Timing of Drought Affected the Growth, Physiology, and Mortality of Mongolian Pine Saplings
Abstract
:1. Introduction
2. Materials and Methods
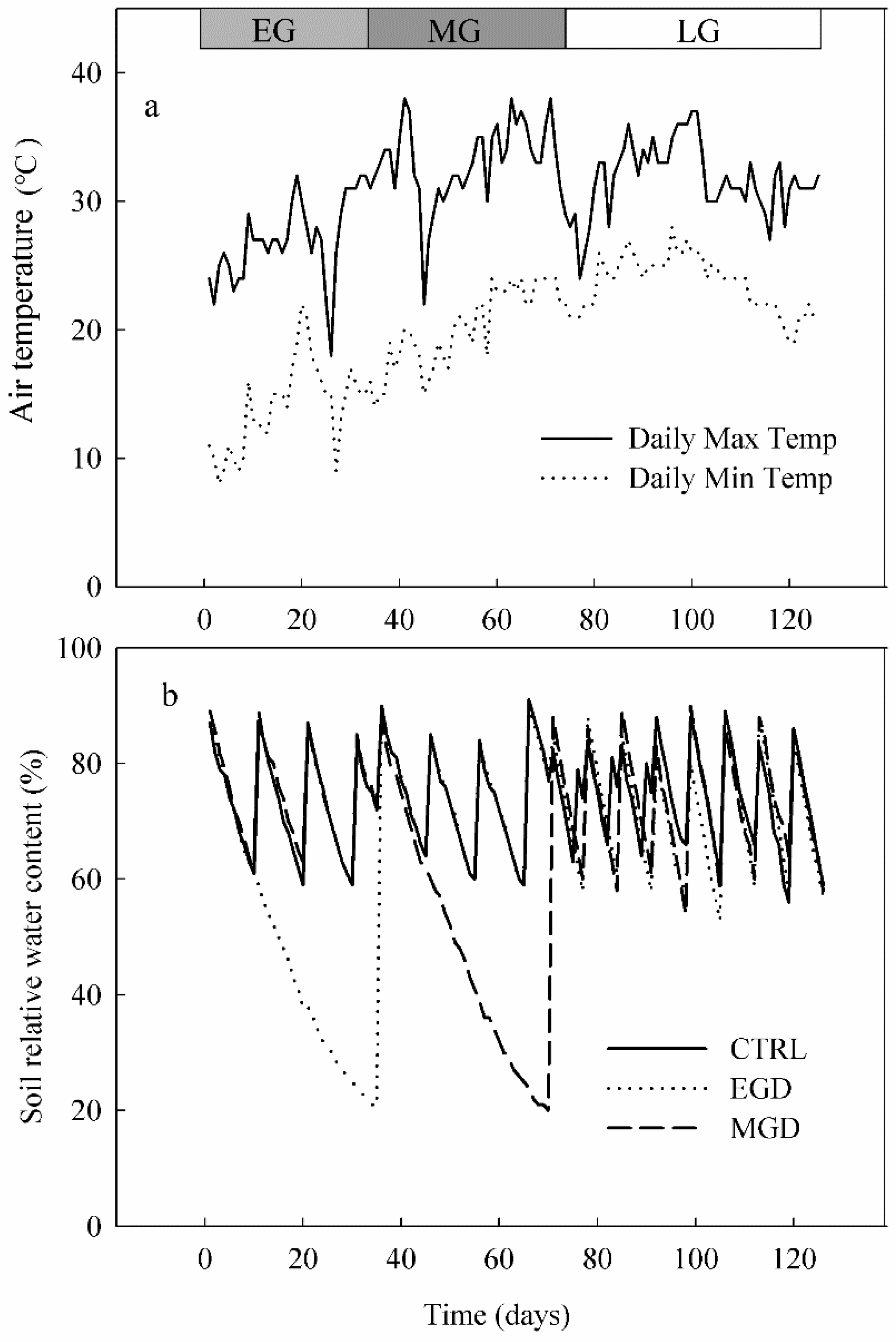
2.1. Plant Cultivation and Drought Treatments
2.2. Physiological and Growth Measurements
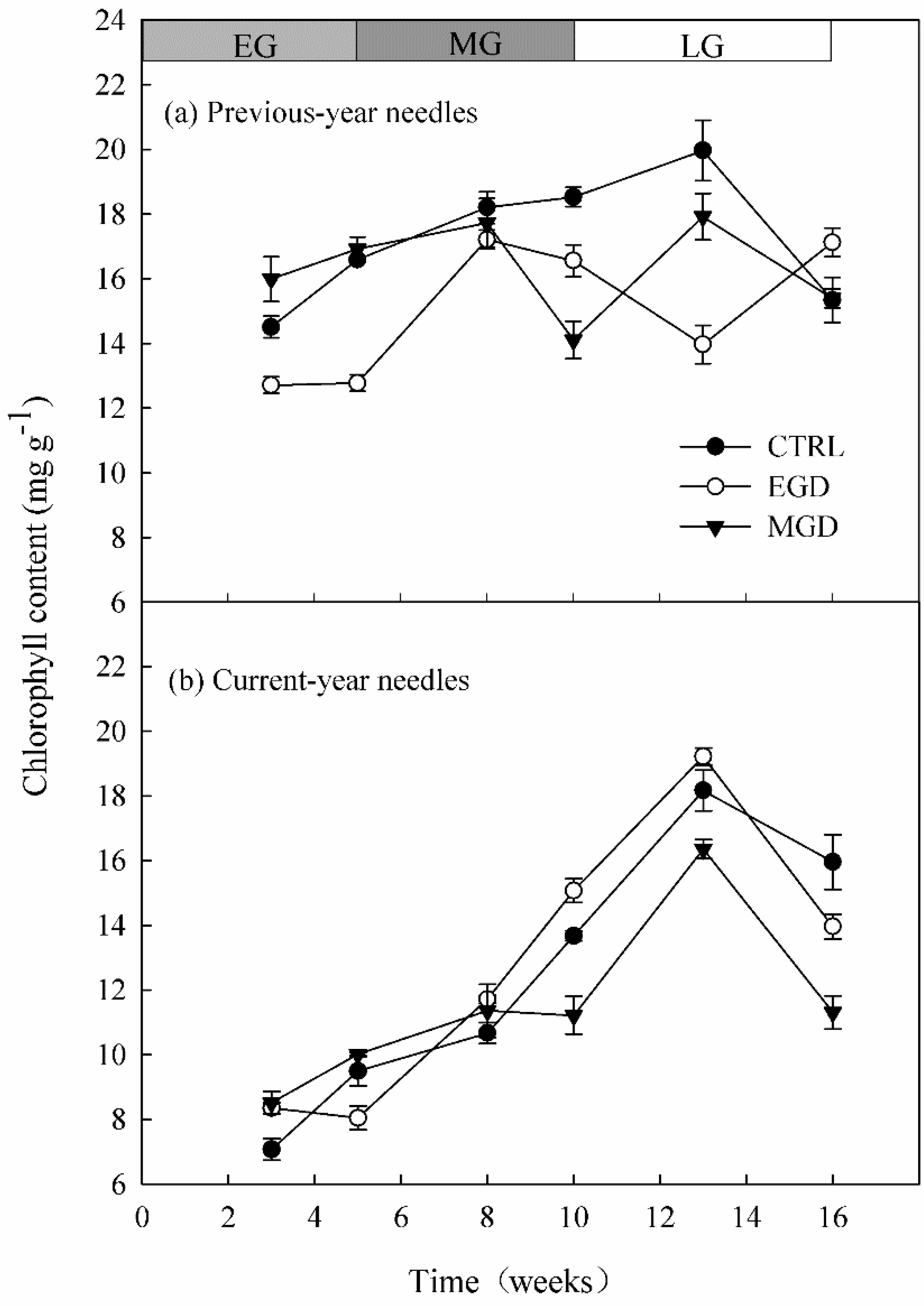
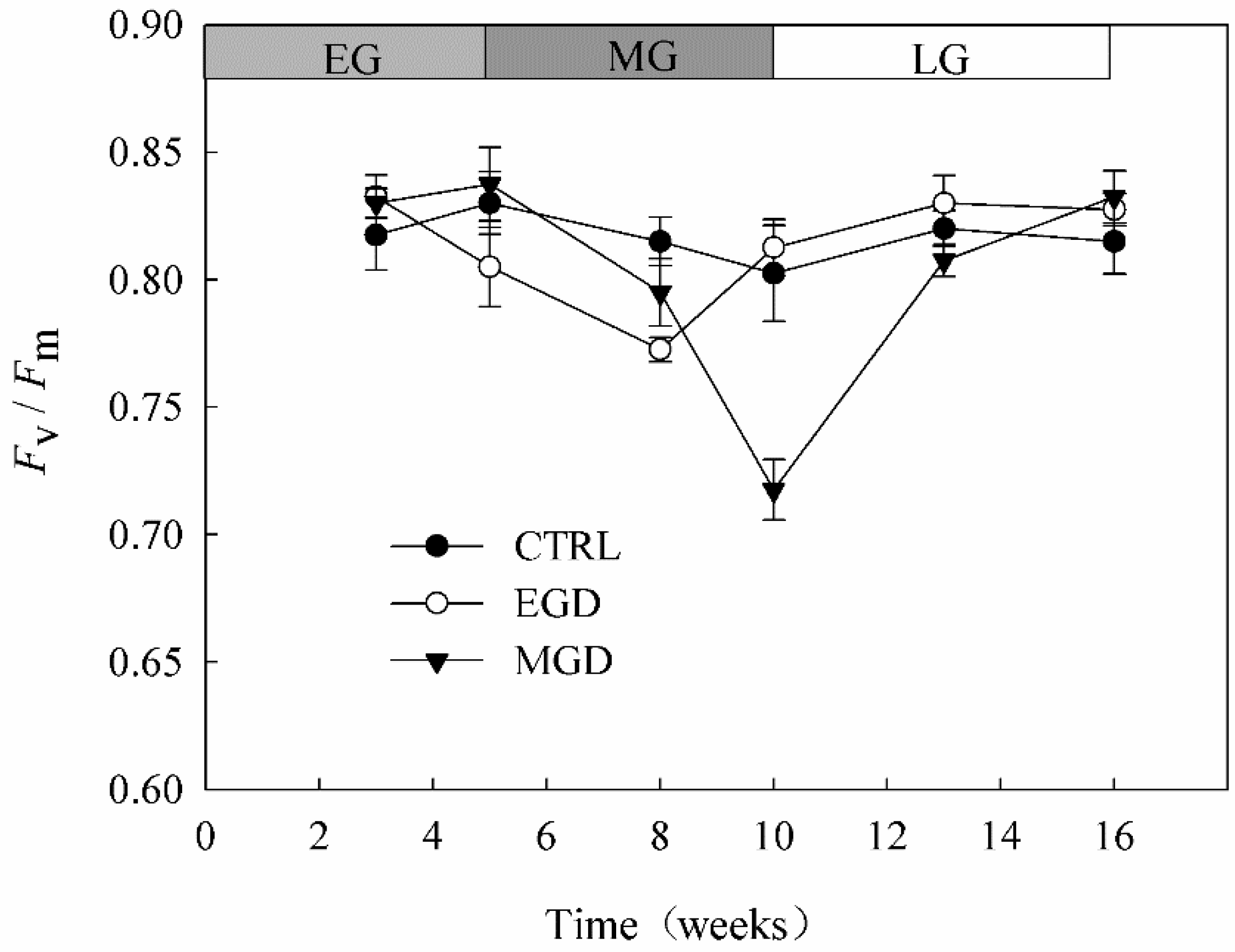
2.2.1. Chlorophyll Content and Chlorophyll Fluorescence of Needles
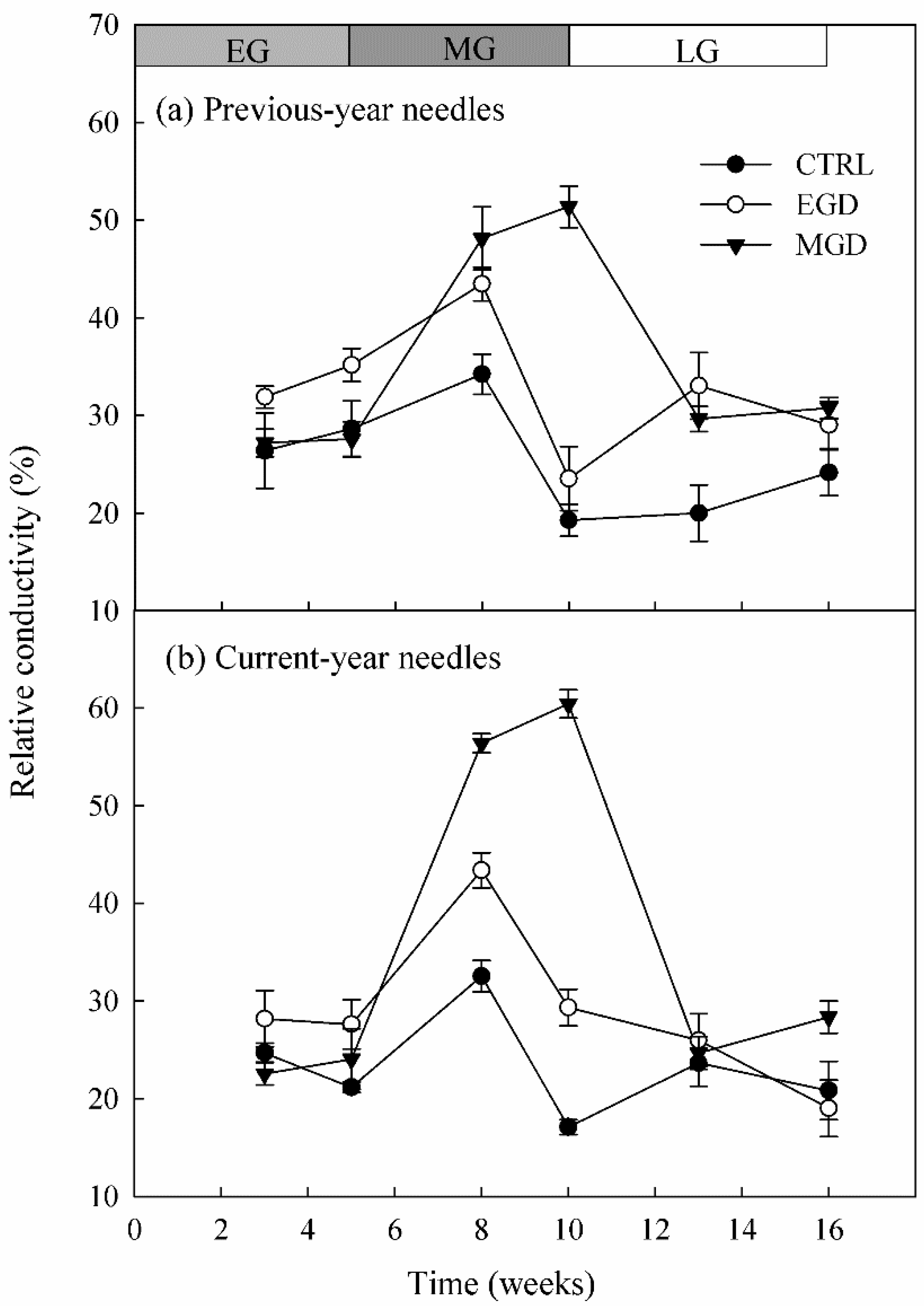
2.2.2. Relative Electrolyte Leakage (REL) of Needles
2.2.3. Soluble Sugar and Starch Content
2.2.4. Abscisic Acid (ABA) Determination
2.2.5. Sapling Growth
2.2.6. Sapling Mortality
2.3. Statistical Analysis
3. Results
3.1. Chlorophyll Content and Chlorophyll Fluorescence of Needles
3.2. Relative Electrolyte Leakage (REL) of Needles
3.3. Non-Structural Carbohydrates (NSC) in Roots, Stems and Needles
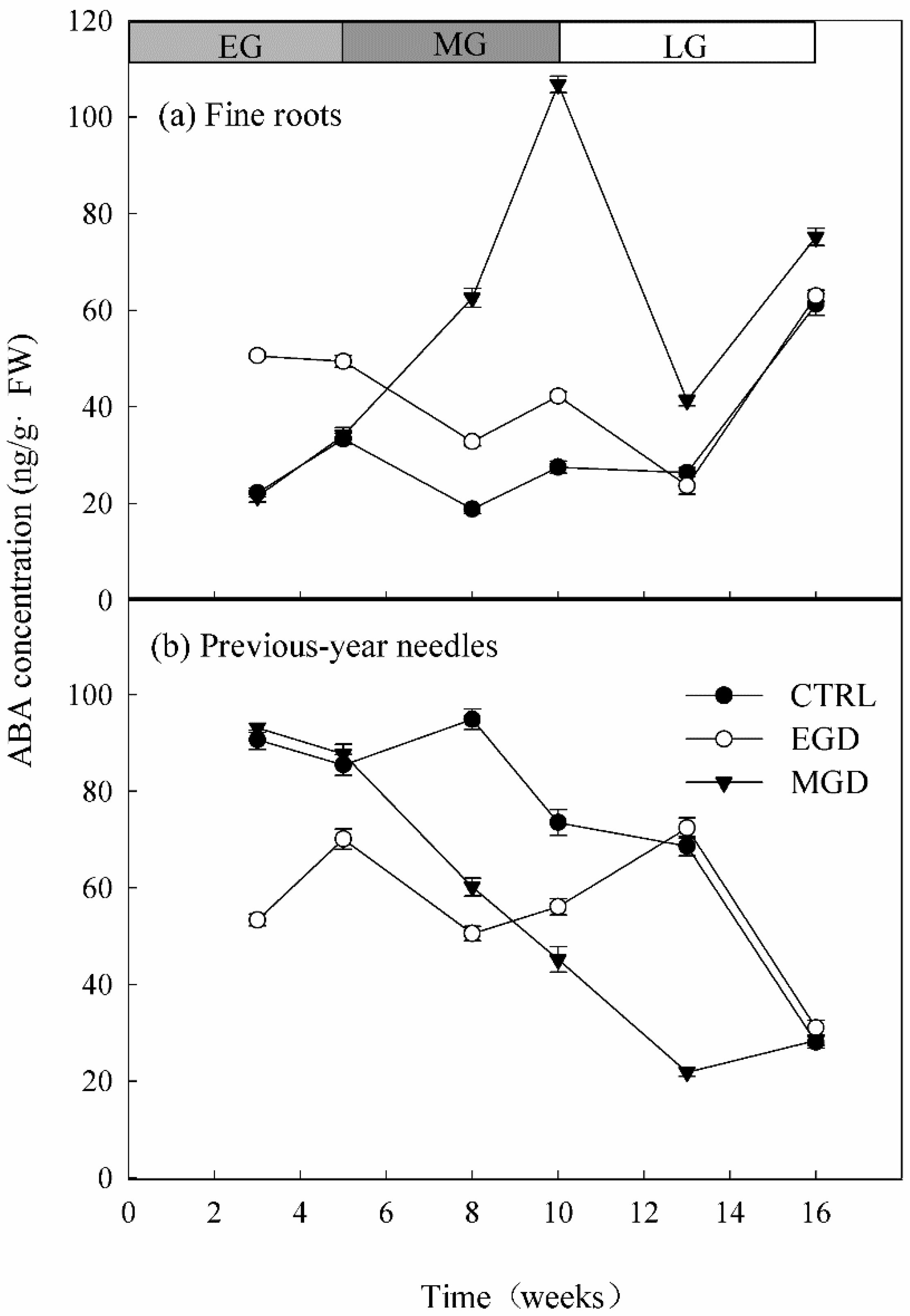
3.4. ABA Concentration in Roots and Needles
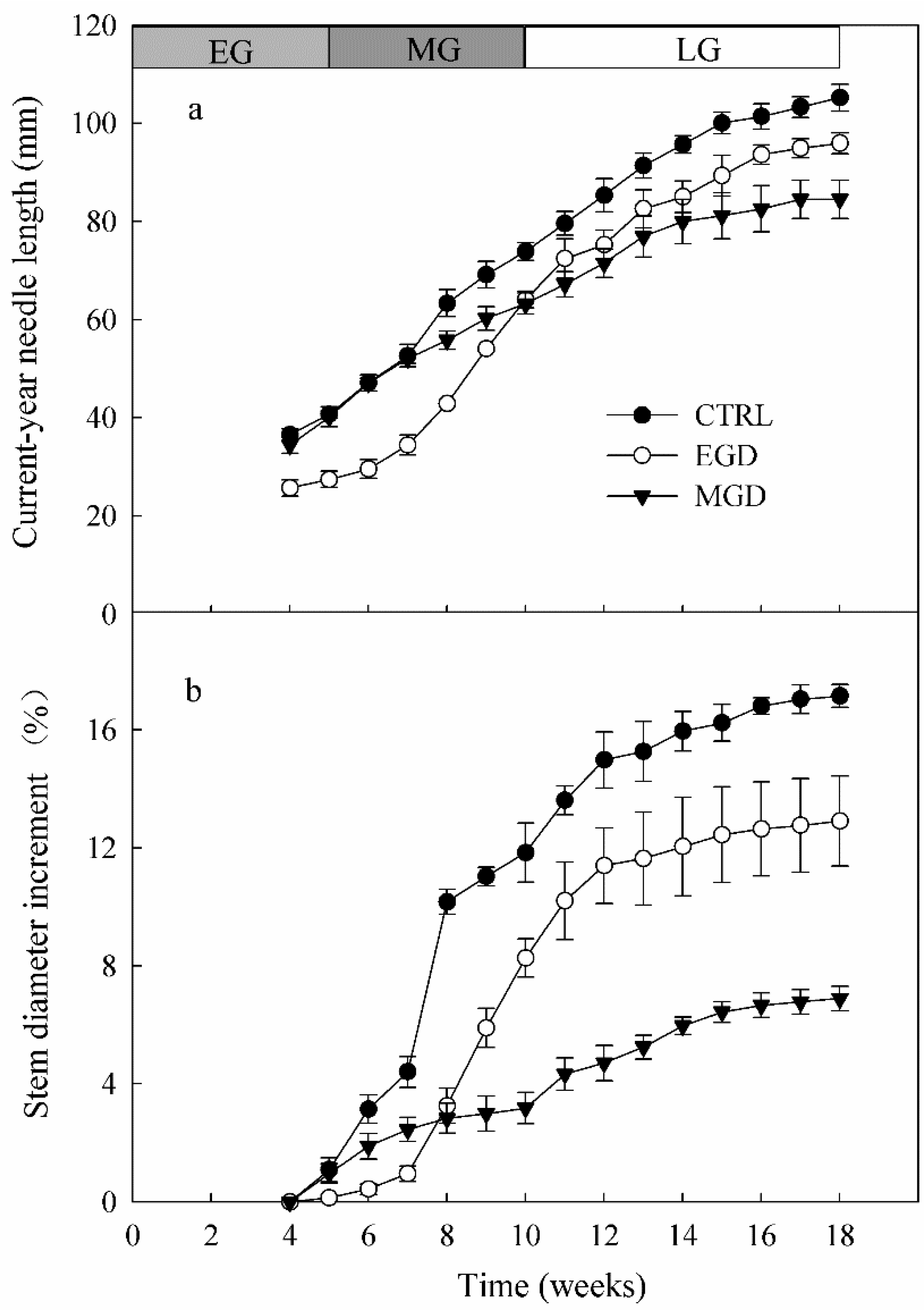
3.5. Length of Current-Year Needles
3.6. Basal Stem Diameter Increment
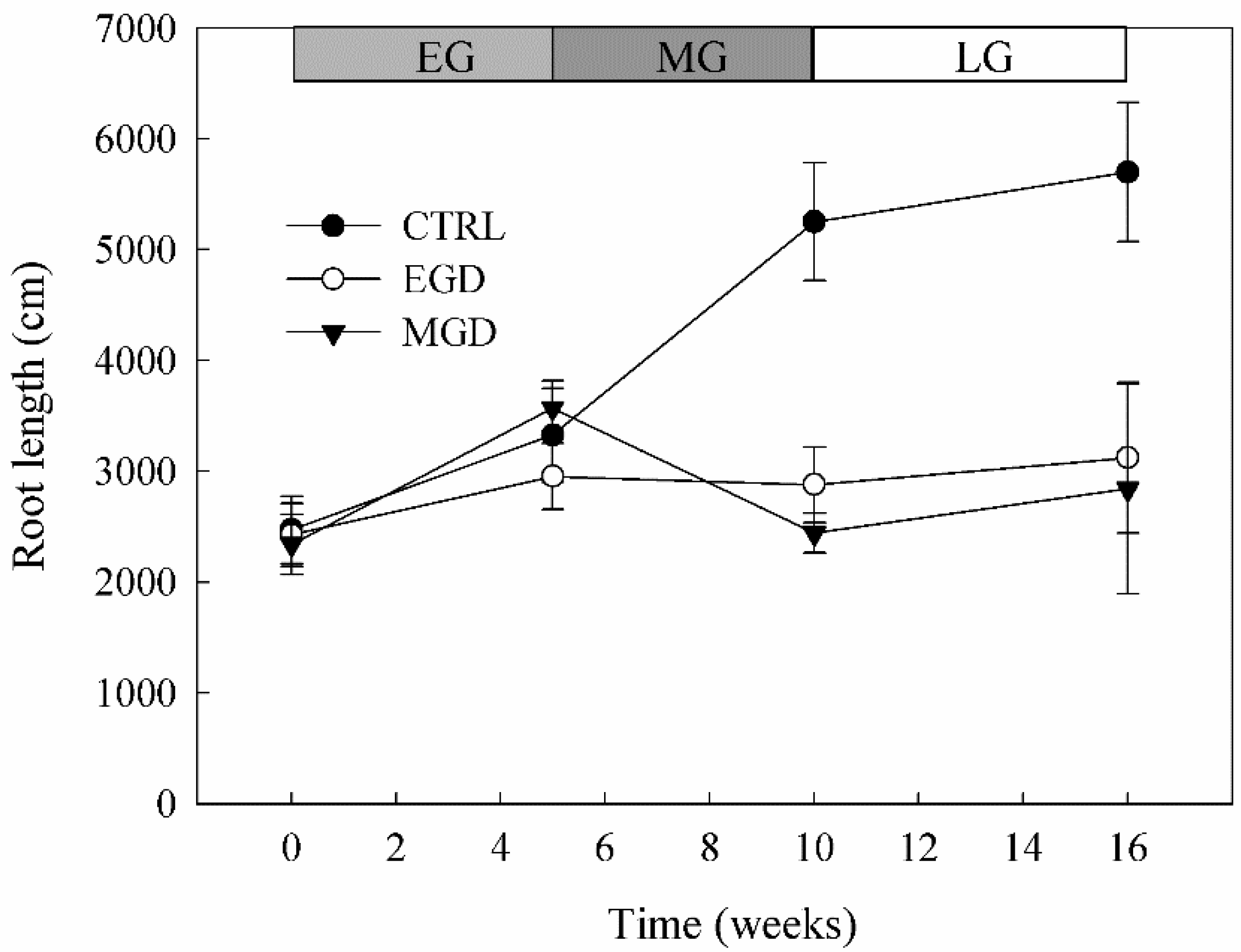
3.7. Root Length
3.8. Sapling Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, A. Characteristics and trends in various forms of the Palmer Drought Severity Index during 1900–2008. JGR-Atmos. 2011, 116, D12115. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Running, S.W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xiao, J.; Zhou, Y.; Zheng, Y.; Li, J.; Xiao, H. Drought events and their effects on vegetation productivity in China. Ecosphere 2016, 7, e01591. [Google Scholar] [CrossRef]
- Huang, M.; Wang, X.; Keenan, T.F.; Piao, S. Drought timing influences the legacy of tree growth recovery. Glob. Chang. Biol. 2018, 24, 3546–3559. [Google Scholar] [CrossRef] [Green Version]
- Misson, L.; Limousin, J.M.; Rodriguez, R.; Letts, M.G. Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant Cell Environ. 2010, 33, 1898–1910. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Franquesa, M.; Sanguesa-Barreda, G. Timing of drought triggers distinct growth responses in Holm Oak: Implications to predict warming-induced forest defoliation and growth decline. Forests 2015, 6, 1576–1597. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Alleviation of summer drought boosts establishment success of Pinus sylvestris in a Mediterranean mountain: An experimental approach. Plant. Ecol. 2005, 181, 191. [Google Scholar] [CrossRef]
- Zang, C.; Pretzsch, H.; Rothe, A. size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees 2012, 26, 557–569. [Google Scholar] [CrossRef]
- Muukkonen, P.; Nevalainen, S.; Lindgren, M.; Peltoniemi, M. Spatial occurrence of drought-associated damages in Finnish boreal forests: Results from forest condition monitoring and GIS analysis. Boreal Environ. Res. 2015, 20, 172–180. [Google Scholar]
- Gao, Y.; Markkanen, T.; Aurela, M.; Mammarella, I.; Thum, T.; Tsuruta, A.; Yang, H.; Aalto, T. Response of water use efficiency to summer drought in a boreal Scots pine forest in Finland. Biogeosciences 2017, 14, 4409–4422. [Google Scholar] [CrossRef] [Green Version]
- Vitasse, Y.; Bottero, A.; Cailleret, M.; Bigler, C.; Fonti, P.; Gessler, A.; Lévesque, M.; Rohner, B.; Weber, P.; Rigling, A.; et al. Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Glob. Chang. Biol. 2019, 25, 3781–3792. [Google Scholar] [CrossRef]
- D’Orangeville, L.; Maxwell, J.; Kneeshaw, D.; Pederson, N.; Duchesne, L.; Logan, T.; Houle, D.; Arseneault, D.; Beier, C.M.; Bishop, D.A.; et al. Drought timing and local climate determine the sensitivity of eastern temperate forests to drought. Glob. Chang. Biol. 2018, 24, 2339–2351. [Google Scholar] [CrossRef]
- Zhang, X.; Manzanedo, R.D.; D’Orangeville, L.; Rademacher, T.T.; Li, J.; Bai, X.; Hou, M.; Chen, Z.; Zou, F.; Song, F.; et al. Snowmelt and early to mid-growing season water availability augment tree growth during rapid warming in southern Asian boreal forests. Glob. Chang. Biol. 2019, 25, 3462–3471. [Google Scholar] [CrossRef]
- Li, M.-Y.; Fang, L.-D.; Duan, C.-Y.; Cao, Y.; Yin, H.; Ning, Q.-R.; Hao, G.-Y. Greater risk of hydraulic failure due to increased drought threatens pine plantations in Horqin Sandy Land of northern China. For. Ecol. Manag. 2020, 461, 117980. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, A.-Y.; An, Y.-N.; Lian, P.-Y.; Wu, D.-D.; Zhu, J.-J.; Meinzer, F.C.; Hao, G.-Y. Hydraulics play an important role in causing low growth rate and dieback of aging Pinus sylvestris var. mongolica trees in plantations of Northeast China. Plant Cell Environ. 2018, 41, 1500–1511. [Google Scholar] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Ann. Rev. Plant. Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiley, E.; Huepenbecker, S.; Casper, B.B.; Helliker, B.R. The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiol. 2013, 33, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Camarero, J.J.; Sangüesa-Barreda, G.; Vergarechea, M. Prior height, growth, and wood anatomy differently predispose to drought-induced dieback in two Mediterranean oak species. Ann. For. Sci. 2016, 73, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Blake, J.; Ferrell, W.K. The association between soil and xylem water potential, leaf resistance, and abscisic acid content in droughted seedlings of Douglas-fir (Pseudotsuga menziesii). Physiol. Plant. 1977, 39, 106–109. [Google Scholar] [CrossRef]
- Zhang, J.; Davies, W.J. Increased synthesis of ABA in partially dehydrated root tips and ABA transport from roots to leaves. J. Exp. Bot. 1987, 38, 2015–2023. [Google Scholar] [CrossRef]
- Trejo, C.L.; Davies, W.J. Drought-induced closure of Phaseolus vulgaris L. stomata precedes leaf water deficit and any increase in xylem ABA concentration. J. Exp. Bot. 1991, 42, 1507–1515. [Google Scholar] [CrossRef]
- Wang, A.F.; Zhang, G.; Qie, Y.X.; Xiang, D.Y.; Di, B.; Chen, Z.P. Effects of drought on physiological characteristics of needles of Pinus bungeana Zucc. seedlings. Plant. Physiol. J. 2012, 48, 189–196, (In Chinese with English abstract). [Google Scholar]
- Wang, A.-F.; Di, B.; Repo, T.; Roitto, M.; Zhang, G. Responses of parameters for electrical impedance spectroscopy and pressure–volume curves to drought stress in Pinus bungeana seedlings. Forests 2020, 11, 359. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Z. Determination of chlorophyll content in plants—Acetone and ethanol mixture method. Liaoning Agric. Sci. 1986, 3, 26–28. [Google Scholar]
- Zhang, G.; Li, Y.-Q.; Dong, S.-H. Assessing frost hardiness of Pinus bungeana shoots and needles by electrical impedance spectroscopy with and without freezing tests. J. Plant. Ecol. 2010, 3, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.; Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant. Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Forner, A.; Valladares, F.; Bonal, D.; Granier, A.; Grossiord, C.; Aranda, I. Extreme droughts affecting Mediterranean tree species’ growth and water-use efficiency: The importance of timing. Tree Physiol. 2018, 38, 1127–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebauer, R.; Plichta, R.; Urban, J.; Volařík, D.; Hájíčková, M. The resistance and resilience of European beech seedlings to drought stress during the period of leaf development. Tree Physiol. 2020, 40, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, Y.; Wang, Y.; Gao, L.; Wang, M.; Chaumont, F.; Shen, Q.; Guo, S. Root ABA accumulation enhances rice seedling drought tolerance under ammonium supply: Interaction with aquaporins. Front. Plant. Sci. 2016, 7, 1206. [Google Scholar] [CrossRef] [Green Version]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Baigorri, R.; Garnica, M.; Fuentes, M.; Casanova, E.; Zamarreño, A.M.; Iriarte, J.C.; Etayo, D.; et al. Abscisic acid regulation of root hydraulic conductivity and aquaporin gene expression is crucial to the plant shoot growth enhancement caused by rhizosphere humic acids. Plant. Physiol. 2015, 169, 2587–2596. [Google Scholar] [PubMed]
- Zhang, Y.; Xu, F.; Ding, Y.; Du, H.; Zhang, Q.; Dang, X.; Cao, Y.; Dodd, I.C.; Xu, W. Abscisic acid mediates barley rhizosheath formation under mild soil drying by promoting root hair growth and auxin response. Plant Cell Environ. 2021, 44, 1935–1945. [Google Scholar] [CrossRef]
- Jackson, G.E.; Irvine, J.; Grace, J.; Khalil, A.A.M. Abscisic acid concentrations and fluxes in droughted conifer saplings. Plant Cell Environ. 1995, 18, 13–22. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Hartmann, H.; Trumbore, S. Understanding the roles of nonstructural carbohydrates in forest trees—From what we can measure to what we want to know. New Phytol. 2016, 211, 386–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant. Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, M.; Saarinen, M.; Nummelin, L.; Heiskanen, J.; Roitto, M.; Sarjala, T.; Laine, J. Tolerance of peat-grown Scots pine seedlings to waterlogging and drought: Morphological, physiological, and metabolic responses to stress. For. Ecol. Manag. 2013, 307, 43–53. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, K.A.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Ma, Z.; Lei, X.; Zhu, Q.; Chen, H.; Wang, W.; Liu, S.; Li, W.; Fang, X.; Zhou, X. A drought-induced pervasive increase in tree mortality across Canada’s boreal forests. Nat. Clim. Chang. 2011, 1, 467–471. [Google Scholar] [CrossRef]
- Zhang, G.W.; Zeng, G.; Yang, X.Y.; Jiang, Z.H. Future changes in extreme high temperature over China at 1.5 °C–5 °C global warming based on CMIP6 simulations. Adv. Atmos. Sci. 2021, 38, 253–267. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Adams, H.D.; Collins, A.D.; Briggs, S.P.; Vennetier, M.; Dickman, L.T.; Sevanto, S.A.; Garcia-Forner, N.; McDowell, N.G. Experimental drought and heat can delay phenological development and reduce foliar and shoot growth in semiarid trees. Glob. Chang. Biol. 2015, 21, 4210–4220. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality duringdrought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Adams, H.D.; Eamus, D.; McDowell, N.G.; Law, D.J.; Will, R.E.; Williams, A.P.; Zou, C.B. The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. Front. Plant. Sci. 2013, 4, 266. [Google Scholar] [CrossRef] [Green Version]

| Time (Weeks) | Sapling Cumulative Mortality (%) | ||
|---|---|---|---|
| CTRL | EGD | MGD | |
| 4 | 4 | 12.9 | 4.8 |
| 6 | 4 | 16.1 | 6.5 |
| 9 | 4.8 | 21.4 | 8.9 |
| 11 | 4.8 | 21.4 | 19.4 |
| 14 | 4.8 | 22.6 | 34.7 |
| 17 | 6.5 | 22.6 | 45.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, H.; Dong, A.-M.; Roitto, M.; Xiang, D.-Y.; Zhang, G.; Repo, T.; Wang, A.-F. Timing of Drought Affected the Growth, Physiology, and Mortality of Mongolian Pine Saplings. Forests 2021, 12, 1491. https://doi.org/10.3390/f12111491
Qian H, Dong A-M, Roitto M, Xiang D-Y, Zhang G, Repo T, Wang A-F. Timing of Drought Affected the Growth, Physiology, and Mortality of Mongolian Pine Saplings. Forests. 2021; 12(11):1491. https://doi.org/10.3390/f12111491
Chicago/Turabian StyleQian, Hui, Ai-Mei Dong, Marja Roitto, Di-Ying Xiang, Gang Zhang, Tapani Repo, and Ai-Fang Wang. 2021. "Timing of Drought Affected the Growth, Physiology, and Mortality of Mongolian Pine Saplings" Forests 12, no. 11: 1491. https://doi.org/10.3390/f12111491
APA StyleQian, H., Dong, A. -M., Roitto, M., Xiang, D. -Y., Zhang, G., Repo, T., & Wang, A. -F. (2021). Timing of Drought Affected the Growth, Physiology, and Mortality of Mongolian Pine Saplings. Forests, 12(11), 1491. https://doi.org/10.3390/f12111491






