Fire Regime Has a Greater Impact Than Selective Timber Harvesting on Vegetation in a Sub-Tropical Australian Eucalypt Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Site and Experiment
2.2. Vegetation and Soil Sampling
2.3. Statistical Analysis
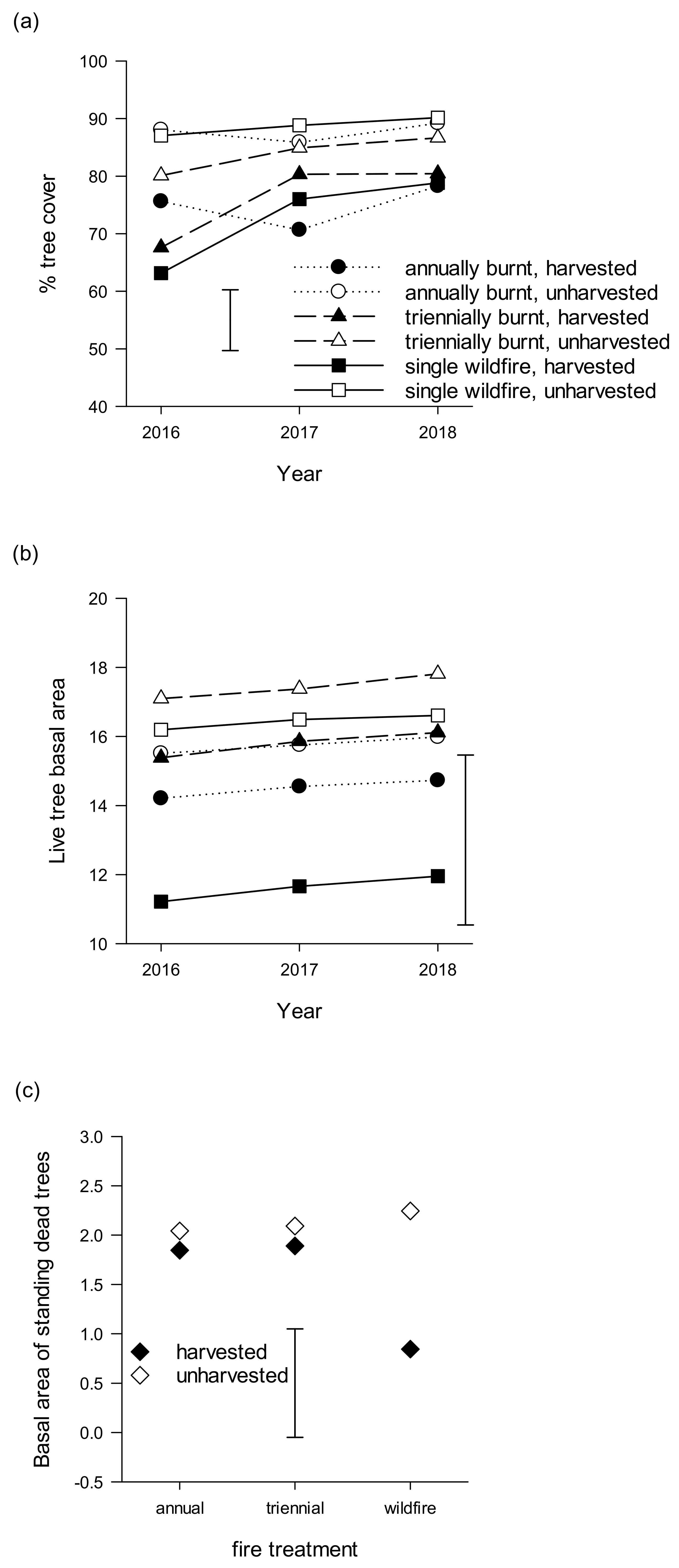
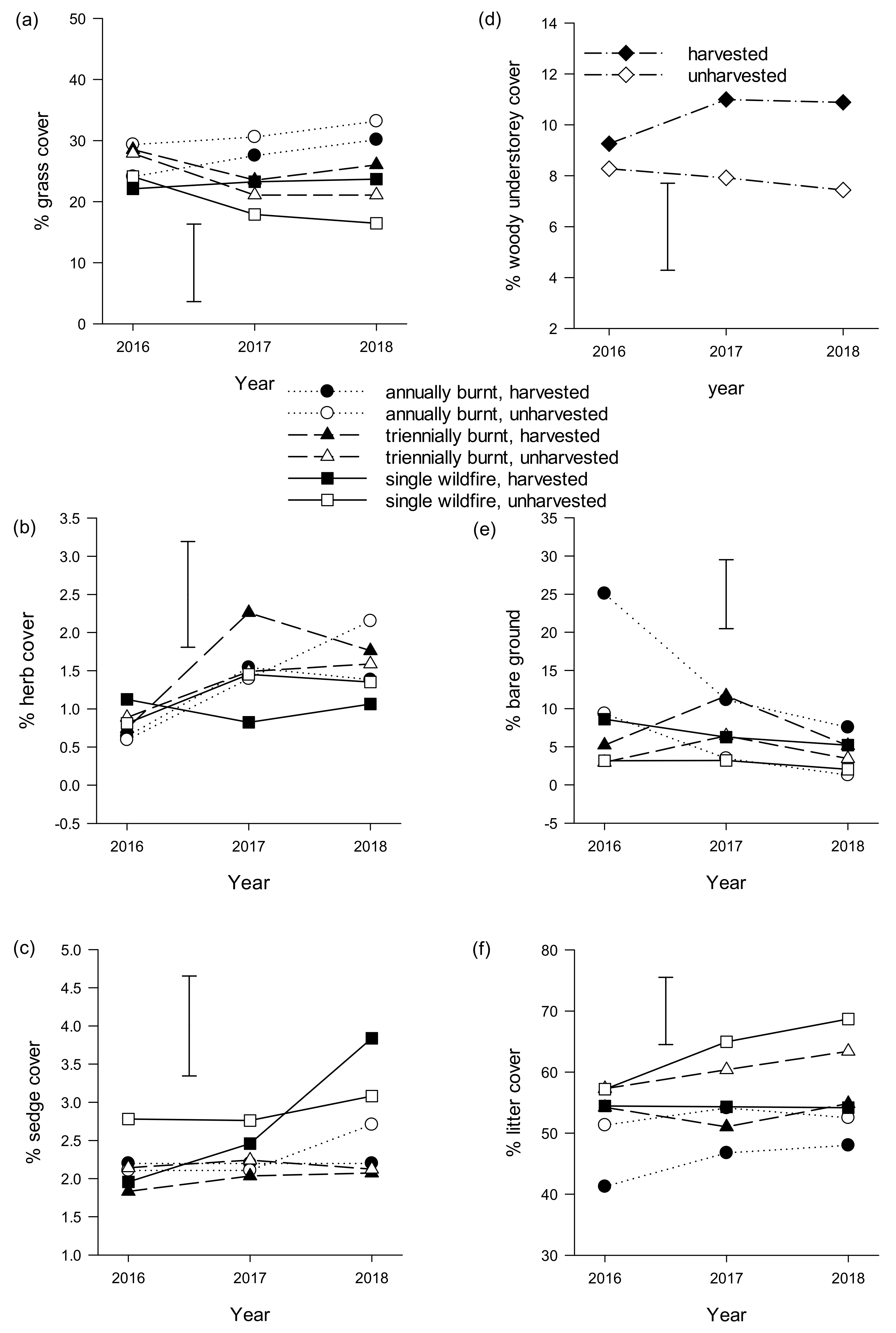
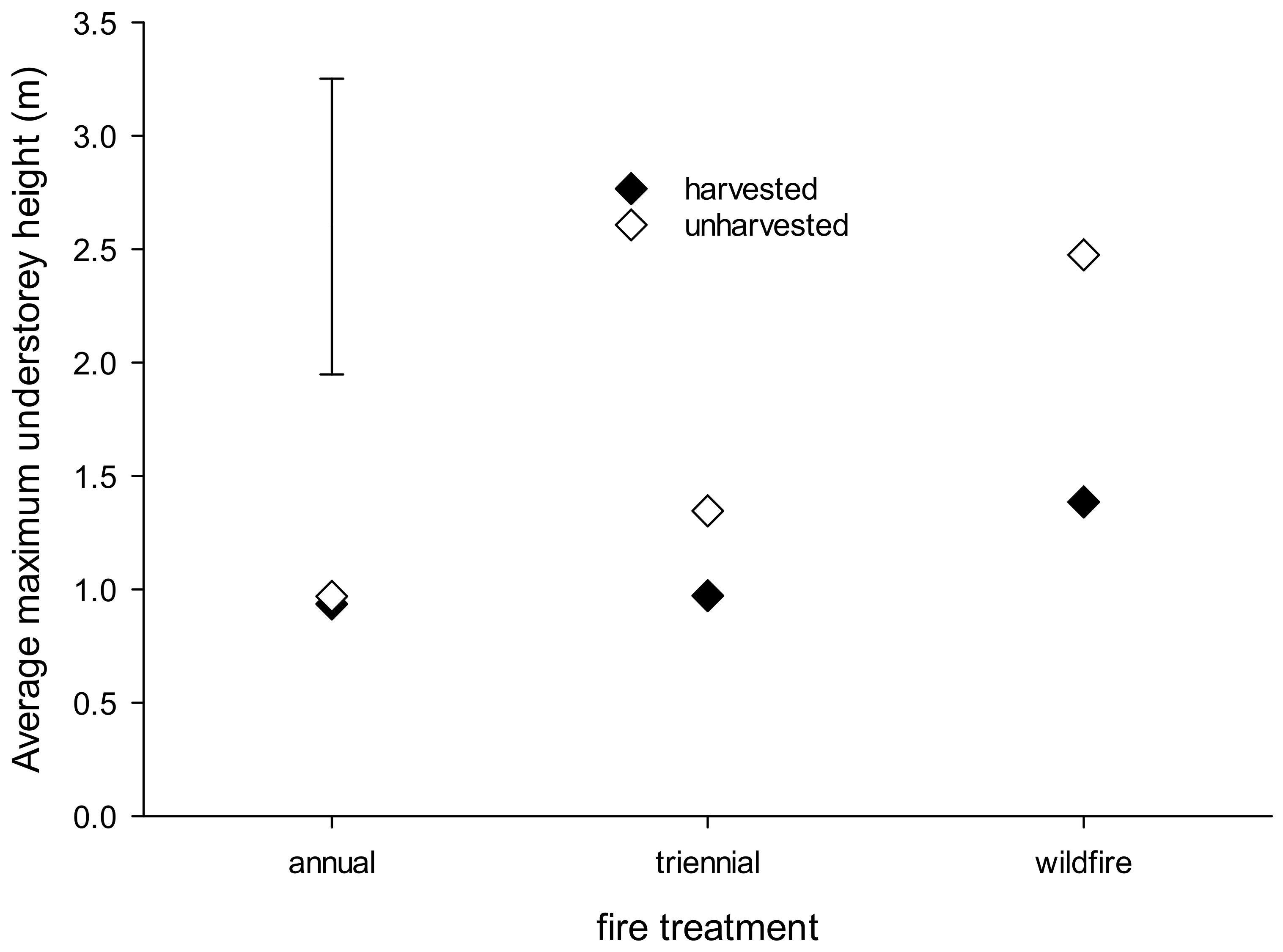
3. Results
3.1. Tree Layer
3.2. Near-Ground Temperature, Vegetation Cover, Structure and Composition
3.3. Bare Ground and Debris
3.4. Topsoil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Response Variable | Time | Fire Treatment | Harvesting | Fire × Harvesting | Time × Fire | Time × Harvesting | Time × Fire × Harvesting |
|---|---|---|---|---|---|---|---|
| Tree canopy cover (%) | <0.001 | NS | <0.001 | NS | <0.001 | <0.001 | <0.001 |
| Tree density (stems/ha) | <0.001 | NS | <0.001 | NS | NS | NS | NS |
| Tree richness (number per transect) | NS | NS | NS | NS | NS | NS | NS |
| Live tree basal area (m2/ha) | <0.001 | 0.007 | 0.010 | NS | NS | NS | NS |
| Dead tree basal area (m2/ha) | 0.013 | NS | 0.001 | 0.009 | NS | NS | NS |
| Near ground temperature (°C) | <0.001 | NS | NS | <0.001 | NS | NS | NS |
| Ground-layer biomass (t/ha) | NS | NS | 0.003 | NS | NS | NS | NS |
| Grass cover (%) | <0.001 | <0.001 | NS | 0.042 | <0.001 | <0.001 | 0.045 |
| Herb cover (%) | <0.001 | NS | NS | NS | 0.006 | NS | 0.017 |
| Sedge cover (%) | <0.001 | <0.001 | NS | NS | 0.002 | NS | 0.002 |
| Woody understorey cover (%) | NS | NS | 0.006 | NS | <0.001 | <0.001 | NS |
| Understorey plant species richness (number per transect) | NS | NS | NS | NS | NS | NS | NS |
| Understorey vegetation height (m) | NS | 0.027 | 0.004 | 0.030 | NS | NS | NS |
| Bar ground cover (%) | <0.001 | <0.001 | 0.001 | 0.005 | <0.001 | <0.001 | <0.001 |
| Litter cover (%) | <0.001 | <0.001 | <0.001 | NS | 0.026 | 0.003 | <0.001 |
| Coarse woody debris volume per transect | NS | NS | 0.004 | NS | NS | NS | NS |
| Topsoil %C | NS | 0.013 | NS | NS | NS | NS | NS |
| Topsoil %N | 0.022 | 0.030 | 0.044 | NS | NS | NS | NS |
| Topsoil effective cation exchange capacity (cmol+/Kg) | NS | NS | NS | NS | NS | NS | NS |
| Topsoil Bray P (mg/kg) | <0.001 | 0.027 | NS | NS | NS | NS | NS |
References
- Shlisky, A.; Alencar, A.A.C.; Nolasco, M.M.; Curran, L.M. Overview: Global fire regime conditions, threats, and opportunities for fire management in the tropics. In Tropical Fire Ecology; Springer: New York, NY, USA, 2009; pp. 65–83. [Google Scholar]
- Wright, S.J. The future of tropical forests. Ann. N. Y. Acad. Sci. 2010, 1195, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Stork, N.E.; Goosem, S.; Turton, S.M. Status and Threats in the Dynamic Landscapes of Northern Australia’s Tropical Rainforest Biodiversity Hotspot: The Wet Tropics. In Biodiversity Hotspots; Springer: London, UK, 2011; pp. 311–332. [Google Scholar]
- Le Page, Y.; Morton, D.; Hartin, C.; Bond-Lamberty, B.; Pereira, J.M.C.; Hurtt, G.; Asrar, G. Synergy between land use and climate change increases future fire risk in Amazon forests. Earth Syst. Dyn. 2017, 8, 1237–1246. [Google Scholar] [CrossRef]
- Florence, R. Ecology and Silviculture of Eucalypt Forests; CSIRO Publishing: Collingwood, ON, Canada, 1996. [Google Scholar]
- Metzger, F.; Schultz, J. Spring Ground Layer Vegetation 50 Years After Harvesting in Northern Hardwood Forests. Am. Midl. Nat. 1981, 105, 44. [Google Scholar] [CrossRef]
- Reader, R. Loss of species from deciduous forest understorey immediately following selective tree harvesting. Biol. Conserv. 1987, 42, 231–244. [Google Scholar] [CrossRef]
- Fredericksen, T.S.; Ross, B.D.; Hoffman, W.; Morrison, M.L.; Beyea, J.; Johnson, B.N.; Lester, M.B.; Ross, E. Short-term understory plant community responses to timber-harvesting intensity on non-industrial private forestlands in Pennsylvania. Ecol. Manag. 1999, 116, 129–139. [Google Scholar] [CrossRef]
- Schumann, M.E.; White, A.S.; Witham, J.W. The effects of harvest-created gaps on plant species diversity, composition, and abundance in a Maine oak–pine forest. Ecol. Manag. 2003, 176, 543–561. [Google Scholar] [CrossRef]
- Burke, D.M.; Elliott, K.A.; Holmes, S.B.; Bradley, D. The effects of partial harvest on the understory vegetation of southern Ontario woodlands. Ecol. Manag. 2008, 255, 2204–2212. [Google Scholar] [CrossRef]
- ABC 2019. Available online: https://www.abc.net.au/news/2018-12-09/forestry-survey-rejects-native-forest-logging/10597490 (accessed on 20 February 2019).
- Dare, L.; Schirmer, J.; Mylek, M.; Private Native Forest Owner Attitudinal Survey—Northern NSW. Understanding Forest Owners Value and Use of Their Forest Resource. Prepared for the NSW Department of Primary Industries. 2017. Available online: https://www.dpi.nsw.gov.au/forestry/private-native-forestry (accessed on 20 September 2021).
- Eyre, T.J.; Butler, D.W.; Kelly, A.L.; Wang, J. Effects of forest management on structural features important for biodiversity in mixed-age hardwood forests in Australia’s subtropics. Ecol. Manag. 2010, 259, 534–546. [Google Scholar] [CrossRef]
- Watson, G.; French, K.; Collins, L. Timber harvest and frequent prescribed burning interact to affect the demography of Eucalypt species. Ecol. Manag. 2020, 475, 118463. [Google Scholar] [CrossRef]
- Penman, T.D.; Binns, D.L.; Shiels, R.J.; Allen, R.M.; Kavanagh, R.P. Changes in understorey plant species richness following logging and prescribed burning in shrubby dry sclerophyll forests of south-eastern Australia. Austral Ecol. 2008, 33, 197–210. [Google Scholar] [CrossRef]
- Law, B.; Chidel, M.; Britton, A.; Brassil, T. Response of eastern pygmy possums, Cercartetus nanus, to selective logging in New South Wales: Home range, habitat selection and den use. Wildl. Res. 2013, 40, 470–481. [Google Scholar] [CrossRef]
- Eyre, T.J.; Ferguson, D.J.; Kennedy, M.; Rowland, J.; Maron, M. Long term thinning and logging in Australian cypress pine forest: Changes in habitat attributes and response of fauna. Biol. Conserv. 2015, 186, 83–96. [Google Scholar] [CrossRef]
- Law, B.S.; Chidel, M.; Law, P. Forest bat population dynamics over 14 years at a climate refuge: Effects of timber harvesting and weather extremes. PLoS ONE 2018, 13, e0191471. [Google Scholar] [CrossRef]
- Monarrez-Gonzalez, J.C.; Gonzalez-Elizondo, M.S.; Marquez-Linares, M.A.; Gutierrez-Yurrita, P.J.; Perez-Verdin, G. Effect of forest management on tree diversity in temperate ecosystem forests in northern Mexico. PLoS ONE 2020, 15, e0233292. [Google Scholar] [CrossRef]
- Putz, F.E.; Zuidema, P.; Synnott, T.; Peña-Claros, M.; Pinard, M.A.; Sheil, D.; Vanclay, J.; Sist, P.; Gourlet-Fleury, S.; Griscom, B.; et al. Sustaining conservation values in selectively logged tropical forests: The attained and the attainable. Conserv. Lett. 2012, 5, 296–303. [Google Scholar] [CrossRef]
- Fox, M.D.; Fox, B.J. The effect of fire frequency on the structure and floristic composition of a woodland understorey. Austral Ecol. 1986, 11, 77–85. [Google Scholar] [CrossRef]
- Whelan, R.J. The Ecology of Fire; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Peterson, D.W.; Reich, P.B. Prescribed fire in oak savanna: Fire frequency effects on stand structure and dynamics. Ecol. Appl. 2001, 11, 914–927. [Google Scholar] [CrossRef]
- Watson, P.; Wardell-Johnson, G. Fire frequency and time-since-fire effects on the open-forest and woodland flora of Girraween National Park, south-east Queensland, Australia. Austral Ecol. 2004, 29, 225–236. [Google Scholar] [CrossRef]
- Lewis, T.; Debuse, V.J. Resilience of a eucalypt forest woody understorey to long-term (34–55 years) repeated burning in subtropical Australia. Int. J. Wildland Fire 2012, 21, 980–991. [Google Scholar] [CrossRef]
- Russell, M.; Roberts, B. Effects of Four Low-Intensity Burns Over 14 Years on the Floristics of a Blackbutt (Eucalyptus pilularis) Forest in Southern Queensland. Aust. J. Bot. 1996, 44, 315–329. [Google Scholar] [CrossRef]
- Russell-Smith, J.; Whitehead, P.J.; Cook, G.D.; Hoare, J.L. Response of Eucalyptus-Dominated Savanna to Frequent Fires: Lessons from Munmarlary, 1973–1996. Ecol. Monogr. 2003, 73, 349–375. [Google Scholar] [CrossRef]
- Fairfax, R.; Fensham, R.; Butler, D.; Quinn, K.; Sigley, B.; Holman, J. Effects of multiple fires on tree invasion in montane grasslands. Landsc. Ecol. 2009, 24, 1363–1373. [Google Scholar] [CrossRef]
- Lewis, T.; Reif, M.; Prendergast, E.; Tran, C. The effect of long-term repeated burning and fire exclusion on above- and below-ground Blackbutt (Eucalyptus pilularis) forest vegetation assemblages. Austral Ecol. 2012, 37, 767–778. [Google Scholar] [CrossRef]
- Pellegrini, A.F.A.; Refsland, T.; Averill, C.; Terrer, C.; Staver, A.C.; Brockway, D.G.; Caprio, A.; Clatterbuck, W.; Coetsee, C.; Haywood, J.D.; et al. Decadal changes in fire frequencies shift tree communities and functional traits. Nat. Ecol. Evol. 2021, 5, 504–512. [Google Scholar] [CrossRef]
- Reich, P.B.; Peterson, D.W.; Wedin, D.A.; Wrage, K. Fire and vegetation effects on productivity and nitrogen cycling across a forest–grassland continuum. Ecology 2001, 82, 1703–1719. [Google Scholar] [CrossRef]
- Burton, J.A.; Hallgren, S.W.; Fuhlendorf, S.D.; Leslie, D.M. Understory response to varying fire frequencies after 20 years of prescribed burning in an upland oak forest. Plant Ecol. 2011, 212, 1513–1525. [Google Scholar] [CrossRef]
- Spencer, R.; Baxter, G.S. Effects of fire on the structure and composition of open eucalypt forests. Austral Ecol. 2006, 31, 638–646. [Google Scholar] [CrossRef]
- Burgess, E.E.; Moss, P.; Haseler, M.; Maron, M. The influence of a variable fire regime on woodland structure and composition. Int. J. Wildland Fire 2015, 24, 59–69. [Google Scholar] [CrossRef]
- Collins, L.; Bradstock, R.; Ximenes, F.; Horsey, B.; Sawyer, R.; Penman, T. Aboveground forest carbon shows different responses to fire frequency in harvested and unharvested forests. Ecol. Appl. 2019, 29, e01815. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Hughes, T.P. Catastrophes, Phase Shifts, and Large-Scale Degradation of a Caribbean Coral Reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Paine, R.T.; Tegner, M.J.; Johnson, E.A. Compounded Perturbations Yield Ecological Surprises. Ecosystems 1998, 1, 535–545. [Google Scholar] [CrossRef]
- Keane, R.E.; Loehman, R.; Clark, J.; Smithwick, E.A.H.; Miller, C. Exploring Interactions among Multiple Disturbance Agents in Forest Landscapes: Simulating Effects of Fire, Beetles, and Disease under Climate Change. In Simulation Modeling of Forest Landscape Disturbances; Perera, A.H., Sturtevant, B.R., Buse, L.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 201–231. [Google Scholar]
- Buma, B.; Wessman, C.A. Disturbance interactions can impact resilience mechanisms of forests. Ecosphere 2011, 2, art64. [Google Scholar] [CrossRef]
- Guinto, D.F.; Xu, Z.H.; House, A.P.N.; Saffigna, P.G. Soil chemical properties and forest floor nutrients under repeated prescribed-burning in eucalypt forests of south-east Queensland, Australia. N. Z. J. For. Sci. 2001, 31, 170–187. [Google Scholar]
- Lemmon, P.E. A spherical densiometer for estimating forest overstory density. For. Sci. 1956, 2, 314–320. [Google Scholar] [CrossRef]
- Goodall, D. Some Considerations in the Use of Point Quadrats for the Analysis of Vegetation. Aust. J. Biol. Sci. 1952, 5, 1–41. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods–Australasia; CSIRO Publishing: Melbourne, Australia, 2010. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Biometris, Wageningen University and Research Centre: Wageningen, The Netherlands, 2002. [Google Scholar]
- Legendre, P. Spatial Autocorrelation: Trouble or New Paradigm? Ecology 1993, 74, 1659–1673. [Google Scholar] [CrossRef]
- Department of Environment and Science. Code of Practice for Native Forest Timber Production on Queensland’s State Forest Estate 2020. 2020. Available online: https://parks.des.qld.gov.au/__data/assets/pdf_file/0012/160104/cop-native-forest-timber-production-qpws-estate.pdf (accessed on 20 September 2021).
- Webb, A.A. Can timber and water resources be sustainably co-developed in south-eastern New South Wales, Australia? Environ. Dev. Sustain. 2012, 14, 233–252. [Google Scholar] [CrossRef]
- Harmon, M.E.; Franklin, J.F.; Swanson, F.J.; Sollins, P.; Gregory, S.; Lattin, J.; Anderson, N.H.; Cline, S.P.; Aumen, N.G.; Sedell, J.; et al. Ecology of coarse woody debris in temperate ecosystems. Adv. Ecol. Res. 2004, 34, 59–234. [Google Scholar] [CrossRef]
- Grove, S. Tree basal area and dead wood as surrogate indicators of saproxylic insect faunal integrity: A case study from the Australian lowland tropics. Ecol. Indic. 2002, 1, 171–188. [Google Scholar] [CrossRef]
- Eyre, T.J. Hollow-bearing trees in large glider habitat in south-east Queensland, Australia: Abundance, spatial distribution and management. Pac. Conserv. Biol. 2005, 11, 23–37. [Google Scholar] [CrossRef]
- Butts, S.R.; McComb, W.C. Associations of Forest-Floor Vertebrates with Coarse Woody Debris in Managed Forests of Western Oregon. J. Wildl. Manag. 2000, 64, 95. [Google Scholar] [CrossRef]
- Mac Nally, R.; Parkinson, A.; Horrocks, G.; Conole, L.; Tzaros, C. Relationships between terrestrial vertebrate diversity, abundance and availability of coarse woody debris on south-eastern Australian floodplains. Biol. Conserv. 2001, 99, 191–205. [Google Scholar] [CrossRef]
- Grove, S.; Meggs, J. Coarse woody debris, biodiversity and management: A review with particular reference to Tasmanian wet eucalypt forests. Aust. For. 2003, 66, 258–272. [Google Scholar] [CrossRef]
- Savadogo, P.; Tiveau, D.; Sawadogo, L.; Tigabu, M. Herbaceous species responses to long-term effects of prescribed fire, grazing and selective tree cutting in the savanna-woodlands of West Africa. Perspect. Plant Ecol. Evol. Syst. 2008, 10, 179–195. [Google Scholar] [CrossRef]
- Barefoot, C.R.; Willson, K.G.; Hart, J.L.; Schweitzer, C.J.; Dey, D.C. Effects of thinning and prescribed fire frequency on ground flora in mixed Pinus-hardwood stands. Ecol. Manag. 2019, 432, 729–740. [Google Scholar] [CrossRef]
- Franklin, C.M.; Nielsen, S.E.; Macdonald, S.E. Understory vascular plant responses to retention harvesting with and without prescribed fire. Can. J. Res. 2019, 49, 1087–1100. [Google Scholar] [CrossRef]
- O’Hara, K.; Ramage, B. Silviculture in an uncertain world: Utilizing multi-aged management systems to integrate disturbance. For. Int. J. For. Res. 2013, 86, 401–410. [Google Scholar] [CrossRef]
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Gonzalez-Olabarria, J.R.; Koricheva, J.; Meurisse, N.; Brockerhoff, E.G. Tree Diversity Drives Forest Stand Resistance to Natural Disturbances. Curr. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- VanDerWoude, C.; De Bruyn, L.A.L.; House, A.P.N. Long-term ant community responses to selective harvesting of timber from Spotted Gum (Corymbia variegata)-dominated forests in south-east Queensland. Ecol. Manag. Restor. 2000, 1, 204–214. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, S.Q.; Liu, S.; Oeding, J. A meta-analysis on the impacts of partial cutting on forest structure and carbon storage. Biogeosciences 2013, 10, 3691–3703. [Google Scholar] [CrossRef]
- Deal, R.L. The effects of partial cutting on forest plant communities of western hemlock—Sitka spruce stands in southeast Alaska. Can. J. Res. 2001, 31, 2067–2079. [Google Scholar] [CrossRef]
- Kern, C.C.; Palik, B.J.; Strong, T.F. Ground-layer plant community responses to even-age and uneven-age silvicultural treatments in Wisconsin northern hardwood forests. Ecol. Manag. 2006, 230, 162–170. [Google Scholar] [CrossRef]
- Bradstock, R.; Keith, D.; Auld, T. Fire and Conservation: Imperatives and Constraints on Managing for Diversity. In Conserving Biodiversity: Threats and Solutions; Bradstock, R.A., Auld, T.D., Keith, D.A., Kingsford, R.T., Lunney, D., Sivertsen, D.P., Eds.; Surrey Beatty & Sons: Sydney, Australia, 1995; pp. 323–333. [Google Scholar]
- Morrison, D.A.; Cary, G.J.; Pengelly, S.M.; Ross, D.G.; Mullins, B.J.; Thomas, C.R.; Anderson, T.S. Effects of fire frequency on plant species composition of sandstone communities in the Sydney region: Inter-fire interval and time-since-fire. Aust. J. Ecol. 1995, 20, 239–247. [Google Scholar] [CrossRef]
- Brockway, D.G.; Lewis, C.E. Long-term effects of dormant-season prescribed fire on plant community diversity, structure and productivity in a longleaf pine wiregrass ecosystem. Ecol. Manag. 1997, 96, 167–183. [Google Scholar] [CrossRef]
- Williams, P.R.; Congdon, R.A.; Grice, A.C.; Clarke, P.J. Effect of fire regime on plant abundance in a tropical eucalypt savanna of north-eastern Australia. Austral Ecol. 2003, 28, 327–338. [Google Scholar] [CrossRef]
- Pellegrini, A.; Ahlström, A.; Hobbie, S.E.; Reich, P.; Nieradzik, L.P.; Staver, A.C.; Scharenbroch, B.C.; Jumpponen, A.; Anderegg, W.R.L.; Randerson, J.T.; et al. Fire frequency drives decadal changes in soil carbon and nitrogen and ecosystem productivity. Nat. Cell Biol. 2018, 553, 194–198. [Google Scholar] [CrossRef]
- Lewis, T. Very frequent burning encourages tree growth in sub-tropical Australian eucalypt forest. Ecol. Manag. 2020, 459, 117842. [Google Scholar] [CrossRef]
- Guinto, D.F.; Xu, Z.H.; Saffigna, P.G.; House, A.P.N.; Perera, M.C.S. Soil nitrogen mineralisation and organic matter composition revealed by 13C NMR spectroscopy under repeated prescribed burning in eucalypt forests of south-east Queensland. Soil Res. 1999, 37, 123. [Google Scholar] [CrossRef]
- Muqaddas, B.; Zhou, X.; Lewis, T.; Wild, C.; Chen, C. Long-term frequent prescribed fire decreases surface soil carbon and nitrogen pools in a wet sclerophyll forest of Southeast Queensland, Australia. Sci. Total Environ. 2015, 536, 39–47. [Google Scholar] [CrossRef]
- Muqaddas, B.; Lewis, T.; Esfandbod, M.; Chen, C. Responses of labile soil organic carbon and nitrogen pools to long-term prescribed burning regimes in a wet sclerophyll forest of southeast Queensland, Australia. Sci. Total Environ. 2019, 647, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Butler, O.; Elser, J.J.; Lewis, T.; Mackey, B.; Chen, C. The phosphorus-rich signature of fire in the soil-plant system: A global meta-analysis. Ecol. Lett. 2018, 21, 335–344. [Google Scholar] [CrossRef] [PubMed]


| Product Type | Annually Burnt | Triennially Burnt | No Prescribed Fire (Single Wildfire) |
|---|---|---|---|
| Compulsory sawlog 1 | 9.7 m3/ha | 13.2 m3/ha | 19.0 m3/ha |
| Optional sawlog 2 | 4.5 m3/ha | 4.3 m3/ha | 3.2 m3/ha |
| Salvage (landscaping) | 3.8 m3/ha | 2.2 m3/ha | 3.0 m3/ha |
| Split posts | nil | nil | 32.4 pieces/ha |
| Round posts | nil | nil | 2.9 lm/ha |
| Poles | 27.4 lm/ha | 21 lm/ha | nil |
| Girders | 2.9 lm/ha | 5.6 lm/ha | 4.0 lm/ha |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, T.; Menzies, T.; Pachas, A.N. Fire Regime Has a Greater Impact Than Selective Timber Harvesting on Vegetation in a Sub-Tropical Australian Eucalypt Forest. Forests 2021, 12, 1478. https://doi.org/10.3390/f12111478
Lewis T, Menzies T, Pachas AN. Fire Regime Has a Greater Impact Than Selective Timber Harvesting on Vegetation in a Sub-Tropical Australian Eucalypt Forest. Forests. 2021; 12(11):1478. https://doi.org/10.3390/f12111478
Chicago/Turabian StyleLewis, Tom, Tracey Menzies, and Anibal Nahuel Pachas. 2021. "Fire Regime Has a Greater Impact Than Selective Timber Harvesting on Vegetation in a Sub-Tropical Australian Eucalypt Forest" Forests 12, no. 11: 1478. https://doi.org/10.3390/f12111478
APA StyleLewis, T., Menzies, T., & Pachas, A. N. (2021). Fire Regime Has a Greater Impact Than Selective Timber Harvesting on Vegetation in a Sub-Tropical Australian Eucalypt Forest. Forests, 12(11), 1478. https://doi.org/10.3390/f12111478






