Investigating Masking Effects of Age Trends on the Correlations among Tree Ring Proxies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Detrending
2.2. Correlation Analysis
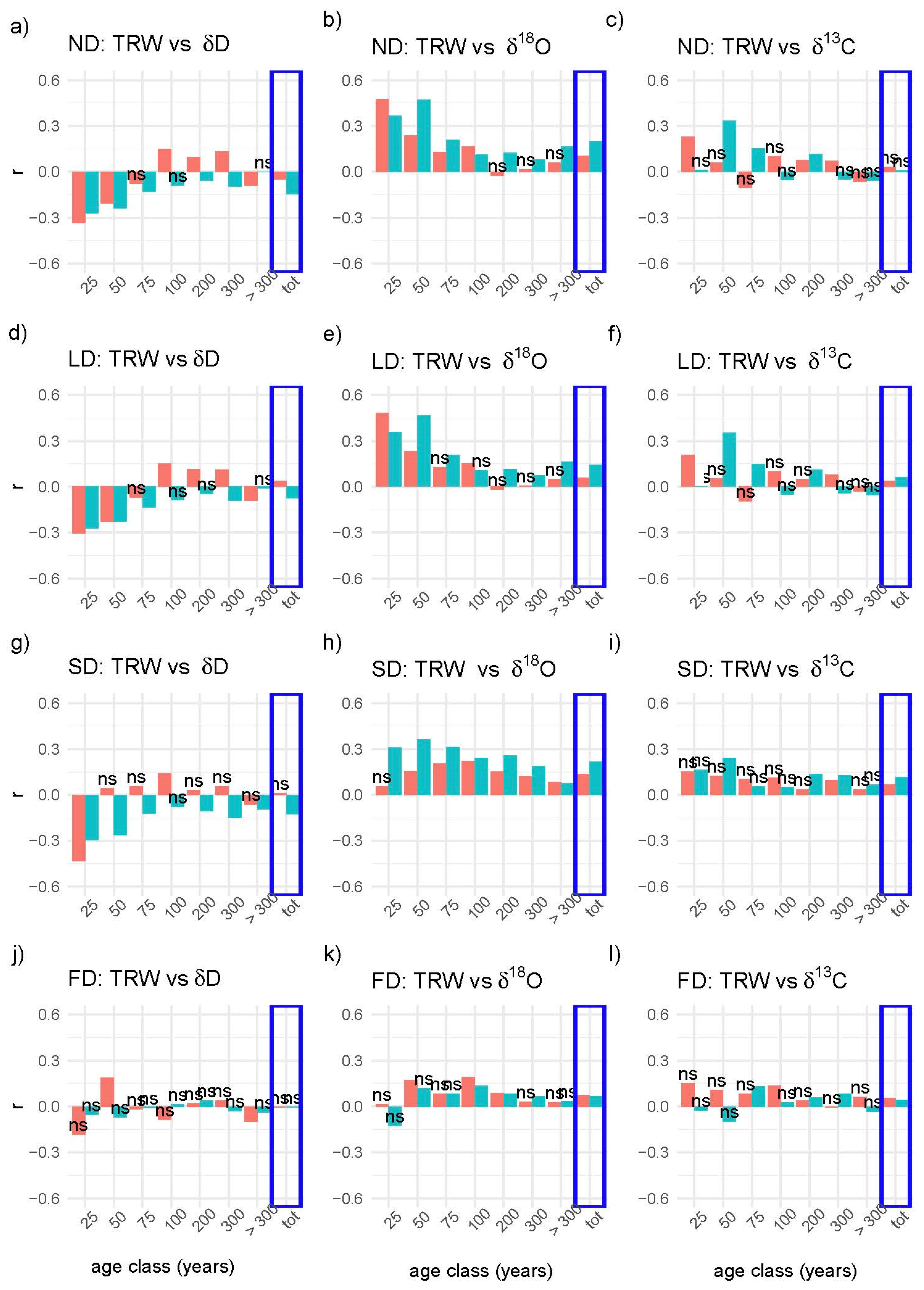
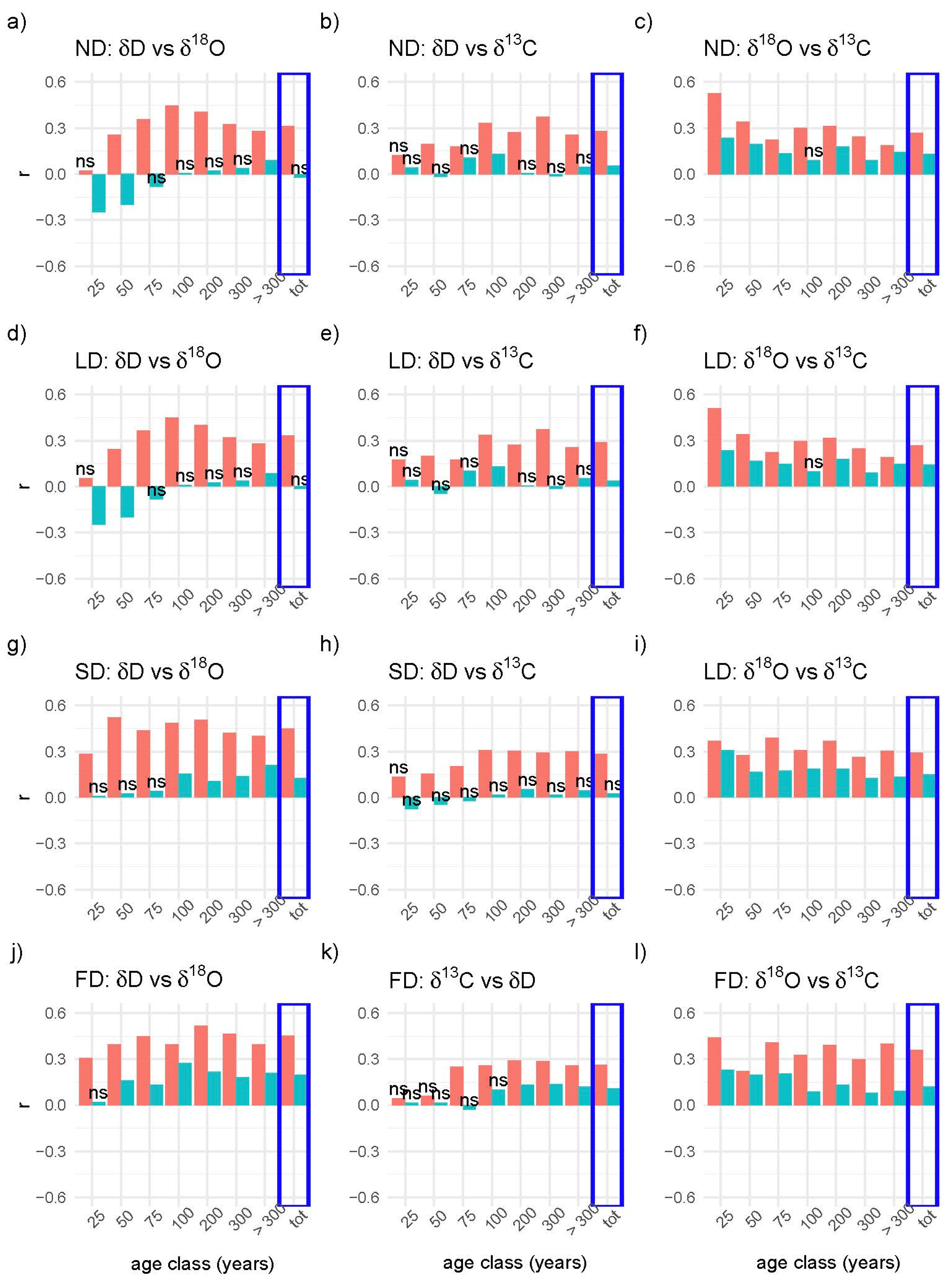
3. Results
4. Discussion
4.1. Correlations between TRW and Isotopes in the Three Scenarios
4.2. Correlations between the Isotopes in the Four Scenarios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Büntgen, U.; Kolář, T.; Rybníček, M.; Koňasová, E.; Trnka, M.; Ač, A.; Krusic, P.J.; Esper, J.; Treydte, K.; Reinig, F.; et al. No Age Trends in Oak Stable Isotopes. Paleoceanogr. Paleoclimatology 2020, 35, e2019PA003831. [Google Scholar] [CrossRef]
- Fritz, H. Tree Rings: Basics and Applications of Dendrochronology; Springer Science & Business Media: Berlin, Germany, 1976. [Google Scholar]
- Loader, N.J.; Young, G.H.F.; McCarroll, D.; Davies, D.; Miles, D.; Bronk Ramsey, C. Summer Precipitation for the England and Wales Region, 1201–2000 CE, from Stable Oxygen Isotopes in Oak Tree Rings. J. Quat. Sci. 2020, 35, 731–736. [Google Scholar] [CrossRef]
- Cherubini, P.; Battipaglia, G.; Innes, J.L. Tree vitality and forest health: Can tree-ring stable isotopes be used as indicators? Curr. For. Rep. 2021, 7, 69–80. [Google Scholar]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Grießinger, J.; Bräuning, A.; Helle, G.; Schleser, G.H.; Hochreuther, P.; Meier, W.J.-H.; Zhu, H. A Dual Stable Isotope Approach Unravels Common Climate Signals and Species-Specific Responses to Environmental Change Stored in Multi-Century Tree-Ring Series from the Tibetan Plateau. Geosciences 2019, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, M.M.; Vitali, V.; Schuler, P.; Leuenberger, M.; Saurer, M. More than Climate: Hydrogen Isotope Ratios in Tree Rings as Novel Plant Physiological Indicator for Stress Conditions. Dendrochronologia 2021, 65, 125788. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Qin, D.; Chen, T.; Sun, W.; An, W.; Wang, W.; Wu, G.; Zeng, X.; Ren, J. Drought History Inferred from Tree Ring δ13C and δ18O in the Central Tianshan Mountains of China and Linkage with the North Atlantic Oscillation. Theor. Appl. Climatol. 2014, 116, 385–401. [Google Scholar] [CrossRef]
- Esper, J.; Frank, D.C.; Battipaglia, G.; Büntgen, U.; Holert, C.; Treydte, K.; Siegwolf, R.; Saurer, M. Low-Frequency Noise in δ13 C and δ18O Tree Ring Data: A Case Study of Pinus Uncinata in the Spanish Pyrenees: Noise in δ13C and δ18O data. Global Biogeochem. Cycles 2010, 24, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Helama, S.; Arppe, L.; Timonen, M.; Mielikäinen, K.; Oinonen, M. Age-Related Trends in Subfossil Tree-Ring Δ13C Data. Chem. Geol. 2015, 416, 28–35. [Google Scholar] [CrossRef]
- Xu, G.; Wu, G.; Liu, X.; Chen, T.; Wang, B.; Hudson, A.; Trouet, V. Age-Related Climate Response of Tree-Ring Δ13C and Δ18O from Spruce in Northwestern China, with Implications for Relative Humidity Reconstructions. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005513. [Google Scholar] [CrossRef]
- Duffy, J.E.; McCarroll, D.; Loader, N.J.; Young, G.H.; Davies, D.; Miles, D.; Bronk Ramsey, C. Absence of Age-Related Trends in Stable Oxygen Isotope Ratios from Oak Tree Rings. Glob. Biogeochem. Cycles 2019, 33, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Szejner, P.; Clute, T.; Anderson, E.; Evans, M.N.; Hu, J. Reduction in Lumen Area Is Associated with the Δ18O Exchange between Sugars and Source Water during Cellulose Synthesis. New Phytol. 2020, 226, 1583–1593. [Google Scholar] [CrossRef]
- Arosio, T.; Ziehmer, M.M.; Nicolussi, K.; Schlüchter, C.; Leuenberger, M. Alpine Holocene Tree-Ring Dataset: Age-Related Trends in the Stable Isotopes of Cellulose Show Species-Specific Patterns. Biogeosciences 2020, 17, 4871–4882. [Google Scholar] [CrossRef]
- Roden, J.S.; Lin, G.; Ehleringer, J.R. A Mechanistic Model for Interpretation of Hydrogen and Oxygen Isotope Ratios in Tree-Ring Cellulose. Geochim. Cosmochim. Acta 2000, 64, 21–35. [Google Scholar] [CrossRef]
- Sidorova, O.V.; Saurer, M.; Guillet, S.; Corona, C.; Fonti, P.; Myglan, V.S.; Kirdyanov, A.V.; Naumova, O.V.; Ovchinnikov, D.V.; Shashkin, A.V. Siberian Tree-Ring and Stable Isotope Proxies as Indicators of Temperature and Moisture Changes after Major Stratospheric Volcanic Eruptions. Clim. Past 2019, 15, 685–700. [Google Scholar]
- Deshpande, A.G.; Boutton, T.W.; Hyodo, A.; Lafon, C.W.; Moore, G.W. Bottomland Hardwood Forest Growth and Stress Response to Hydroclimatic Variation: Evidence from Dendrochronology and Tree Ring Δ13C Values. Biogeosciences 2020, 17, 5639–5653. [Google Scholar] [CrossRef]
- Shestakova, T.A.; Voltas, J.; Saurer, M.; Berninger, F.; Esper, J.; Andreu-Hayles, L.; Daux, V.; Helle, G.; Leuenberger, M.; Loader, N.J. Spatio-Temporal Patterns of Tree Growth as Related to Carbon Isotope Fractionation in European Forests under Changing Climate. Glob. Ecol. Biogeogr. 2019, 28, 1295–1309. [Google Scholar] [CrossRef]
- Shestakova, T.A.; Camarero, J.J.; Ferrio, J.P.; Knorre, A.A.; Gutiérrez, E.; Voltas, J. Increasing Drought Effects on Five European Pines Modulate Δ13C-Growth Coupling along a Mediterranean Altitudinal Gradient. Funct. Ecol. 2017, 31, 1359–1370. [Google Scholar] [CrossRef] [Green Version]
- Nakatsuka, T.; Sano, M.; Li, Z.; Xu, C.; Tsushima, A.; Shigeoka, Y.; Sho, K.; Ohnishi, K.; Sakamoto, M.; Ozaki, H. A 2600-Year Summer Climate Reconstruction in Central Japan by Integrating Tree-Ring Stable Oxygen and Hydrogen Isotopes. Clim. Past 2020, 16, 2153–2172. [Google Scholar] [CrossRef]
- Hafner, P.; Robertson, I.; McCarroll, D.; Loader, N.J.; Gagen, M.; Bale, R.J.; Jungner, H.; Sonninen, E.; Hilasvuori, E.; Levanič, T. Climate Signals in the Ring Widths and Stable Carbon, Hydrogen and Oxygen Isotopic Composition of Larix Decidua Growing at the Forest Limit in the Southeastern European Alps. Trees 2011, 25, 1141–1154. [Google Scholar] [CrossRef]
- Kirdyanov, A.V.; Vaganov, E.A.; Hughes, M.K. Separating the Climatic Signal from Tree-Ring Width and Maximum Latewood Density Records. Trees 2007, 21, 37–44. [Google Scholar] [CrossRef]
- Schollaen, K.; Heinrich, I.; Neuwirth, B.; Krusic, P.J.; D’Arrigo, R.D.; Karyanto, O.; Helle, G. Multiple Tree-Ring Chronologies (Ring Width, Δ13C and Δ18O) Reveal Dry and Rainy Season Signals of Rainfall in Indonesia. Quat. Sci. Rev. 2013, 73, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Weigl, M.; Grabner, M.; Helle, G.; Schleser, G.H.; Wimmer, R. Variability of Latewood-Widths and-Stable Isotope Ratios in a Sessile Oak Tree (Quercus Petraea (Matt.) Liebl.). Dendrochronologia 2007, 24, 117–122. [Google Scholar] [CrossRef]
- Sidorova, O.V.; Siegwolf, R.T.; Saurer, M.; Naurzbaev, M.M.; Shashkin, A.V.; Vaganov, E.A. Spatial Patterns of Climatic Changes in the Eurasian North Reflected in Siberian Larch Tree-Ring Parameters and Stable Isotopes. Glob. Chang. Biol. 2010, 16, 1003–1018. [Google Scholar] [CrossRef] [Green Version]
- Nicolussi, K.; Kaufmann, M.; Melvin, T.M.; Van Der Plicht, J.; Schießling, P.; Thurner, A. A 9111 Year Long Conifer Tree-Ring Chronology for the European Alps: A Base for Environmental and Climatic Investigations. Holocene 2009, 19, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Nicolussi, K.; Bortenschlager, S.; Körner, C. Increase in Tree-Ring Width in Subalpine Pinus Cembra from the Central Alps That May Be CO2-Related. Trees 1995, 9, 181–189. [Google Scholar] [CrossRef]
- Ziehmer, M.M.; Nicolussi, K.; Schlüchter, C.; Leuenberger, M. Preliminary Evaluation of the Potential of Tree-Ring Cellulose Content as a Novel Supplementary Proxy in Dendroclimatology. Biogeosciences 2018, 15, 1047–1064. [Google Scholar] [CrossRef] [Green Version]
- Boettger, T.; Haupt, M.; Knöller, K.; Weise, S.M.; Waterhouse, J.S.; Rinne, K.T.; Loader, N.J.; Sonninen, E.; Jungner, H.; Masson-Delmotte, V. Wood Cellulose Preparation Methods and Mass Spectrometric Analyses of Δ13C, Δ18O, and Nonexchangeable Δ2H Values in Cellulose, Sugar, and Starch: An Interlaboratory Comparison. Anal. Chem. 2007, 79, 4603–4612. [Google Scholar] [CrossRef]
- Filot, M.S.; Leuenberger, M.; Pazdur, A.; Boettger, T. Rapid Online Equilibration Method to Determine the D/H Ratios of Non-Exchangeable Hydrogen in Cellulose. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up Minute Res. Mass Spectrom. 2006, 20, 3337–3344. [Google Scholar] [CrossRef]
- Loader, N.J.; Street-Perrott, F.A.; Daley, T.J.; Hughes, P.D.M.; Kimak, A.; Levanic, T.; Mallon, G.; Mauquoy, D.; Robertson, I.; Roland, T.P. Simultaneous Determination of Stable Carbon, Oxygen, and Hydrogen Isotopes in Cellulose. Anal. Chem. 2015, 87, 376–380. [Google Scholar] [CrossRef]
- Leuenberger, M. To What Extent Can Ice Core Data Contribute to the Understanding of Plant Ecological Developments of the Past? Terr. Ecol. 2007, 1, 211–233. [Google Scholar]
- Coplen, T.B. Reporting of Stable Hydrogen, Carbon, and Oxygen Isotopic Abundances (Technical Report). Pure Appl. Chem. 1994, 66, 273–276. [Google Scholar] [CrossRef]
- Bunn, A.G. A Dendrochronology Program Library in R (DplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Zar, J.H. Significance Testing of the Spearman Rank Correlation Coefficient. J. Am. Stat. Assoc. 1972, 67, 578–580. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Arosio, T.; Ziehmer-Wenz, M.M.; Nicolussi, K.; Schlüchter, C.; Leuenberger, M. Larch Cellulose Shows Significantly Depleted Hydrogen Isotope Values with Respect to Evergreen Conifers in Contrast to Oxygen and Carbon Isotopes. Front. Earth Sci. 2020, 8, 579. [Google Scholar] [CrossRef]
- Li, M.; Zhu, J.; Zhang, M. Foliar Carbon Isotope Discrimination and Related Traits along Light Gradients in Two Different Functional-Type Tree Species. Eur. J. For. Res. 2013, 132, 815–824. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carrer, M. Age-Dependent Xylogenesis in Timberline Conifers. New Phytol. 2008, 177, 199–208. [Google Scholar] [CrossRef]
- Bräker, O.-U. Der Alterstrend Bei Jahrringdichten Und Jahrringbreiten von Nadelhoebyzern und Sein Ausgleich; Mitteilungen der Forstlichen Bundesversuchsanstalt: Vienna, Austria, 1981. [Google Scholar]
- Farquhar, G.D.; Lloyd, J. Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In Stable Isotopes and Plant Carbon-Water Relations; Elsevier: Amsterdam, The Netherlands, 1993; pp. 47–70. [Google Scholar]
- Sternberg, L.D.S.L.O. Oxygen Stable Isotope Ratios of Tree-Ring Cellulose: The next Phase of Understanding. New Phytol. 2009, 181, 553–562. [Google Scholar] [CrossRef]
- Tranquillini, W. Climatic resistance and damage of trees at timberline. In Physiological Ecology of the Alpine Timberline; Springer: Berlin/Heidelberg, Germany, 1979; pp. 91–111. [Google Scholar]
- Gagen, M.; McCarroll, D.; Edouard, J.-L. Combining Ring Width, Density and Stable Carbon Isotope Proxies to Enhance the Climate Signal in Tree-Rings: An Example from the Southern French Alps. Clim. Chang. 2006, 78, 363–379. [Google Scholar] [CrossRef]
- Treydte, K.; Schleser, G.H.; Schweingruber, F.H.; Winiger, M. The Climatic Significance of δ13C in Subalpine Spruces (Lötschental, Swiss Alps): A Case Study with Respect to Altitude, Exposure and Soil Moisture. Tellus B Chem. Phys. Meteorol. 2001, 53, 593–611. [Google Scholar] [CrossRef]
- Gessler, A.; Ferrio, J.P.; Hommel, R.; Treydte, K.; Werner, R.A.; Monson, R.K. Stable Isotopes in Tree Rings: Towards a Mechanistic Understanding of Isotope Fractionation and Mixing Processes from the Leaves to the Wood. Tree Physiol. 2014, 34, 796–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimak, A.; Kern, Z.; Leuenberger, M. Qualitative Distinction of Autotrophic and Heterotrophic Processes at the Leaf Level by Means of Triple Stable Isotope (C–O–H) Patterns. Front. Plant Sci. 2015, 6, 1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tcherkez, G.; Mahé, A.; Hodges, M. 12C/13C Fractionations in Plant Primary Metabolism. Trends Plant Sci. 2011, 16, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Cormier, M.-A.; Werner, R.A.; Leuenberger, M.C.; Kahmen, A. 2 H-Enrichment of Cellulose and n-Alkanes in Heterotrophic Plants. Oecologia 2019, 189, 365–373. [Google Scholar] [CrossRef] [PubMed]

| N Samples | N Trees | |||
|---|---|---|---|---|
| Age Class | PICE | LADE | PICE | LADE |
| 1–25 | 124 | 78 | 35 | 25 |
| 26–50 | 225 | 171 | 54 | 40 |
| 51–75 | 323 | 230 | 70 | 50 |
| 76–100 | 334 | 264 | 73 | 57 |
| 101–200 | 1329 | 1048 | 85 | 68 |
| 201–300 | 896 | 776 | 68 | 57 |
| >300 | 801 | 830 | 33 | 34 |
| total | 4133 | 3471 | 108 | 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arosio, T.; Ziehmer-Wenz, M.M.; Nicolussi, K.; Schlüchter, C.; Leuenberger, M.C. Investigating Masking Effects of Age Trends on the Correlations among Tree Ring Proxies. Forests 2021, 12, 1523. https://doi.org/10.3390/f12111523
Arosio T, Ziehmer-Wenz MM, Nicolussi K, Schlüchter C, Leuenberger MC. Investigating Masking Effects of Age Trends on the Correlations among Tree Ring Proxies. Forests. 2021; 12(11):1523. https://doi.org/10.3390/f12111523
Chicago/Turabian StyleArosio, Tito, Malin Michelle Ziehmer-Wenz, Kurt Nicolussi, Christian Schlüchter, and Markus Christian Leuenberger. 2021. "Investigating Masking Effects of Age Trends on the Correlations among Tree Ring Proxies" Forests 12, no. 11: 1523. https://doi.org/10.3390/f12111523






