The Responses of Leaf Litter Calcium, Magnesium, and Manganese Dynamics to Simulated Nitrogen Deposition and Reduced Precipitation Vary with Different Decomposition Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Design
2.3. Leaf Litter Manipulation
2.4. Calculations and Statistical Analysis
3. Results
3.1. Litter Temperature and Water Content during Decomposition
3.2. Ca, Mg, and Mn Concentrations during Decomposition
3.3. Ca, Mg, and Mn Releases during Decomposition
3.4. Relationships between Ca, Mg, and Mn Concentrations and Mass Loss
4. Discussion and Conclusions
4.1. Ca, Mg, and Mn Dynamics during Decomposition
4.2. Responses of Ca, Mg, and Mn to N Deposition and Reduced Precipitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Yu, G.; Jia, Y.; He, N.; Zhu, J.; Chen, Z.; Wang, Q.; Piao, S.; Liu, X.; He, H.; Guo, X.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, F.; Ciais, P.; Miao, C.; Yang, T.; Jia, Y.; Zhou, X.; Klaus, B.-B.; Yang, T.; Yu, G. Human activities aggravate nitrogen-deposition pollution to inland water over China. Natl. Sci. Rev. 2020, 7, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Fang, Y.; Gundersen, P.; Vogt, R.D.; Koba, K.; Chen, F.; Chen, X.Y.; Yoh, M. Atmospheric deposition and leaching of nitrogen in Chinese forest ecosystems. J. For. Res. 2011, 16, 341–350. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, H.; Liu, T.; Mao, P.; Zhang, W.; Shao, Y.; Fu, S. An increase in precipitation exacerbates negative effects of nitrogen deposition on soil cations and soil microbial communities in a temperate forest. Environ. Pollut. 2018, 235, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Asbjornsen, H.; Campbell, J.L.; Jennings, K.A.; Vadeboncoeur, M.; McIntire, C.; Templer, P.H.; Phillips, R.P.; Bauerle, T.L.; Dietze, M.; Frey, S.D.; et al. Guidelines and considerations for designing field experiments simulating precipitation extremes in forest ecosystems. Methods Ecol. Evol. 2018, 9, 2310–2325. [Google Scholar] [CrossRef] [Green Version]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.; et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Summary for policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Li, G.; Kim, S.; Han, S.H.; Chang, H.; Son, Y. Effect of soil moisture on the response of soil respiration to open-field experimental warming and precipitation manipulation. Forests 2017, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Stocker, B.D.; Zscheischler, J.; Keenan, T.F.; Prentice, I.C.; Seneviratne, S.I.; Peñuelas, J. Drought impacts on terrestrial primary production underestimated by satellite monitoring. Nat. Geosci. 2019, 12, 264–270. [Google Scholar] [CrossRef]
- Zhao, M.; Running, S.W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.J.; Ma, S.; He, H.S.; Liu, Z.; Thompson, F.R.; Jin, W.; Wu, Z.F.; A. Spetich, M.; Wang, L.; Xue, S.; et al. Effects of rising atmospheric CO2, climate change, and nitrogen deposition on aboveground net primary production in a temperate forest. Environ. Res. Lett. 2019, 14, 104005. [Google Scholar] [CrossRef]
- Wu, Y.; Kwak, J.-H.; Karst, J.; Ni, M.; Yan, Y.; Lv, X.; Xu, J.; Chang, S.X. Long-term nitrogen and sulfur deposition increased root-associated pathogen diversity and changed mutualistic fungal diversity in a boreal forest. Soil Biol. Biochem. 2021, 155, 108163. [Google Scholar] [CrossRef]
- Beaumelle, L.; de Laender, F.; Eisenhauer, N. Biodiversity mediates the effects of stressors but not nutrients on litter decomposition. eLife 2020, 9, e55659. [Google Scholar] [CrossRef]
- Zhou, S.-X.; Huang, C.-D.; Han, B.-H.; Xiao, Y.-X.; Tang, J.-D.; Xiang, Y.-B.; Luo, C. Simulated nitrogen deposition significantly suppresses the decomposition of forest litter in a natural evergreen broad-leaved forest in the rainy area of Western China. Plant Soil 2017, 420, 135–145. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, C.; Xiang, Y.; Tie, L.; Han, B.; Scheu, S. Effects of reduced precipitation on litter decomposition in an evergreen broad-leaved forest in western China. For. Ecol. Manag. 2018, 430, 219–227. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Bretscher, D. Isopod effects on decomposition of litter produced under elevated CO2, N deposition and different soil types. Glob. Chang. Biol. 2001, 7, 565–579. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.; Lu, X.; Yang, Y.; Hartley, I.P.; Zhang, W.; Mo, J.; Yu, G.; Zhou, J.; Kuzyakov, Y. Long-term nitrogen addition modifies microbial composition and functions for slow carbon cycling and increased sequestration in tropical forest soil. Glob. Chang. Biol. 2019, 25, 3267–3281. [Google Scholar] [CrossRef] [Green Version]
- Couˆteaux, M.-M.; Bottner, P.; Berg, B. Litter decomposition, climate and liter quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Mori, A.S.; Cornelissen, J.H.C.; Fujii, S.; Okada, K.-I.; Isbell, F. A meta-analysis on decomposition quantifies afterlife effects of plant diversity as a global change driver. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Berg, B. Decomposing litter; limit values; humus accumulation, locally and regionally. Appl. Soil Ecol. 2018, 123, 494–508. [Google Scholar] [CrossRef] [Green Version]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Zhou, S.; Butenschoen, O.; Barantal, S.; Handa, I.T.; Makkonen, M.; Vos, V.; Aerts, R.; Berg, M.P.; McKie, B.; van Ruijven, J.; et al. Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna. J. Ecol. 2020, 108, 2283–2297. [Google Scholar] [CrossRef]
- Makkonen, M.; Berg, M.P.; Handa, I.T.; Hättenschwiler, S.; van Ruijven, J.; van Bodegom, P.M.; Aerts, R. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 2012, 15, 1033–1041. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W. Control of climate and litter quality on leaf litter decomposition in different climatic zones. J. Plant Res. 2015, 128, 791–802. [Google Scholar] [CrossRef] [PubMed]
- García-Palacios, P.; McKie, B.G.; Handa, I.T.; Frainer, A.; Hättenschwiler, S. The importance of litter traits and decomposers for litter decomposition: A comparison of aquatic and terrestrial ecosystems within and across biomes. Funct. Ecol. 2016, 30, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Berg, B.; Erhagen, B.; Johansson, M.-B.; Vesterdal, L.; Faituri, M.; Sanborn, P.; Nilsson, M. Manganese dynamics in decomposing needle and leaf litter—A synthesis. Can. J. For. Res. 2013, 43, 1127–1136. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.; Harmon, M.E.; Mao, J.; Pett-Ridge, J.; Kleber, M. Long-term litter decomposition controlled by manganese redox cycling. Proc. Natl. Acad. Sci. USA 2015, 112, E5253–E5260. [Google Scholar] [CrossRef] [Green Version]
- Berg, B.; Johansson, M.-B.; Liu, C.; Faituri, M.; Sanborn, P.; Vesterdal, L.; Ni, X.; Hansen, K.; Ukonmaanaho, L. Calcium in decomposing foliar litter—A synthesis for boreal and temperate coniferous forests. For. Ecol. Manag. 2017, 403, 137–144. [Google Scholar] [CrossRef]
- Dauer, J.M.; Perakis, S.S. Calcium oxalate contribution to calcium cycling in forests of contrasting nutrient status. For. Ecol. Manag. 2014, 334, 64–73. [Google Scholar] [CrossRef]
- Sun, T.; Cui, Y.; Berg, B.; Zhang, Q.; Dong, L.; Wu, Z.; Zhang, L. A test of manganese effects on decomposition in forest and cropland sites. Soil Biol. Biochem. 2019, 129, 178–183. [Google Scholar] [CrossRef]
- Yue, K.; Ni, X.; Fornara, D.A.; Peng, Y.; Liao, S.; Tan, S.; Wang, D.; Wu, F.; Yang, Y. Dynamics of calcium, magnesium, and manganese during litter decomposition in alpine forest aquatic and terrestrial ecosystems. Ecosystems 2021, 24, 516–529. [Google Scholar] [CrossRef]
- Trum, F.; Titeux, H.; Ponette, Q.; Berg, B. Influence of manganese on decomposition of common beech (Fagus sylvatica L.) leaf litter during field incubation. Biogeochemistry 2015, 125, 349–358. [Google Scholar] [CrossRef]
- Berg, B.; Sun, T.; Johansson, M.-B.; Sanborn, P.; Ni, X.; Åkerblom, S.; Lönn, M. Magnesium dynamics in decomposing foliar litter—A synthesis. Geoderma 2021, 382, 114756. [Google Scholar] [CrossRef]
- Tie, L.; Wei, S.; Peñuelas, J.; Sardans, J.; Peguero, G.; Zhou, S.; Liu, X.; Hu, J.; Huang, C. Phosphorus addition reverses the negative effect of nitrogen addition on soil arthropods during litter decomposition in a subtropical forest. Sci. Total Environ. 2021, 781, 146786. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, C.; Xiang, Y.; Xiao, Y.; Tang, J.; Han, B.; Luo, C. Response of carbon and nitrogen release to simulated nitrogen deposition in natural evergreen broad-leaved forests in a rainy area in Western China. Acta Ecol. Sin. 2017, 37, 258–264. [Google Scholar] [CrossRef]
- Xu, Z.; Tu, L.; Hu, T.; Schädler, M. Implications of greater than average increases in nitrogen deposition on the western edge of the Szechwan Basin, China. Environ. Pollut. 2013, 177, 201–202. [Google Scholar] [CrossRef]
- Tu, L.-H.; Hu, T.-X.; Zhang, J.; Li, X.-W.; Hu, H.-L.; Liu, L.; Xiao, Y.-L. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Peng, Y.; Song, S.-Y.; Li, Z.-Y.; Li, S.; Chen, G.-T.; Hu, H.-L.; Xie, J.-L.; Chen, G.; Xiao, Y.-L.; Liu, L.; et al. Influences of nitrogen addition and aboveground litter-input manipulations on soil respiration and biochemical properties in a subtropical forest. Soil Biol. Biochem. 2020, 142, 107694. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Jiang, H.; Li, X.; Gao, C. Analysis on the time series of annual precipitation in lately 50 years of Ya’an City. Sichaun For. Explor. Des. 2016, 4, 16–21. [Google Scholar]
- Berg, B.; McClaugherty, C. Plant Litter; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Parsons, S.A.; Congdon, R.A.; Lawler, I.R. Determinants of the pathways of litter chemical decomposition in a tropical region. New Phytol. 2014, 203, 873–882. [Google Scholar] [CrossRef]
- Osono, T.; Takeda, H. Potassium, calcium, and magnesium dynamics during litter decomposition in a cool temperate forest. J. For. Res. 2004, 9, 23–31. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Fan, Y.; Mo, Q.; Li, Y.; Li, Y.; Li, Z.; Wang, F. Effect of nitrogen and phosphorus addition on litter decomposition and nutrients release in a tropical forest. Plant Soil 2020, 454, 139–153. [Google Scholar] [CrossRef]
- Cromack, K.; Todd, R.L.; Monk, C.D. Patterns of basidiomycete nutrient accumulation in conifer and deciduous forest litter. Soil Biol. Biochem. 1975, 7, 265–268. [Google Scholar] [CrossRef]
- Aponte, C.; García, L.V.; Marañón, T. Tree species effect on litter decomposition and nutrient release in Mediterranean oak forests changes over time. Ecosystems 2012, 15, 1204–1218. [Google Scholar] [CrossRef] [Green Version]
- Lovett, G.M.; Arthur, M.A.; Crowley, K.F. Effects of calcium on the rate and extent of litter decomposition in a northern hardwood forest. Ecosystems 2015, 19, 87–97. [Google Scholar] [CrossRef]
- Norris, V.; Chen, M.; Goldberg, M.; Voskuil, J.; McGurk, G.; Holland, I.B. Calcium in bacteria: A solution to which problem? Mol. Microbiol. 1991, 5, 775–778. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, C. The responses of recalcitrant components to nitrogen deposition and reduced precipitation during litter decomposition. 2021; Unpublished work. [Google Scholar]
- Blair, J.M. Nutrient release from decomposing foliar litter of three tree species with spicial reference to calcium, magnesium and potassium dynamics. Plant Soil 1988, 110, 49–55. [Google Scholar] [CrossRef]
- Ma, Z.-L.; Gao, S.; Yang, W.-Q.; Wu, F.-Z. Seasonal release characteristics of Ca, Mg and Mn of foliar litter of six tree species in subtropical evergreen broadleaved forest. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2015, 26, 2913–2920. [Google Scholar]
- Lu, X.K.; Vitousek, P.M.; Mao, Q.G.; Gilliam, F.S.; Luo, Y.Q.; Zhou, G.Y.; Zou, X.; Bai, E.; Scanlon, T.M.; Hou, E.; et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest. Proc. Natl. Acad. Sci. USA 2018, 115, 5187–5192. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Chen, H.; Xiang, Y.; Zhou, S.; Huang, C. Effects of simulated nitrogen deposition on the releases of potassium, calcium, and magnesium during litter decomposition in a natural evergreen broadleaved forest in the rainy area of western China. For. Res. 2020, 33, 124–131. [Google Scholar]
- Zhou, S.; Xiang, Y.; Tie, L.; Han, B.; Huang, C. Simulated nitrogen deposition significantly reduces soil respiration in an evergreen broadleaf forest in western China. PLoS ONE 2018, 13, e0204661. [Google Scholar] [CrossRef]
- Wei, S.; Tie, L.; Liao, J.; Liu, X.; Du, M.; Lan, S.; Li, X.; Li, C.; Zhan, H.; Huang, C. Nitrogen and phosphorus co-addition stimulates soil respiration in a subtropical evergreen broad-leaved forest. Plant Soil 2020, 450, 171–182. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, S.; Tie, L.; Huang, C. The response of mesofauna to nitrogen deposition and reduced precipitation during litter decomposition. 2021; Unpublished work. [Google Scholar]
- Trentini, C.P.; Villagra, M.; Pámies, D.G.; Laborde, V.B.; Bedano, J.C.; Campanello, P. Effect of nitrogen addition and litter removal on understory vegetation, soil mesofauna, and litter decomposition in loblolly pine plantations in subtropical Argentina. For. Ecol. Manag. 2018, 429, 133–142. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.Y.H.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Salamanca, E.F.; Kaneko, N.; Katagiri, S. Rainfall manipulation effects on litter decomposition and the microbial biomass of the forest floor. Appl. Soil Ecol. 2003, 22, 271–281. [Google Scholar] [CrossRef]
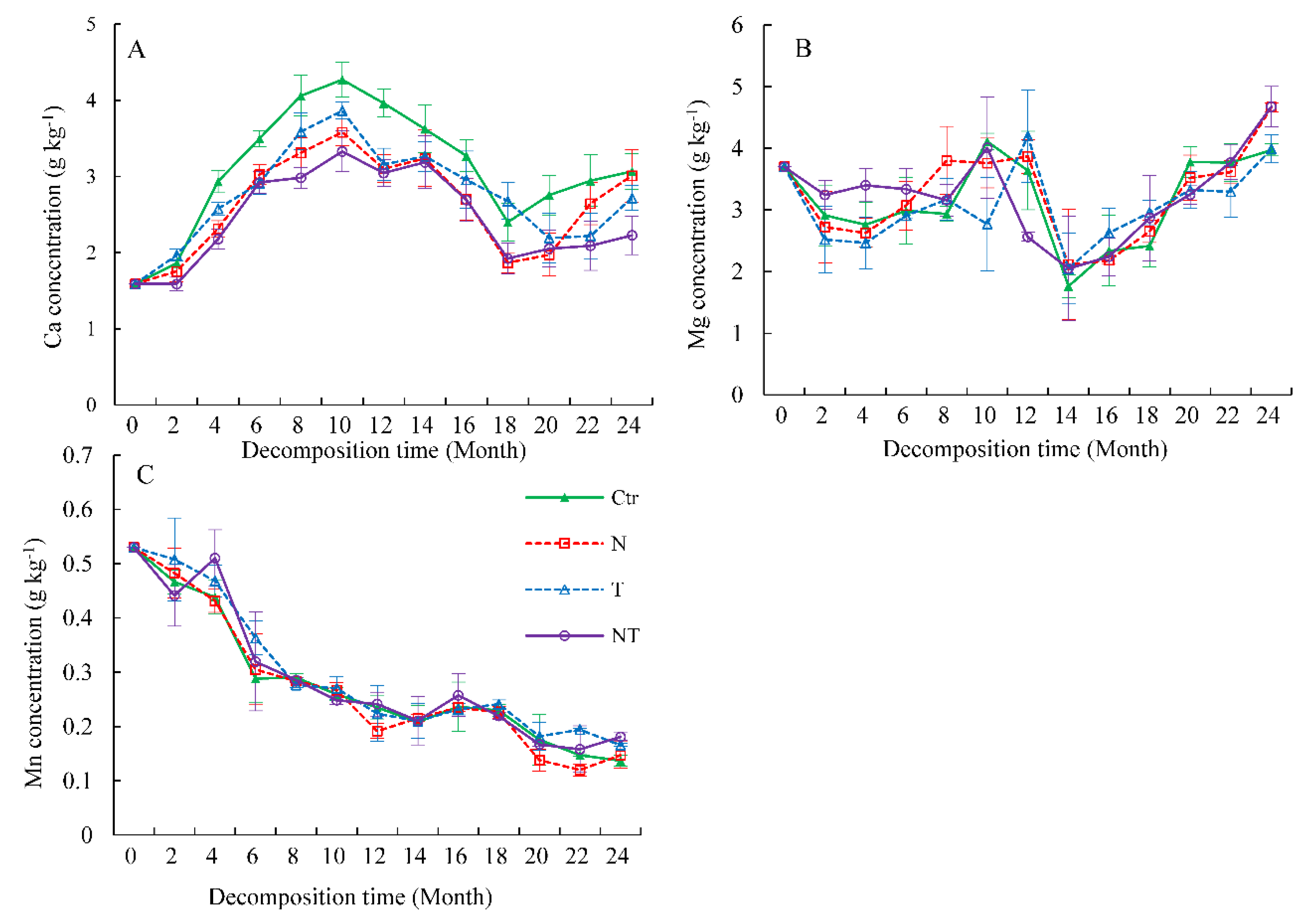
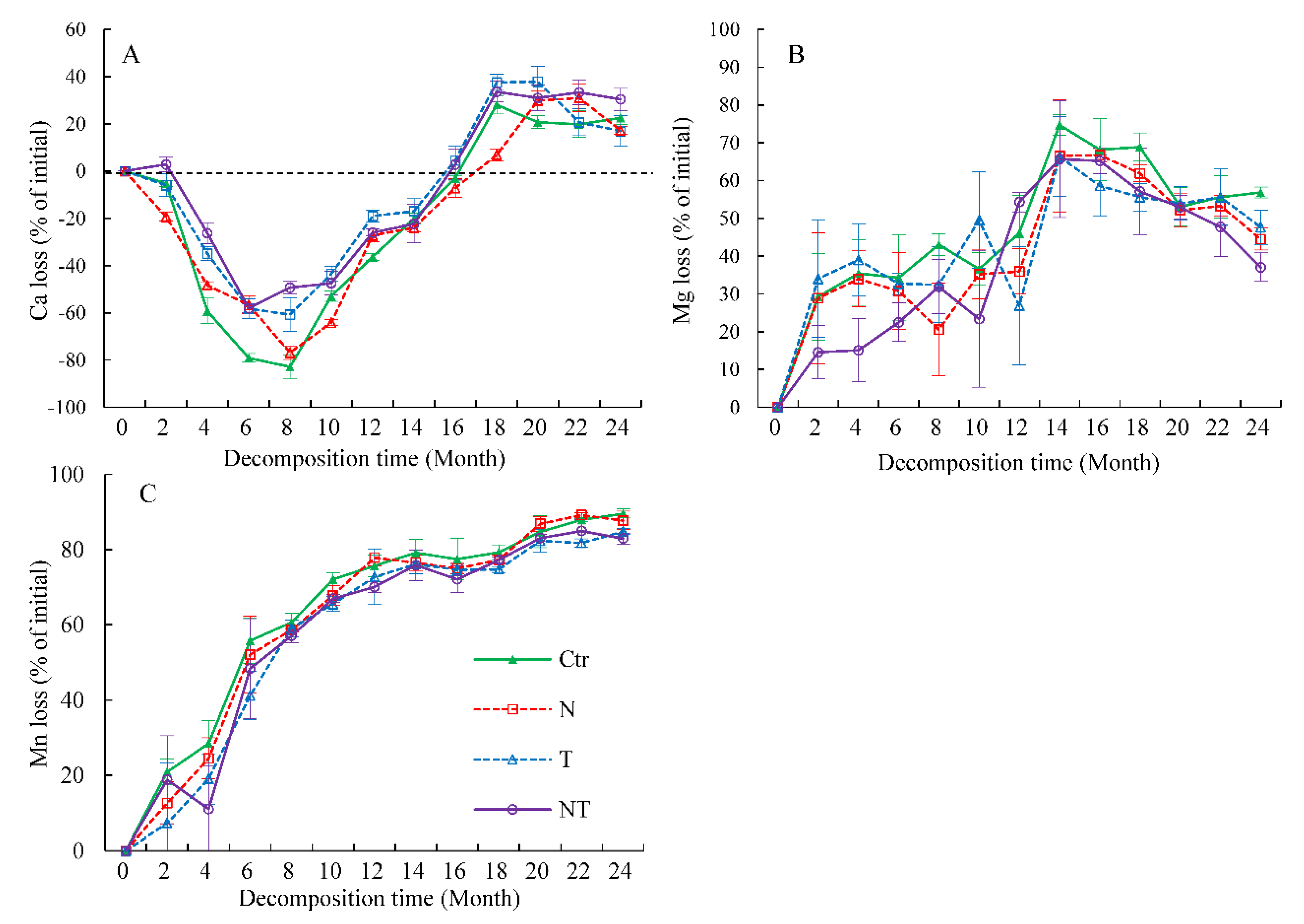
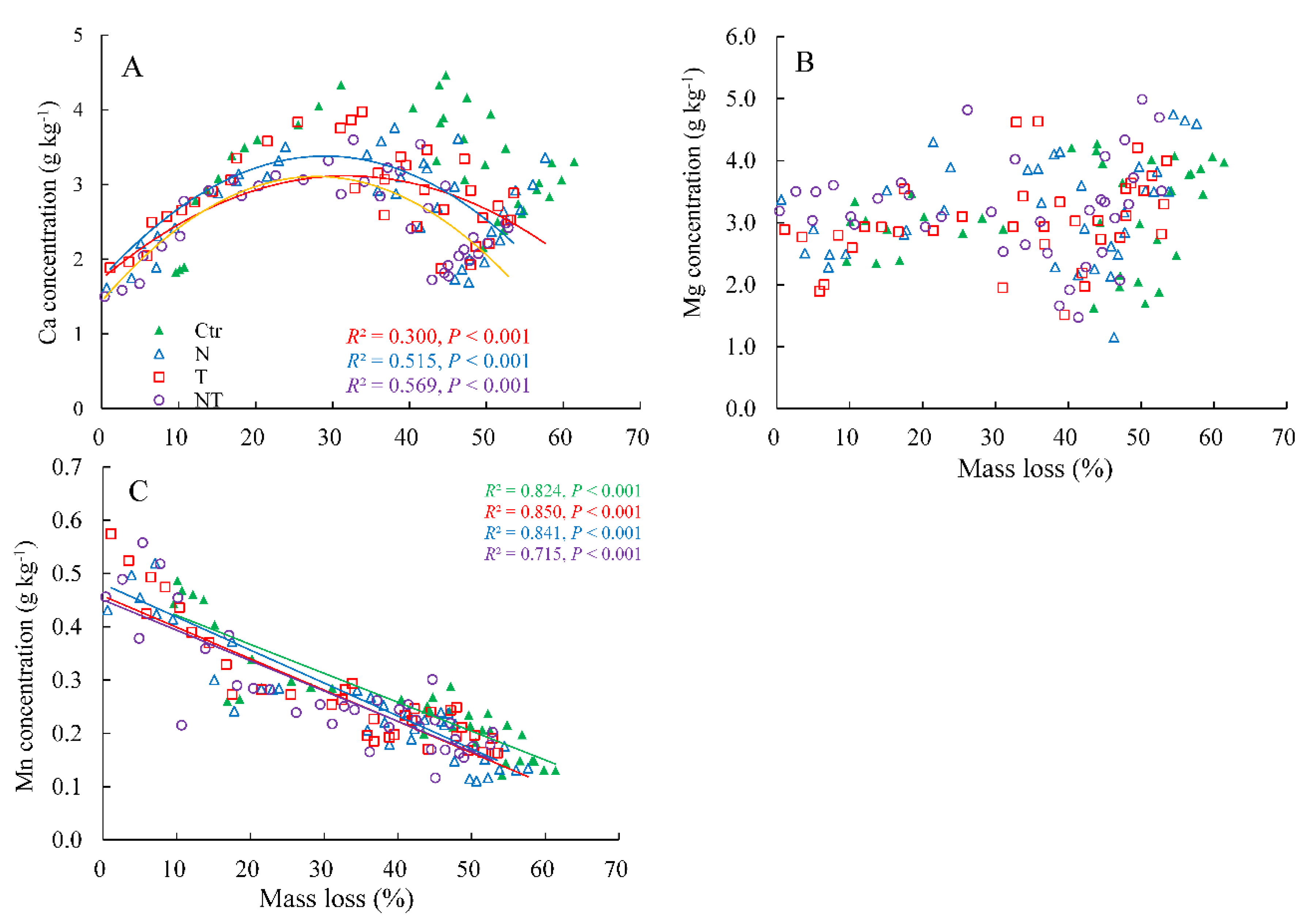
| Sources | Whole Decomposition Stage (0–24 Months) | Early Decomposition Stage (0–10 Months) | Late Decomposition Stage (10–24 Months) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | d.f. | F | p | |
| Ca concentration (g kg−1) | |||||||||
| Nitrogen deposition | 1 | 11.4 | 0.0096 | 1 | 28.4 | 0.0007 | 1 | 6.7 | 0.0322 |
| Throughfall reduction | 1 | 5.4 | 0.0490 | 1 | 10.0 | 0.0132 | 1 | 3.9 | 0.0839 |
| Sampling time | 11 | 531.6 | <0.0001 | 4 | 1539.9 | <0.0001 | 6 | 517.4 | <0.0001 |
| Nitrogen deposition × Throughfall reduction | 1 | 0.6 | 0.4629 | 1 | 0.8 | 0.4117 | 1 | 0.5 | 0.4853 |
| Nitrogen deposition × Sampling time | 11 | 11.3 | <0.0001 | 4 | 26.4 | <0.0001 | 6 | 14.8 | <0.0001 |
| Throughfall reduction × Sampling time | 11 | 18.5 | <0.0001 | 4 | 13.2 | <0.0001 | 6 | 45.0 | <0.0001 |
| Nitrogen deposition × Throughfall reduction × Sampling time | 11 | 12.1 | <0.0001 | 4 | 11.3 | <0.0001 | 6 | 27.5 | <0.0001 |
| Mg concentration (g kg−1) | |||||||||
| Nitrogen deposition | 1 | 4.2 | 0.0441 | 1 | 7.7 | 0.0244 | 1 | 0.0 | 0.9633 |
| Throughfall reduction | 1 | 0.6 | 0.4346 | 1 | 0.4 | 0.5482 | 1 | 0.1 | 0.7139 |
| Sampling time | 11 | 27.2 | <0.0001 | 4 | 8.6 | 0.0001 | 6 | 46.7 | <0.0001 |
| Nitrogen deposition × Throughfall reduction | 1 | 0.4 | 0.5317 | 1 | 5.5 | 0.0466 | 1 | 1.9 | 0.2058 |
| Nitrogen deposition × Sampling time | 11 | 2.2 | 0.0178 | 4 | 0.1 | 0.9677 | 6 | 3.1 | 0.0112 |
| Throughfall reduction × Sampling time | 11 | 1.4 | 0.1991 | 4 | 1.8 | 0.1572 | 6 | 1.5 | 0.2144 |
| Nitrogen deposition × Throughfall reduction × Sampling time | 11 | 3.6 | 0.0003 | 4 | 4.0 | 0.0099 | 6 | 2.7 | 0.0240 |
| Mn concentration (g kg−1) | |||||||||
| Nitrogen deposition | 1 | 1.4 | 0.2397 | 1 | 0.3 | 0.6153 | 1 | 1.2 | 0.3088 |
| Throughfall reduction | 1 | 10.2 | 0.0019 | 1 | 2.7 | 0.1076 | 1 | 6.8 | 0.0312 |
| Sampling time | 11 | 130.1 | <0.0001 | 4 | 74.1 | <0.0001 | 6 | 28.6 | <0.0001 |
| Nitrogen deposition × Throughfall reduction | 1 | 0.0 | 0.9183 | 1 | 0.8 | 0.3807 | 1 | 0.8 | 0.4099 |
| Nitrogen deposition × Sampling time | 11 | 0.7 | 0.7225 | 4 | 0.4 | 0.8256 | 6 | 1.7 | 0.1422 |
| Throughfall reduction × Sampling time | 11 | 1.5 | 0.1523 | 4 | 1.8 | 0.1469 | 6 | 1.5 | 0.1987 |
| Nitrogen deposition × Throughfall reduction × Sampling time | 11 | 1.2 | 0.3027 | 4 | 1.3 | 0.3020 | 6 | 1.0 | 0.4292 |
| Sources | Whole Decomposition Stage (0–24 Months) | Early Decomposition Stage (0–10 Months) | Late Decomposition Stage (10–24 Months) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | d.f. | F | p | d.f. | F | p | |
| Ca loss (% of initial) | |||||||||
| Nitrogen deposition | 1 | 25.9 | 0.0009 | 1 | 69.8 | <0.0001 | 1 | 10.0 | 0.0134 |
| Throughfall reduction | 1 | 0.4 | 0.5272 | 1 | 4.0 | 0.0799 | 1 | 0.0 | 0.9038 |
| Sampling time | 11 | 5549.2 | <0.0001 | 4 | 3021.0 | <0.0001 | 6 | 2234.9 | <0.0001 |
| Nitrogen deposition × Throughfall reduction | 1 | 0.1 | 0.7502 | 1 | 0.3 | 0.5837 | 1 | 0.0 | 0.8563 |
| Nitrogen deposition × Sampling time | 11 | 59.8 | <0.0001 | 4 | 66.5 | <0.0001 | 6 | 31.0 | <0.0001 |
| Throughfall reduction × Sampling time | 11 | 66.4 | <0.0001 | 4 | 93.8 | <0.0001 | 6 | 55.8 | <0.0001 |
| Nitrogen deposition × Throughfall reduction × Sampling time | 11 | 54.3 | <0.0001 | 4 | 87.6 | <0.0001 | 6 | 46.9 | <0.0001 |
| Mg (% of initial) | |||||||||
| Nitrogen deposition | 1 | 6.0 | 0.0400 | 1 | 11.2 | 0.0101 | 1 | 0.5 | 0.4927 |
| Throughfall reduction | 1 | 2.8 | 0.1341 | 1 | 1.0 | 0.3415 | 1 | 2.6 | 0.1484 |
| Sampling time | 11 | 38.3 | <0.0001 | 4 | 1.5 | 0.2219 | 6 | 29.2 | <0.0001 |
| Nitrogen deposition × Throughfall reduction | 1 | 0.0 | 0.9070 | 1 | 2.4 | 0.157 | 1 | 2.4 | 0.1613 |
| Nitrogen deposition × Sampling time | 11 | 2.1 | 0.0299 | 4 | 0.2 | 0.9184 | 6 | 3.0 | 0.0144 |
| Throughfall reduction × Sampling time | 11 | 0.6 | 0.8495 | 4 | 0.4 | 0.7936 | 6 | 1.0 | 0.4111 |
| Nitrogen deposition × Throughfall reduction × Sampling time | 11 | 3.7 | 0.0002 | 4 | 3.0 | 0.0335 | 6 | 3.6 | 0.0048 |
| Mn (% of initial) | |||||||||
| Nitrogen deposition | 1 | 0.3 | 0.5729 | 1 | 0.3 | 0.5908 | 1 | 0.1 | 0.7410 |
| Throughfall reduction | 1 | 15.5 | 0.0043 | 1 | 7.5 | 0.0252 | 1 | 16.1 | 0.0039 |
| Sampling time | 11 | 285.4 | <0.0001 | 4 | 134.4 | <0.0001 | 6 | 55.4 | <0.0001 |
| Nitrogen deposition × Throughfall reduction | 1 | 1.6 | 0.2474 | 1 | 2.3 | 0.1707 | 1 | 0.0 | 0.8556 |
| Nitrogen deposition × Sampling time | 11 | 0.7 | 0.7591 | 4 | 0.6 | 0.6533 | 6 | 1.5 | 0.2079 |
| Throughfall reduction × Sampling time | 11 | 1.1 | 0.3984 | 4 | 1.1 | 0.3963 | 6 | 1.0 | 0.4372 |
| Nitrogen deposition × Throughfall reduction × Sampling time | 11 | 1.4 | 0.1816 | 4 | 1.3 | 0.2802 | 6 | 1.1 | 0.3898 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Yan, G.; Hu, J.; Liu, X.; Zou, X.; Tie, L.; Yuan, R.; Yang, Y.; Xiao, L.; Cui, X.; et al. The Responses of Leaf Litter Calcium, Magnesium, and Manganese Dynamics to Simulated Nitrogen Deposition and Reduced Precipitation Vary with Different Decomposition Stages. Forests 2021, 12, 1473. https://doi.org/10.3390/f12111473
Zhou S, Yan G, Hu J, Liu X, Zou X, Tie L, Yuan R, Yang Y, Xiao L, Cui X, et al. The Responses of Leaf Litter Calcium, Magnesium, and Manganese Dynamics to Simulated Nitrogen Deposition and Reduced Precipitation Vary with Different Decomposition Stages. Forests. 2021; 12(11):1473. https://doi.org/10.3390/f12111473
Chicago/Turabian StyleZhou, Shixing, Gang Yan, Junxi Hu, Xiong Liu, Xingcheng Zou, Liehua Tie, Rongze Yuan, Yudie Yang, Lin Xiao, Xinglei Cui, and et al. 2021. "The Responses of Leaf Litter Calcium, Magnesium, and Manganese Dynamics to Simulated Nitrogen Deposition and Reduced Precipitation Vary with Different Decomposition Stages" Forests 12, no. 11: 1473. https://doi.org/10.3390/f12111473







