Water Absorption Properties in Transverse Direction of Heat-Treated Chinese Fir Wood Determined Using TD-NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. TD-NMR Experiments
3. Results and Discussion
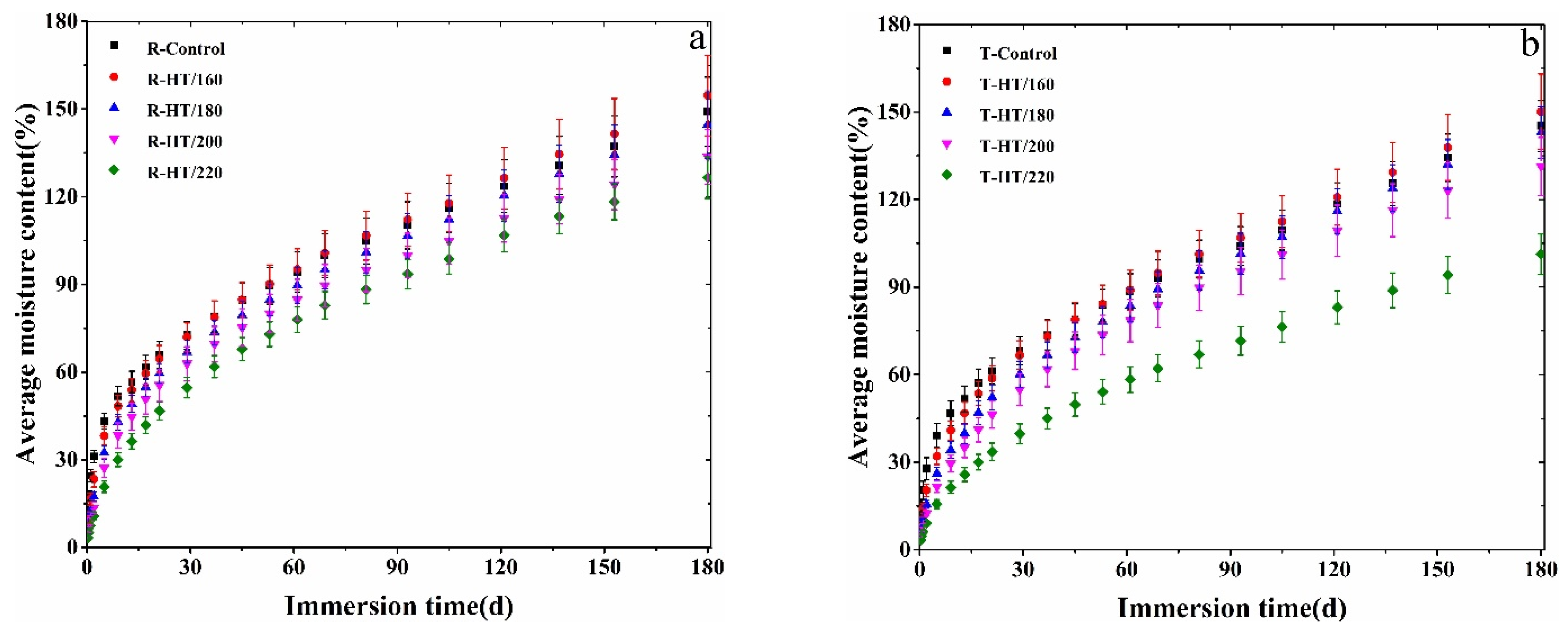
3.1. MC Change during Water Absorption Process
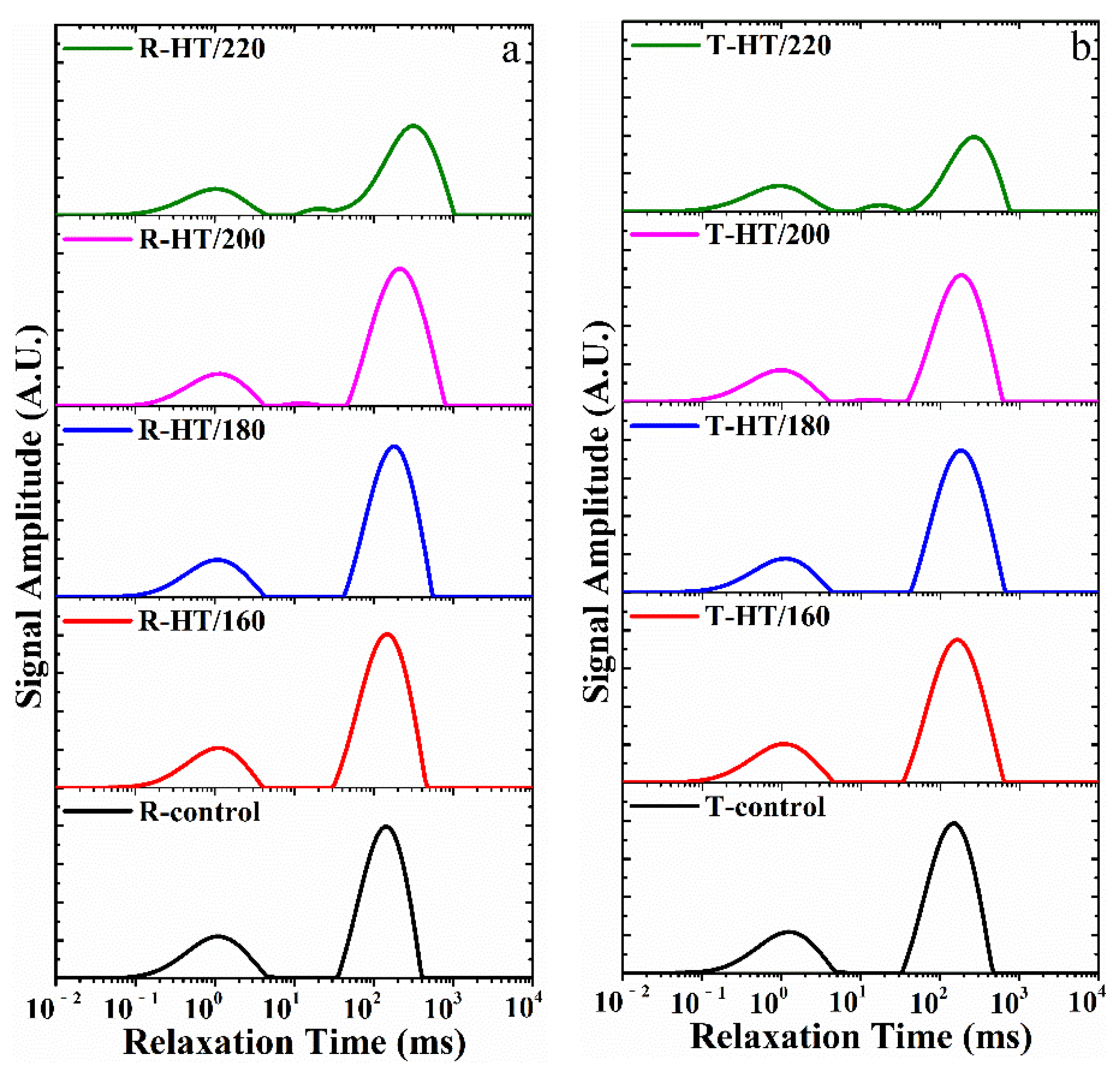
3.2. Determination of the Water States
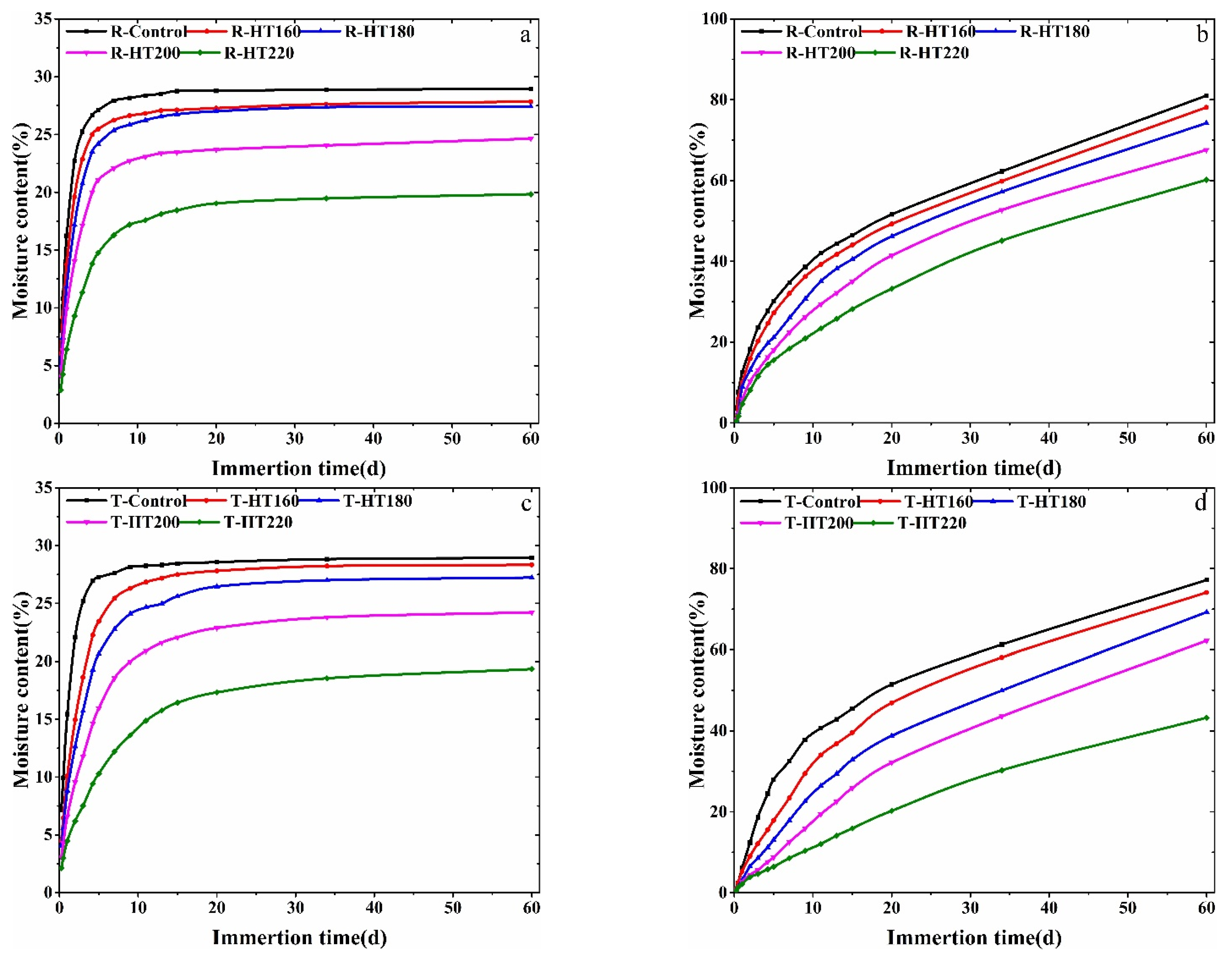
3.3. Amount of Bound Water and Free Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamperidou, V. The Biological Durability of Thermally- and Chemically-Modified Black Pine and Poplar Wood Against Basidiomycetes and Mold Action. Forests 2019, 10, 1111. [Google Scholar] [CrossRef] [Green Version]
- Kartal, S.N.; Hwang, W.J.; Imamura, Y. Water absorption of boron-treated and heat-modified wood. J. Wood Sci. 2007, 53, 454–457. [Google Scholar] [CrossRef]
- Lu, J.; Xu, K.; Liu, Y.; Wu, Y.; Li, X. Research progresses on reinforced modifi cation of poplar wood from fastgrowing plantation. J. Cent. South Univ. For. Technol. 2014, 34, 99–103. (In Chinese) [Google Scholar]
- Herrera-Builes, J.F.; Sepúlveda-Villarroel, V.; Osorio, J.A.; Salvo-Sepúlveda, L.; Ananías, R.A. Effect of Thermal Modification Treatment on Some Physical and Mechanical Properties of Pinus oocarpa Wood. Forests 2021, 12, 249. [Google Scholar] [CrossRef]
- Gu, L.; Ding, T.; Jiang, N. Development of wood heat treatment research and industrialization. J. For. Eng. 2019, 22, 9–19. (In Chinese) [Google Scholar]
- Altgen, M.; Hofmann, T.; Militz, H. Wood moisture content during the thermal modification process affects the improvement in hygroscopicity of Scots pine sapwood. Wood Sci. Technol. 2016, 50, 1181–1195. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 99–127. [Google Scholar]
- Akgül, M.; Korkut, S. The effect of heat treatment on some chemical properties and colour in Scots pine and Uludağ fir wood. Int. J. Phys. Sci. 2012, 7, 2854–2859. [Google Scholar]
- Chao, L.; Minghui, Z.; Jianfang, Y. Determining wood moisture content by free induction decay of nuclear magnetic resonance. J. Beijing For. Univ. 2012, 34, 142–145. [Google Scholar]
- Gao, Y.; Zhang, M. Moisture Sorption in Wood Studied by Time Domain Nuclear Magnetic Resonance. Chin. J. Magn. Reson. 2016, 33, 295–304. (In Chinese) [Google Scholar]
- Nanassy, A.J. Use of wide line NMR for measurement of moisture content in wood. Wood Sci. Technol. 1973, 5, 187–193. [Google Scholar]
- Xu, Y.; Araujo, C.D.; Mackay, A.L.; Whittall, K.P. Proton Spin–Lattice Relaxation in Wood— T 1 Related to Local Specific Gravity Using a Fast-Exchange Model. J. Magn. Reson. 1996, 110, 55–64. [Google Scholar] [CrossRef]
- Xu, K.; Lv, J.; Gao, Y.; Wu, Y.; Li, X. Determination of Moisture Content and Moisture Content Profiles in Wood during Drying by Low Field Nuclear Magnetic Resonance. Dry. Technol. 2017, 35, 1909–1918. [Google Scholar] [CrossRef]
- Kekkonen, P.M.; Ylisassi, A.; Telkki, V.V. Absorption of Water in Thermally Modified Pine Wood As Studied by Nuclear Magnetic Resonance. J. Phys. Chem. C 2014, 118, 2146–2153. [Google Scholar] [CrossRef]
- Gao, Y.; Li, X.; Lei, P.; Zhang, M. Water Distribution in Poplar during High-Temperature Drying Process Studied by Time-Domain Nuclear Magnetic Resonance. J. Magn. Reson. 2016, 33, 479–490. (In Chinese) [Google Scholar]
- Gao, Y.; Xu, K.; Peng, H.; Jiang, J.; Lu, J. Effect of Heat Treatment on Water Absorption of Chinese fir Using TD-NMR. Appl. Sci. 2018, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.A.; Morén, T. Moisture properties of heat-treated Scots pine and Norway spruce sapwood impregnated with wood preservatives. Wood Fiber Sci. 2012, 44, 85–93. [Google Scholar]
- Metsä-Kortelainen, S.; Viitanen, H. Wettability of sapwood and heartwood of thermally modifi ed Norway spruce and Scots pine. Eur. J. Wood Prod. 2011, 70, 135–139. [Google Scholar] [CrossRef]
- Scheiding, W.; Direske, M.; Zauer, M. Water absorption of untreated and thermally modified sapwood and heartwood of Pinus sylvestris L. Eur. J. Wood Wood Prod. 2016, 74, 585–589. [Google Scholar] [CrossRef]
- Biziks, V.; Beļkova, Ļ.; Kapača, E.; Militz, H. Changes in the microstructure of birch wood after hydrothermal treatment. Wood Sci. Technol. 2013, 47, 717–735. [Google Scholar] [CrossRef]
- Biziks, V.; Andersons, B.; Sansonetti, E.; Andersone, I.; Militz, H.; Grinins, J. One-stage thermo-hydro treatment (THT) of hardwoods: An analysis of form stability after five soaking-drying cycles. Holzforschung 2015, 69, 563–571. [Google Scholar] [CrossRef]
- Popper, R.; Niemz, P.; Eberle, G. Investigations on the sorption and swelling properties of thermally treated wood. Holz Roh Werkst. 2005, 63, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, X.; Zhou, Y.; Gao, Y. Water Status Change in Wood Drying Studied By Time-Domain NMR. Sci. Silvae Sin. 2014, 50, 109–113. (In Chinese) [Google Scholar]
- Brownstein, K.R.; Tarr, C.E. Importance of classical diffusion in NMR studies of water in biological cells. Phys. Rev. A 1979, 19, 2446–2453. [Google Scholar] [CrossRef]
- Yan, Y.; Li, F.; Zhang, K.; Gu, X.; Guo, M. Dimensional Stability and finishing performance of heat-treated Pinus koraiensis and Rubber Wood. J. Northeast For. Univ. 2018, 46, 45–48. (In Chinese) [Google Scholar]
- Pfriem, A.; Zauer, M.; Wagenführ, A. Alteration of the pore structure of spruce (Picea abies (L.) Karst.) and maple (Acer pseudoplatanus L.) due to thermal treatment as determined by helium pycnometry and mercury intrusion porosimetry. Holzforschung 2009, 63, 94–98. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Wang, Q.; Mu, J.; Chu, D. Study on Water Status and Distribution in 2 Fast-growing Woods During Drying Based on LF-NMR and MRI. J. Southwest For. Univ. 2020, 40, 143–150. (In Chinese) [Google Scholar]
- Boonstra, M.J.; Acker, J.V.; Tjeerdsma, B.F.; Kegel, E.V. Strength properties of thermally modified softwoods and its relation to polymeric structural wood constituents. Ann. For. Sci. 2007, 64, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Araujo, C.D.; Avramidis, S.; Mackay, A.L. Behaviour of Solid Wood and Bound Water as a Function of Moisture Content. A Proton Magnetic Resonance Study. Holzforschung 1994, 48, 69–74. [Google Scholar] [CrossRef]
- Labbé, N.; Jéso, B.D.; Lartigue, J.-C.; Daudé, G.; Pétraud, M.; Ratier, M. Time-domain 1H NMR characterization of the liquid phase in greenwood. Holzforschung 2006, 60, 265–270. [Google Scholar] [CrossRef]
- Stamm, A.J. Thermal Degradation of Wood and Cellulose. Ind. Eng. Chem. 1956, 48, 413–417. [Google Scholar] [CrossRef]
- Källbom, S.; Rautkari, L.; Wålinder, M.; Johansson, L.S.; Campbell, J.M.; Segerholm, K.; Jones, D.; Laine, K. Water vapour sorption characteristics and surface chemical composition of thermally modified spruce (Picea abies karst). Int. Wood Prod. J. 2016, 7, 116–123. [Google Scholar] [CrossRef]
- Jalaludin, Z.; Hill, C.A.S.; Xie, Y.; Samsi, H.W.; Husain, H.; Awang, K.; Curling, S.F. Analysis of the water vapour sorption isotherms of thermally modified acacia and sesendok. Wood Mater. Sci. Eng. 2010, 5, 194–203. [Google Scholar] [CrossRef]

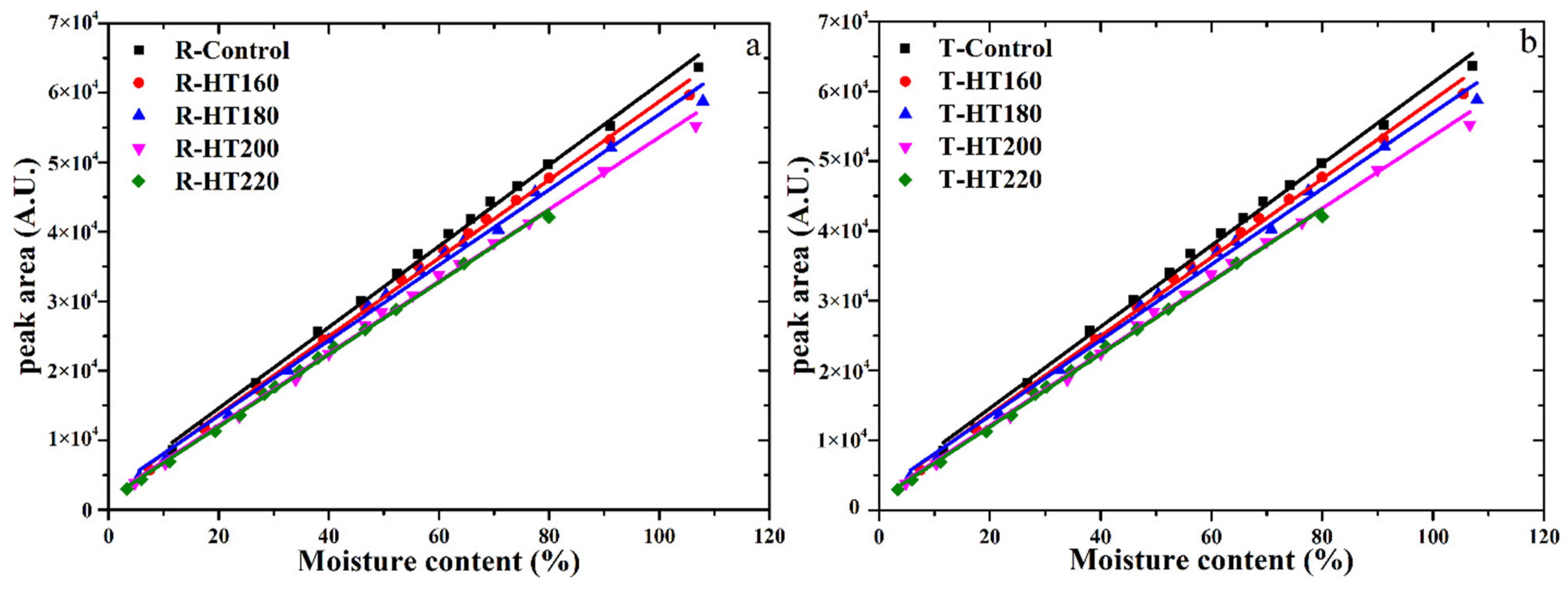
| Samples | Regression Equations | Adj.R2 |
|---|---|---|
| R-Control | Y = 583.72x + 2900.81 | 0.997 |
| R-HT/160 | Y = 563.53x + 2386.21 | 0.996 |
| R-HT/180 | Y = 542.72x + 2635.67 | 0.995 |
| R-HT/200 | Y = 518.51x + 1751.49 | 0.997 |
| R-HT/220 | Y = 520.24x + 1520.01 | 0.998 |
| Samples | Regression Equations | Adj.R2 |
|---|---|---|
| T-Control | Y = 554.04x + 2263.00 | 0.998 |
| T-HT/160 | Y = 571.94x + 2493.09 | 0.998 |
| T-HT/180 | Y = 482.26x + 2466.21 | 0.997 |
| T-HT/200 | Y = 515.90x + 1849.79 | 0.997 |
| T-HT/220 | Y = 531.70x + 1363.97 | 0.999 |
| Samples | R-Control | R-HT/160 | R-HT/180 | R-HT/200 | R-HT/220 |
|---|---|---|---|---|---|
| BW Content | 28.92% | 27.84% | 27.43% | 24.64% | 19.83% |
| FW Content | 81.00% | 78.10% | 74.20% | 67.56% | 60.17% |
| Samples | T-Control | T-HT/160 | T-HT/180 | T-HT/200 | T-HT/220 |
|---|---|---|---|---|---|
| BW Content | 28.93% | 28.32% | 27.23% | 24.21% | 19.33% |
| FW Content | 77.20% | 74.12% | 69.25% | 62.20% | 43.23% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhao, L.; Jiang, J.; Li, Z.; Lyu, J. Water Absorption Properties in Transverse Direction of Heat-Treated Chinese Fir Wood Determined Using TD-NMR. Forests 2021, 12, 1545. https://doi.org/10.3390/f12111545
Gao Y, Zhao L, Jiang J, Li Z, Lyu J. Water Absorption Properties in Transverse Direction of Heat-Treated Chinese Fir Wood Determined Using TD-NMR. Forests. 2021; 12(11):1545. https://doi.org/10.3390/f12111545
Chicago/Turabian StyleGao, Yulei, Liyuan Zhao, Jinghui Jiang, Zhu Li, and Jianxiong Lyu. 2021. "Water Absorption Properties in Transverse Direction of Heat-Treated Chinese Fir Wood Determined Using TD-NMR" Forests 12, no. 11: 1545. https://doi.org/10.3390/f12111545
APA StyleGao, Y., Zhao, L., Jiang, J., Li, Z., & Lyu, J. (2021). Water Absorption Properties in Transverse Direction of Heat-Treated Chinese Fir Wood Determined Using TD-NMR. Forests, 12(11), 1545. https://doi.org/10.3390/f12111545






