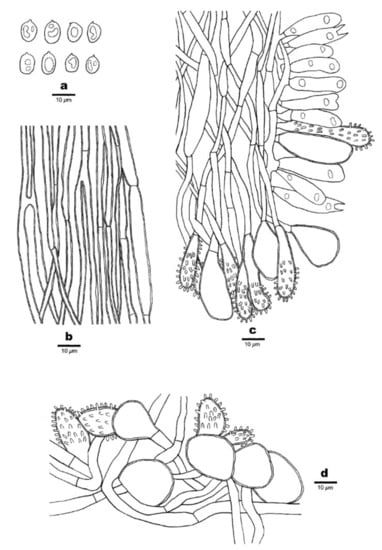
Figure 15.
Microscopic structures of Favolaschia minutissima (Dai 20086, holotype). (a) Basidiospores. (b) Medullary hyphae of the stipe. (c) Hyphae at the edge of pores showing gloeocystidia, acanthocystida, basidia, and basidioles. (d) Hyphae of the pileus cortex, composed of a hymeniform layer of acanthocysts and gloeocystidia.
MycoBank no.: MB 841448.
Diagnosis. Differs from other F. calocera complex species in smaller basidiocarps and smaller pores.
Type. CHINA, Hainan Province, Ledong County, Jianfengling Naturel Reserve, fallen angiosperm branch, 3 July 2019, Dai 20086 (holotype, BJFC031760).
Etymology. Minutissima (Lat.): referring to the species having a very small basidiocarp.
Basidiocarps annual, gregarious, gelatinous. Pileus 2–4 × 1–3 mm, reniform to suborbicular, apricot-orange when fresh, becoming cream buff when dry; pileal surface slightly undulated in a reticulate pattern matching the pores below, sometimes faintly pruinose when dry. Hymenophore concolorous with pileal surface, poroid, 30–90 pores per basidiocarp; pores 0.2–0.5 mm in diameter, pentagonal to hexagonal and sometimes irregularly elongated, larger near the base and smaller near the edge, the marginal pores often incomplete, pore edges pruinose when dry. Stipe often scarcely developed, laterally attached, concolorous with pileus, short and curved, cylindric, very finely velutinate under a lens, 0.5–4 mm long.
Basidiospores (6–)7.5–11(–11.5) × (4.8–)5–7.2(–7.8) μm, L = 9.16 μm, W = 6.10 μm, Q = 1.46–1.56 (n = 90/3), broadly ellipsoid to ovoid, hyaline, thin-walled, smooth, with some guttules, faintly IKI+, CB−. Basidia 25–37 × 7–9.5 μm, clavate, few fusoid, contain some guttules, 2–spored, sterigmata 5–7 μm long; basidioles in shape similar to basidia, but slightly smaller. Gloeocystidia present at the edges of pores, in hymenium and pileipellis, slightly thick-walled, smooth, contents dense and yellow-orange, those in hymenium clavate to vesiculose, 30–42 × 10–12.5 μm; those at the pore edges more or less the same size as those in pileipellis, pyriform, subglobose or ventricose, 20–48 × 10–26 μm. Acanthocysts at the edges of pores, in hymenium and pileipellis, slightly thick-walled, contents dense and yellow-orange, clavate to oblong, 9–45 × 7–12 μm. Tramal hyphae subparallel along tubes, strongly gelatinized, partly branched, hyaline, thin-walled, some slightly inflated, 2–4(–6) μm in diameter. Pileipellis comprising a palisade of acanthocysts and gloeocystidia. Hyphae in stipe parallel along stipe, slightly thick-walled, some slightly inflated, 2–4(–7) µm in diameter. Clamp connections absent.
Additional specimens (paratypes) examined. CHINA, Hainan Province, Changjiang County, Bawangling Nature Reserve, a fallen angiosperm branch, 8 May 2009, Dai 10753 (BJFC004997); Ledong County, Jianfengling Nature Reserve, a fallen angiosperm branch, 3 July 2019, Dai 20085 (BJFC031759), Dai 20088 (BJFC031762).
4. Discussion
Favolaschia calocera, originally described in Madagascar, is a species complex. We recognize six species in the complex: Favolaschia calocera sensu stricto; three new species from China, F. brevibasidiata, F. brevistipitata, F. longistipitata; one new species from Asia, F. minutissima; and a variety raised to species rank Favolaschia claudopus (Singer) Q.Y. Zhang & Y.C. Dai from Oceania, Asia and Africa. Two samples of Favolaschia calocera from the type locality Madagascar form a separate clade; we refer to the description of Singer [8], which is adapted from Heim’s original description and the sequence by Johnston et al. [13]. The members of Favolaschia calocera complex differ from other species in the genus by the bright orange or yellow basidiocarps with a distinct laterally stipe and numerous gloeocystidia and acanthocysts. The main morphological characteristics of species in Favolaschia calocera complex are listed in Table 2.

Table 2.
A comparison of the characteristics of the species in the Favolaschia calocera complex.
In the phylogenetic trees (Figure 1 and Figure 2), our four new species and Favolaschia claudopus are nested in the Favolaschia calocera complex, and form five independent lineages. In addition, Favolaschia brevibasidiata, F. calocera, and F. minutissima are closely related. Morphologically, both Favolaschia brevibasidiata, F. calocera, and F. minutissima have apricot-orange pilei when fresh, but F. calocera differs from F. brevibasidiata by its larger basidiocarps (11 × 9 mm vs. 2–7 × 1.5–4 mm), larger pores (up to 2 mm vs. 0.4–1 mm), and longer basidia (33–36 × 7–10 μm vs. 23–30 × 8–10 μm). Favolaschia minutissima differs from F. brevibasidiata by its smaller basidiocarps (2–4 × 1–3 mm vs. 2–7 × 1.5–4 mm), smaller pores (0.2–0.5 mm vs. 0.4–1 mm), and smaller basidiospores (7.5–11 × 5–7.2 μm vs. 10–12 × 6–7.8 μm). Furthermore, Favolaschia minutissima is easily distinguishable from F. calocera by its smaller basidiocarps (2–4 × 1–3 mm vs. 11 × 9 mm), smaller pores (0.2–0.5 mm vs. up to 2 mm), smaller basidiospores (7.5–11 × 5–7.2 μm vs. 12–12.5 × 8.2–9 μm), and shorter stipes (0.5–4 mm vs. up to 9 mm). Moreover, Favolaschia calocera is distributed in Madagascar, but F. brevibasidiata and F. minutissima occur in Asia (southern China and Thailand).
Favolaschia brevistipitata occurs in Yunnan Province of China and forms a well-supported lineage (Figure 1 and Figure 2). Morphologically, Favolaschia brevistipitata, F. claudopus, and F. longistipitata all have similar pilei (lemon-chrome when fresh, curry yellow when dry). However, Favolaschia claudopus differs from F. brevistipitata by its larger pores (0.5–2.5 mm vs. 0.4–1.5 mm) and longer stipes (3–10 mm vs. 1–4 mm). Favolaschia longistipitata can be easily separated from F. brevistipitata by its larger basidiocarps (2–12 × 1.5–8 mm vs. 2–10 × 1–6 mm) and longer stipes (4–12 mm vs. 1–4 mm). Microscopically, Favolaschia brevistipitata and F. brevibasidiata have similar sized basidiospores, but F. brevibasidiata differs from F. brevistipitata by its smaller basidiocarps (2–7 × 1.5–4 mm vs. 2–10 × 1–6 mm), longer stipes (2–8 mm vs. 1–4 mm), and smaller basidia (23–30 × 8–10 μm vs. 28–46 × 9–12 μm).
According to our study, two specimens of Favolaschia claudopus from Australia clustered together with specimens from Italy, Kenya, and New Zealand; however, our measurements on their basidiospores are slightly longer than those reported by Johnston et al. [11] (9.8–15 × 6–8.8 μm vs. 9–12.5 × 6.5–8.5 μm). In addition, Favolaschia claudopus can be easily separated from other species in F. calocera complex by its largest basidiocarps (4–14 × 3–10 mm) and largest pores (0.5–2.5 mm).
Favolaschia longistipitata is a common species in Yunnan Province of China. Phylogenetically, Favolaschia longistipitata is closely related to Favolaschia claudopus (Figure 1). Morphologically, Favolaschia longistipitata may be confused with Favolaschia claudopus in having lemon-chrome pileus when fresh, curry yellow when dry, and similar sized basidiocarps. However, Favolaschia claudopus differs from F. longistipitata in having larger pores (0.5–2.5 mm vs. 0.5–1.5 mm), longer gloeocystidia in pileipellis (17–52 × 8–26 μm vs. 20–40 × 6–21 μm), and longer sterigmata (6–9 μm vs. 4–6 μm). Both Favolaschia longistipitata, F. brevibasidiata, and F. calocera have similar sized basidiospores under the microscope. However, Favolaschia brevibasidiata is distinguished from F. longistipitata by its smaller basidiocarps (2–7 × 1.5–4 mm vs. 2–12 × 1.5–8 mm), shorter stipes (2–8 mm vs. 4–12 mm), and shorter basidia (23–30 × 8–10 μm vs. 32–47 × 9–13 μm). Favolaschia calocera differs from F. longistipitata by its a lesser pores per basidiocarp (40–70 vs. 40–240), shorter stipes (up to 9 mm vs. 4–12 mm), and longer sterigmata (10–12 μm vs. 4–6 μm).
Three newly sequenced samples Favolaschia minutissima with very small fruiting bodies from Hainan Province of China and one specimen from Thailand (DQ 026240, Johnston et al. 2006) formed a well-supported lineage (Figure 1 and Figure 2). Favolaschia minutissima differs from other F. calocera complex species by its the smallest basidiocarps (2–4 × 1–3 mm), smallest pores (0.2–0.5 mm), and smallest basidiospores (L = 9.16 μm, W = 6.10 μm).
According to the molecular analysis performed by Johnston et al. [13] and Vizzini et al. [14], these collections of Favolaschia calocera from New Zealand, Kenya, and Italy clustered in one group represent very recent, probably human-vectored introductions; on the other hand, samples of Madagascar and Asia (southern China and Thailand) display a higher genetic variability and could represent the natural distribution of this fungus. Our study is consistent with the speculation that the Australian samples cluster with the specimens from New Zealand, Kenya, and Italy and are described as Favolaschia claudopus.
The natural distribution of Favolaschia calocera complex is a controversial question, which is being explored by taxonomists. Two hypotheses have emerged. One hypothesis suggested that Favolaschia calocera as well as other Favolaschia species, during the break-up of Gondwana (about 150 million years ago), drifted away from Madagascar, where they likely evolved, with the India–Seychelles landmass, and subsequently dispersed through Asia. A second hypothesis, contrary to the first, suggested that Favolaschia calocera was accidentally introduced into Madagascar from Asia, by an impressive one-way human migratory flow from Southeast Asia (Borneo) to Madagascar in the centuries between 200 and 500 Anno Domini [14,35,36,37,38]. As in our analysis, these Asian samples of Favolaschia calocera complex show higher variability than Madagascar. Our investigation revealed that Favolaschia calocera complex is common in tropical China and four new species have been discovered. However, to date, only Favolaschia calocera has been recorded in Madagascar in F. calocera complex, as mycologists have been active in Central America since the late1800s and it is unlikely they would have overlooked such a brightly colored and conspicuous fungus. Thus, our data tend to support the second hypothesis, but to confirm these hypotheses, a molecular clock analysis on all the Favolaschia species is needed.
Key to species of the Favolaschia calocera complex.
1. Basidiocarps apricot-orange when fresh.....................................................................................2
1. Basidiocarps lemon-chrome when fresh.....................................................................................4
2. Pileus usually <5 mm..............................................................................................F. minutissima
2. Pileus usually >5 mm....................................................................................................................3
3. Mature pores up to 2 mm in the largest dimension....................................................F. calocera
3. Mature pores 0.4–1 mm........................................................................................F. brevibasidiata
4. Stipe usually <5 mm long......................................................................................F. brevistipitata
4. Stipe usually >5 mm long.............................................................................................................5
5. Basidiospores up to 15 μm in length .......................................................................F. claudopus
5. Basidiospores 9.8–13 × 6–8 μm.............................................................................F. longistipitata
Author Contributions
Conceptualization, Y.-C.D. and Q.-Y.Z.; methodology, Q.-Y.Z.; perform the experiment, Q.-Y.Z.; formal analysis, Q.-Y.Z.; validation, Y.-C.D. and Q.-Y.Z.; investigation, Y.-C.D.; resources, Y.-C.D.; writing—original draft preparation, Q.-Y.Z.; writing—review and editing, Y.-C.D.; visualization, Q.-Y.Z.; supervision, Y.-C.D.; funding acquisition, Y.-C.D. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Second Tibetan Plateau Scientific Expedition and Research Program (STEP), Grant No. 2019QZKK0503.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/; https://www.mycobank.org; http://purl.org/phylo/treebase, submission ID 28714 (accessed on 30 August 2021).
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Patouillard, N. Etude sur le genre Laschia Fr. J. Bot. 1887, 1, 225–231. [Google Scholar]
- Patouillard, N. Essai Taxonomique sur les Families et les Genres des Hyménomycètes; Lucien Declume: Lons-le-Saaunier, France, 1900. [Google Scholar] [CrossRef] [Green Version]
- Singer, R. The Laschia-complex (Basidiomycetes). Lloydia 1945, 8, 170–230. [Google Scholar]
- Clémençon, H. Anatomie der Hymenomyceten (Anatomy of the Hymenomycetes); Flück-Wirth Teufen, Ed.; Universität Lausanne: Lausanne, Switzerland, 1997. [Google Scholar]
- Gillen, K.; Laessoe, T.; Kirschner, R.; Piepenbring, M. Favolaschia species (Agaricales, Basidiomycota) from Ecuador and Panama. Nova Hedwig. 2012, 96, 117–165. [Google Scholar] [CrossRef]
- Thoen, E.; Bugge Harder, C.; Kauserud, H.; Botnen, S.S.; Vik, U.; Taylor, A.F.S.; Menkis, A.; Skrede, I. In vitro evidence of root colonization suggests ecological versatility in the genus Mycena. New Phytol. 2020, 227, 2. [Google Scholar] [CrossRef] [Green Version]
- Bugge Harder, C.; Hesling, E.; Botnen, S.S.; Dima, B.; Bonsdorff-Salminen, T.N.T.; Jarvis, S.G.; Lorberau, K.E.; Ouimette, A.; Hester, A.; Hobbie, E.A.; et al. Mycena species can be opportunist-generalist plant root invaders. bioRxiv 2021, in press. [Google Scholar] [CrossRef]
- Singer, R. A monograph of Favolaschia. Beih. Nova Hedwig. 1974, 50, 1–108. [Google Scholar]
- Parmasto, E. Favolaschia pegleri, sp. nov. (Hymenomycetes). Kew Bull. 1999, 54, 783–788. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, D.W.; Minter Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CABI Europe: Wallingford, UK, 2008. [Google Scholar]
- Heim, R. Les Agarics tropicaux a hyménium tubule (Madagascar, Côte d’lvoire, Guinée, Antilles, Insulinde). Rev. Mycol. 1945, 10, 1–64. [Google Scholar]
- Johnston, P.R.; Buchanan, P.K.; Leathwick, J.; Mortimer, S. Fungal invaders. Australas. Mycol. Newsl. 1998, 17, 48–52. [Google Scholar]
- Johnston, P.R.; Whitton, S.R.; Buchanan, P.K.; Park, D.; Moncalvo, J.M. The basidiomycete genus Favolaschia in New Zealand. N. Zeal. J. Bot. 2006, 44, 65–87. [Google Scholar] [CrossRef]
- Vizzini, A.; Zotti, M.; Mello, A. Alien fungal species distribution: The study case of Favolaschia calocera. Biol. Invasions 2009, 11, 417–429. [Google Scholar] [CrossRef]
- Chuzho, K.; Dkhar, M.S. Ecological determinants of wood-rotting fungal diversity and first report of Favolaschia calocera, an invasive species from India. Proc. Natl. Acad. Sci. USA 2018, 89, 1177–1188. [Google Scholar] [CrossRef]
- Vizzini, A.; Zotti, M. Favolaschia calocera, a tropical species collected in Italy. Mycotaxon 2002, 82, 169–176. [Google Scholar]
- Ainsworth, A.M.; Farley, D.; Gainey, P.; Penna, P.; Suz, L.M. Invasion of the orange ping-pong bats: The rapidly changing distribution of Favolaschia calocera. Field Mycol. 2015, 16, 113–120. [Google Scholar] [CrossRef]
- Cui, B.K.; Li, H.J.; Ji, X.; Zhou, J.L.; Song, J.; Si, J.; Yang, Z.L.; Dai, Y.C. Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers 2019, 97, 137–392. [Google Scholar] [CrossRef]
- Petersen, J.H. Farvekort. The Danish Mycological Society’s Colour-Chart; Foreningen til Svampekundskabens Fremme: Greve, Italy, 1996; pp. 1–6. [Google Scholar]
- Chen, Q.; Du, P.; Vlasák, J.; Wu, F.; Dai, Y.C. Global diversity and phylogeny of Fuscoporia (Hymenochaetales, Basidiomycota). Mycosphere 2020, 11, 1477–1513. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gefand, D.H., Sninsky, J.J., White, M.J.T., Eds.; Academic Press: San Diego, FL, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Dai, Y.C.; Vlasák, J.; Yuan, Y. Molecular phylogeny and global diversity of the genus Haploporus (Polyporales, Basidiomycota). J. Fungi 2021, 7, 96. [Google Scholar] [CrossRef]
- Liu, S.; Han, M.L.; Xu, T.M.; Wang, Y.; Wu, D.M.; Cui, B.K. Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from East Asia. Front. Microbiol 2021, 12, 644979. [Google Scholar] [CrossRef]
- Capelari, M.; Karstedt, F.; Oliveira, J. Favolaschia in remnants of the Atlantic forest, Brazil. Mycoscience 2014, 55, 12–20. [Google Scholar] [CrossRef]
- Jin, J.K.; Hugues, K.W.; Petersen, R.H. Biogeographical patterns in Panellus stypticus. Mycologia 2001, 93, 309–316. [Google Scholar] [CrossRef]
- Chenna, R.; Sugawara, H.; Koike, T.; Lopez, R.; Gibson, T.J.; Higgins, D.G.; Thompson, J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31, 3497–3500. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Page, R.M.D. Treeview: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar] [CrossRef] [Green Version]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Cmethods); Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biodivers. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Ve’rin, P.; Wright, H. Madagascar and Indonesia: New evidence from archaeology and linguistics. Indo Pac. Prehist. Assoc. Bull. 1999, 18, 35–42. [Google Scholar]
- Ducousso, M.; Be´na, G.; Bourgeois, C.; Buyck, B.; Eyssartier, G.; Vincelette, M.; Rabevohitra, R.; Randrihasipara, L.; Dreyfus, B.; Prin, Y. The last common ancestor of Sarcolaenaceae and Asian dipterocarp trees was ectomycorrhizal before the India-Madagascar separation, about 88 million years ago. Mol. Ecol. 2004, 13, 231–236. [Google Scholar] [CrossRef]
- Hurles, M.E.; Sykes, B.C.; Jobling, M.A.; Forster, P. The dual origin of the Malagasy in Island southeast Asia and east Africa: Evidence from maternal and paternal lineages. Am. J. Hum. Genet. 2005, 76, 894–901. [Google Scholar] [CrossRef] [Green Version]
- Subrahmanyam, C.; Chand, S. Evolution of the passive continental margins of India—A geophysical appraisal. Gondwana Res. 2006, 10, 167–178. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).