Cellular and Metabolite Changes in the Secondary Phloem of Chinese Fir (Cuninghamia lanceolata (Lamb.) Hook.) during Dormancy Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tree and Sample Collection
2.2. Anatomical Observations
2.3. Extraction of Metabolites
2.4. Ultra-High Performance Liquid Chromatography (UHPLC)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Cell Structural Characteristics
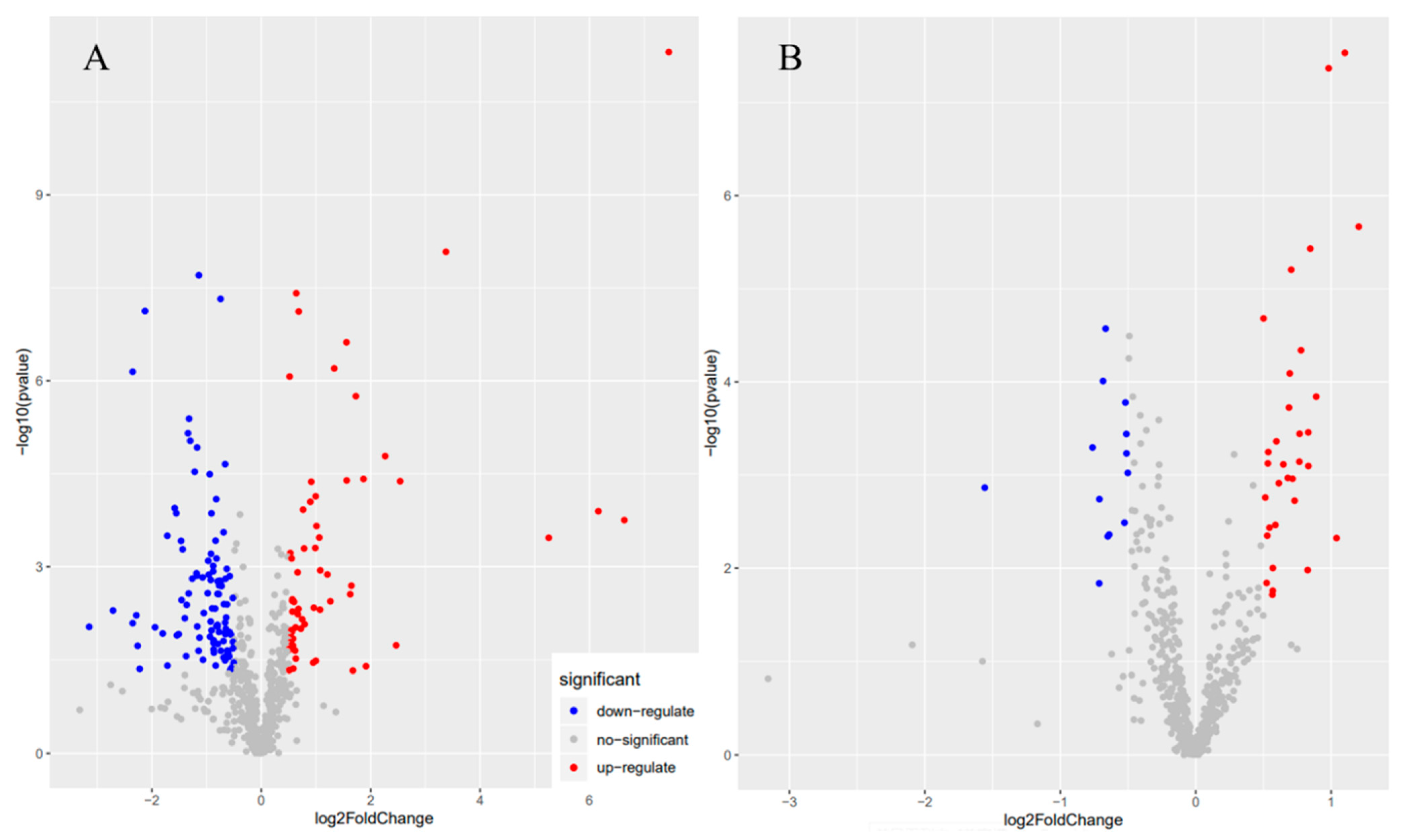
3.2. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
3.3. Metabolite Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Oxford University Press: New York, NY, USA, 2010; ISBN 9780878938667. [Google Scholar]
- Wilson, B.F.; Wodzicki, T.J.; Zahner, R. Differentiation of Cambial Derivatives: Proposed Terminology. For. Sci. 1966, 12, 438–440. [Google Scholar] [CrossRef]
- Prislan, P.; Cufar, K.; Koch, G.; Schmitt, U.; Gricar, J. Review of cellular and subcellular changes in the cambium. IAWA J. 2013, 34, 391–407. [Google Scholar] [CrossRef] [Green Version]
- Delpierre, N.; Lireux, S.; Hartig, F.; Camarero, J.J.; Cheaib, A.; Cufar, K.; Cuny, H.; Deslauriers, A.; Fonti, P.; Gricar, J.; et al. Chilling and forcing temperatures interact to predict the onset of wood formation in northern hemisphere conifers. Glob. Chang. Biol. 2019, 25, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.G.; Ma, Q.Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.Y.; Makinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in northern hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.; Carvalho, A.; Campelo, F. Xylogenesis in the early life stages of maritime pine. For. Ecol. Manag. 2018, 424, 71–77. [Google Scholar] [CrossRef]
- Qi, X.Q.; Wang, Y.L.; Chen, X.Y. (Eds.) Plant Metabolomics: Methods and Application; Chemical Industry Press: Beijing, China, 2011; ISBN 978-7-122-10587-5. [Google Scholar]
- Morris, C.R.; Scott, J.T.; Chang, H.M.; Sederoff, R.R.; O’Malley, D.; Kadla, J.F. Metabolic profiling: a new tool in the study of wood formation. J. Agric. Food Chem. 2004, 52, 1427–1434. [Google Scholar] [CrossRef]
- Abreu, I.N.; Johansson, A.I.; Sokolowska, K.; Niittyla, T.; Sundberg, B.; Hvidsten, T.R.; Street, N.R.; Moritz, T. A metabolite roadmap of the wood-forming tissue in Populus tremula. New Phytol. 2020, 228, 1559–1572. [Google Scholar] [CrossRef]
- Wang, N.W. Comparative Studies on Secondary Phloem in Coniferous Species. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2014. [Google Scholar]
- Shi, J.T.; Liu, H.C.; Peng, J.Y.; Luo, J.Y. Seasonal Changes of Cell Types and Morphology in Secondary Phloem of Broussonetia papyrifera. J. Northwest For. Univ. 2019, 34, 202–207. [Google Scholar] [CrossRef]
- Peng, J.Y.; Lin, Q.; Shi, J.T. Histochemical staining of Pinus sylvestris wood. J. Cent. South. Univ. For. Technol. 2020, 40, 88–94. [Google Scholar] [CrossRef]
- Rensing, K.H.; Samuels, A.L. Cellular changes associated with rest and quiescence in winter-dormant vascular cambium of Pinus contorta. Trees 2004, 18, 373–380. [Google Scholar] [CrossRef]
- Little, C.H.A.; Bonga, J.M. Rest in the cambium of Abies balsamea. Can. J. Bot. 1974, 52, 1723–1730. [Google Scholar] [CrossRef]
- Sundberg, B.; Little, C.H.A.; Riding, R.T.; Sandberg, G. Levels of endogenous indole-3-acetic-acid in the vascular cambium region of Abies balsamea trees during the activity-rest-quiescence transition. Physiol. Plant. 1987, 71, 163–170. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Oribe, Y.; Kubo, T.; Funada, R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata). Ann. Bot. 2007, 100, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Fan, R.W.; Huang, J.S.; Gan, X.H.; Fang, Y.M. Ultrastructure change during the development sieve cell of secondary phloem in Cunninghamia lanceolata. J. Nanjing For. Univ. Nat. Sci. Ed. 2008, 32, 47–51. [Google Scholar]
- Budzinski, I.G.F.; Moon, D.; Linden, P.; Moritz, T.; Labate, C.A. Seasonal Variation of Carbon Metabolism in the Cambial Zone of Eucalyptus grandis. Front. Plant Sci. 2016, 7, 932. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Bucio, J.; Nieto-Jacobo, M.F.; Ramirez-Rodriguez, V.V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. Int. J. Exp. Plant Biol. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Dixon, R.A.; Harrison, M.J.; Lamb, C.J. Early events in the activation of plant defense responses. Annu. Rev. Phytopathol. 1994, 32, 479–501. [Google Scholar] [CrossRef]
- Gallinger, J.; Gross, J. Phloem Metabolites of Prunus Sp. Rather than Infection with Candidatus Phytoplasma Prunorum Influence Feeding Behavior of Cacopsylla pruni Nymphs. J. Chem. Ecol. 2020, 46, 756–770. [Google Scholar] [CrossRef] [Green Version]
- Porterfield, D.M.; Kuang, A.; Smith, P.J.; Crispi, M.L.; Musgrave, M.E. Oxygen-depleted zones inside reproductive structures of Brassicaceae: Implications for oxygen control of seed development. Can. J. Bot. 1999, 77, 1439–1446. [Google Scholar] [CrossRef]
- van Dongen, J.T.; Schurr, U.; Pfister, M.; Geigenberger, P. Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol. 2003, 131, 1529–1543. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar] [CrossRef] [Green Version]
- Baxter, A.; Mittler, R.; Suzuki, N. Ros as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Keller, F. Raffinose in chloroplasts is synthesized in the cytosol and transported across the chloroplast envelope. Plant Cell Physiol. 2009, 50, 2174–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.L. Physiological Characteristics and Metabolomics Reveal the Tolerance Mechanism to Low Nitrogen in Glycine soja Sieb.et Zucc. Seedling Leaves. Master’s Thesis, Northeast Normal University, Changchun, China, 2020. [Google Scholar]
- Abid, G.; Silue, S.; Muhovski, Y.; Jacquemin, J.M.; Toussaint, A.; Baudoin, J.P. Role of myo-inositol phosphate synthase and sucrose synthase genes in plant seed development. Gene 2009, 439, 1–10. [Google Scholar] [CrossRef]
- Fan, Y.D. Study on Serum Metabonomics of Early Colorectal Cancer Based on Liquid Chromatography-Mass Spectrometry. Master’s Thesis, Jilin University, Changchun, China, 2020. [Google Scholar]
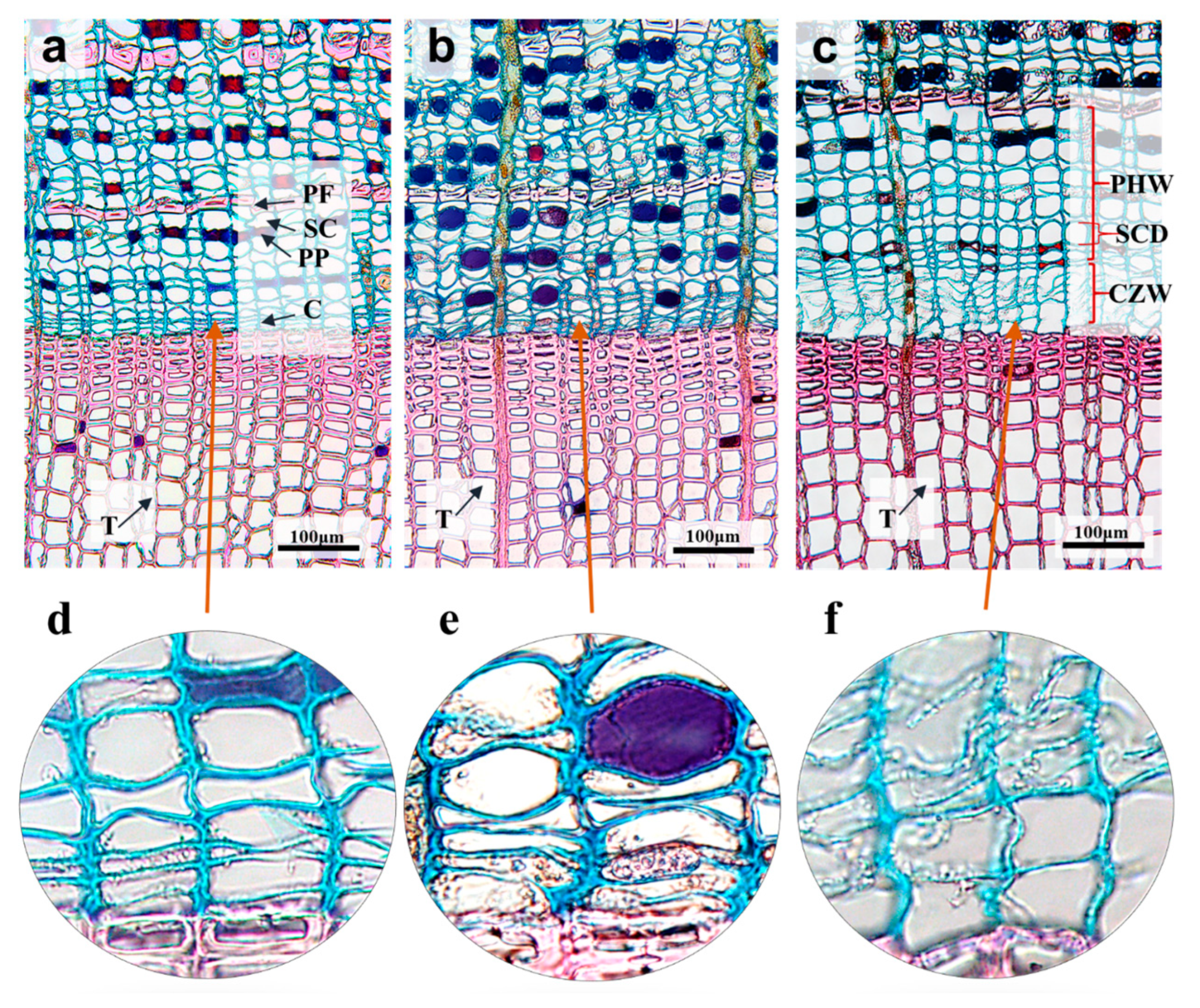


| 2 March (μm) | 28 March (μm) | 13 April (μm) | |
|---|---|---|---|
| CZW | 23.3 ± 2.5 B | 18.7 ± 5.7 B | 76.5 ± 3.0 A |
| PHW | 124.1 ± 13.5 B | 138.9 ± 6.9 B | 197.0 ± 8.5 A |
| SCD | 16.0 ± 7.9 B | 15.4 ± 7.5 B | 21.5 ± 7.4 A |
| Metabolites | Molecular Formula | Retention Time (min) | m/z | Ions Mode | Relative Amount (%) | ||
|---|---|---|---|---|---|---|---|
| 28 March | 13 April | ||||||
| Carbohydrates | Sedoheptulose | C7H14O7 | 0.7225 | 209.0666 | pos | 11.81 ± 2.87 | 24.35 ± 12.10 |
| L-Arabitol | C5H12O5 | 0.6882 | 153.0757 | neg | 118.30 ± 17.22 | 104.98 ± 14.67 | |
| 1-Kestose | C18H32O16 | 0.9513 | 527.1579 | neg | 105.52 ± 17.70 | 174.45 ± 41.79 | |
| Fructose 6-phosphate | C6H13O9P | 0.7401 | 261.0367 | neg | 6.35 ± 2.41 | 5.44 ± 2.36 | |
| Maltotetraose | C24H42O21 | 0.9801 | 689.2101 | neg | 42.10 ± 19.16 | 82.43 ± 23.94 | |
| Raffinose | C18H32O16 | 0.7528 | 522.2025 | neg | 55.93 ± 8.92 | 91.61 ± 28.11 | |
| D-Galactose | C6H12O6 | 0.6752 | 203.0524 | neg | 47.07 ± 18.54 | 106.69 ± 85.93 | |
| Adenosine | C10H13N5O4 | 1.3227 | 268.1037 | neg | 11.72 ± 2.81 | 29.61 ± 12.25 | |
| Guanosine | C10H13N5O5 | 1.3799 | 284.0986 | neg | 0.92 ± 0.36 | 3.23 ± 1.43 | |
| Organic acid | Ascorbic acid | C6H8O6 | 0.9340 | 175.0247 | pos | 1.02 ± 0.20 | 1.93 ± 0.64 |
| Isocitric acid | C6H8O7 | 0.7631 | 191.0197 | pos | 26.87 ± 3.75 | 29.02 ± 12.10 | |
| Oxoglutaric acid | C5H6O5 | 0.7488 | 145.0141 | pos | 0.65 ± 0.12 | 1.46 ± 12.10 | |
| Vanillic acid | C8H8O4 | 2.6059 | 167.0349 | pos | 1.12 ± 0.19 | 2.56 ± 12.10 | |
| 2-Hydroxycinnamic acid | C9H8O3 | 1.1800 | 182.0812 | neg | 4.95 ± 1.93 | 18.62 ± 12.10 | |
| Homogentisic acid | C8H8O4 | 0.6752 | 191.0315 | neg | 10.10 ± 1.97 | 8.43 ± 12.10 | |
| Imidazoleacetic acid | C5H6N2O2 | 0.7266 | 127.0501 | neg | 18.66 ± 18.06 | 8.64 ± 12.10 | |
| Amino Acids | Ornithine | C5H12N2O2 | 0.6841 | 131.0825 | pos | 6.17 ± 1.12 | 5.51 ± 1.49 |
| Citrulline | C6H13N3O3 | 0.6841 | 174.0883 | pos | 33.70 ± 6.49 | 27.67 ± 9.58 | |
| L-Glutamate | C5H9NO4 | 0.6882 | 148.0603 | neg | 81.70 ± 6.16 | 133.13 ± 38.65 | |
| L-leucine | C6H13NO2 | 1.4368 | 132.1019 | neg | 4.67 ± 0.82 | 18.64 ± 29.93 | |
| L-Isoleucine | C6H13NO2 | 1.5941 | 132.1019 | neg | 5.14 ± 1.22 | 19.68 ± 43.20 | |
| L-Phenylalanine | C9H11NO2 | 2.8120 | 166.0862 | neg | 25.48 ± 12.17 | 68.08 ± 30.72 | |
| Fatty acid | 2-Methylcitric acid | C7H10O7 | 1.1351 | 205.0353 | pos | 1.58 ± 0.33 | 3.17 ± 0.76 |
| (S)-dihydrolipoic acid | C8H16O2S2 | 0.6841 | 207.051 | pos | 60.27 ± 26.54 | 67.06 ± 14.35 | |
| (S)-3,4-Dihydroxybutyric acid | C4H8O4 | 0.6970 | 239.0773 | pos | 28.31 ± 9.62 | 49.27 ± 7.55 | |
| Pyruvic acid | C3H4O3 | 0.7358 | 175.0247 | pos | 1.14 ± 0.38 | 2.08 ± 0.43 | |
| 6,7-dihydroxy-4-oxo-2-heptenoic acid | C7H10O5 | 0.8907 | 173.0454 | pos | 30.48 ± 6.31 | 59.65 ± 12.40 | |
| 5-oxo-pentanoic acid | C5H8O3 | 0.6882 | 117.0545 | neg | 75.96 ± 10.51 | 69.57 ± 9.97 | |
| Butyric acid | C4H8O2 | 0.6752 | 106.0861 | neg | 2.35 ± 1.04 | 1.16 ± 0.86 | |
| beta-vinyl acrylic acid | C5H6O2 | 0.7008 | 99.04397 | neg | 35.39 ± 4.67 | 35.34 ± 6.38 | |
| Juniperic acid | C16H32O3 | 9.4623 | 290.2686 | neg | 15.44 ± 13.98 | 1.88 ± 0.63 | |
| (S)-dihydrolipoic acid | C8H16O2S2 | 0.9369 | 226.0919 | neg | 17.45 ± 3.12 | 31.20 ± 6.18 | |
| Amino Alcohols | Sphingosine | C18H37NO2 | 9.7053 | 300.2893 | neg | 26.53 ± 16.52 | 0.22 ± 0.08 |
| Terpenoid | Limonene-1,2-diol | C10H18O2 | 5.9842 | 171.1379 | neg | 0.84 ± 0.29 | 4.33 ± 6.44 |
| Unknow | 4-Hydroxy-6-methyl-2-pyrone | C6H6O3 | 0.7266 | 127.0389 | neg | 147.33 ± 26.44 | 243.62 ± 58.50 |
| Epicatechin | C15H14O6 | 4.6759 | 291.086 | neg | 104.28 ± 36.95 | 345.20 ± 271.99 | |
| 2-Amino-2-methyl-1-propanol | C4H11NO | 5.0353 | 90.0913 | neg | 7.44 ± 2.93 | 7.31 ± 2.86 | |
| Embelin | C17H26O4 | 8.7993 | 293.1768 | pos | 3.10 ± 0.99 | 2.45 ± 037 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Xia, C.; Peng, J.; Liu, X.; Pan, B. Cellular and Metabolite Changes in the Secondary Phloem of Chinese Fir (Cuninghamia lanceolata (Lamb.) Hook.) during Dormancy Release. Forests 2021, 12, 1552. https://doi.org/10.3390/f12111552
Shi J, Xia C, Peng J, Liu X, Pan B. Cellular and Metabolite Changes in the Secondary Phloem of Chinese Fir (Cuninghamia lanceolata (Lamb.) Hook.) during Dormancy Release. Forests. 2021; 12(11):1552. https://doi.org/10.3390/f12111552
Chicago/Turabian StyleShi, Jiangtao, Chongyang Xia, Junyi Peng, Xing Liu, and Biao Pan. 2021. "Cellular and Metabolite Changes in the Secondary Phloem of Chinese Fir (Cuninghamia lanceolata (Lamb.) Hook.) during Dormancy Release" Forests 12, no. 11: 1552. https://doi.org/10.3390/f12111552
APA StyleShi, J., Xia, C., Peng, J., Liu, X., & Pan, B. (2021). Cellular and Metabolite Changes in the Secondary Phloem of Chinese Fir (Cuninghamia lanceolata (Lamb.) Hook.) during Dormancy Release. Forests, 12(11), 1552. https://doi.org/10.3390/f12111552






