Litterfall and Element Return in an Abies faxoniana Forest in Tibet—A Five-Year Study
Abstract
:1. Introduction
2. Materials and Methods
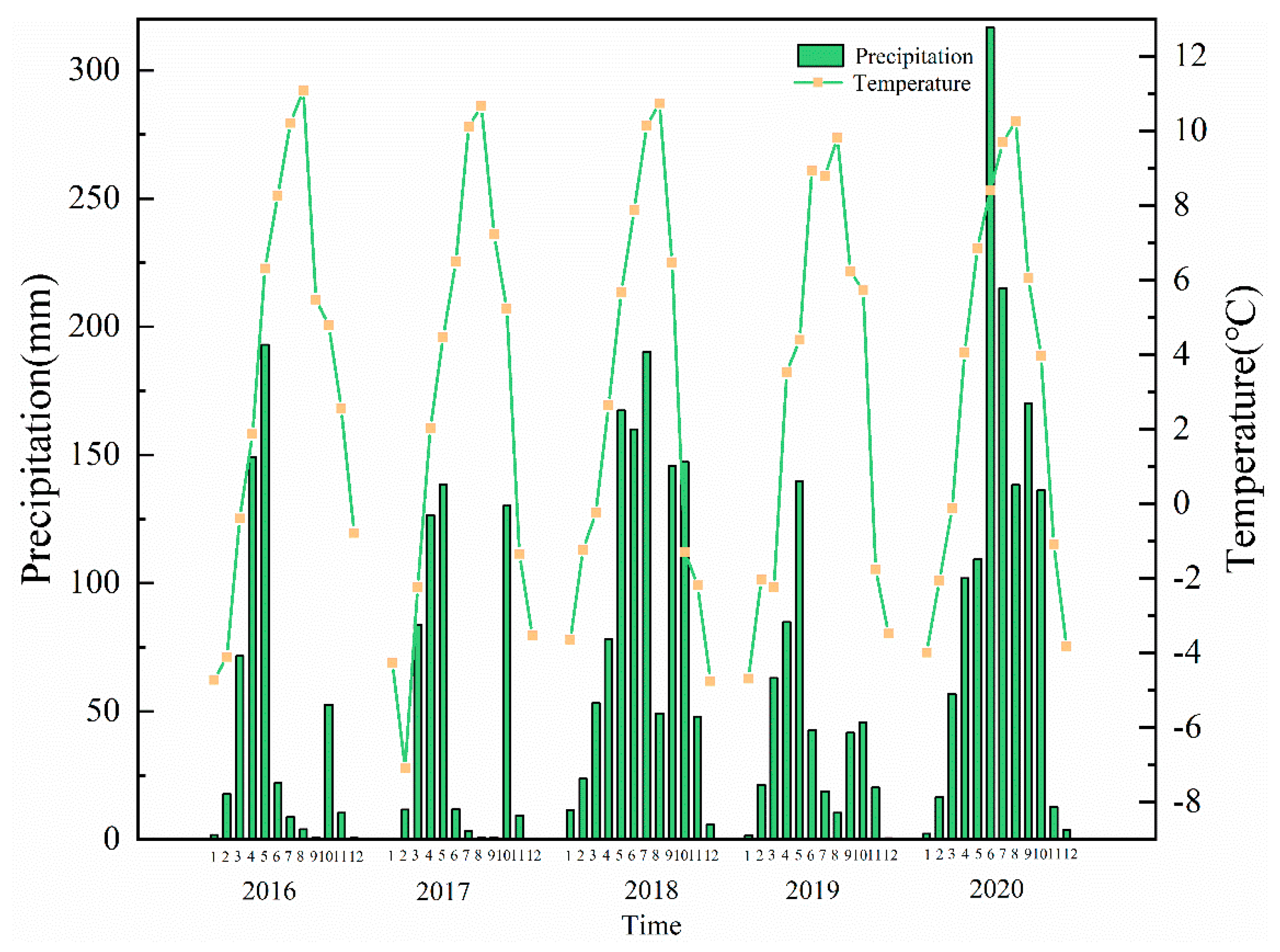
2.1. Study Site
2.2. Sample Plot Design
2.3. Litter Collection and Processing
2.4. Data Analysis
3. Results
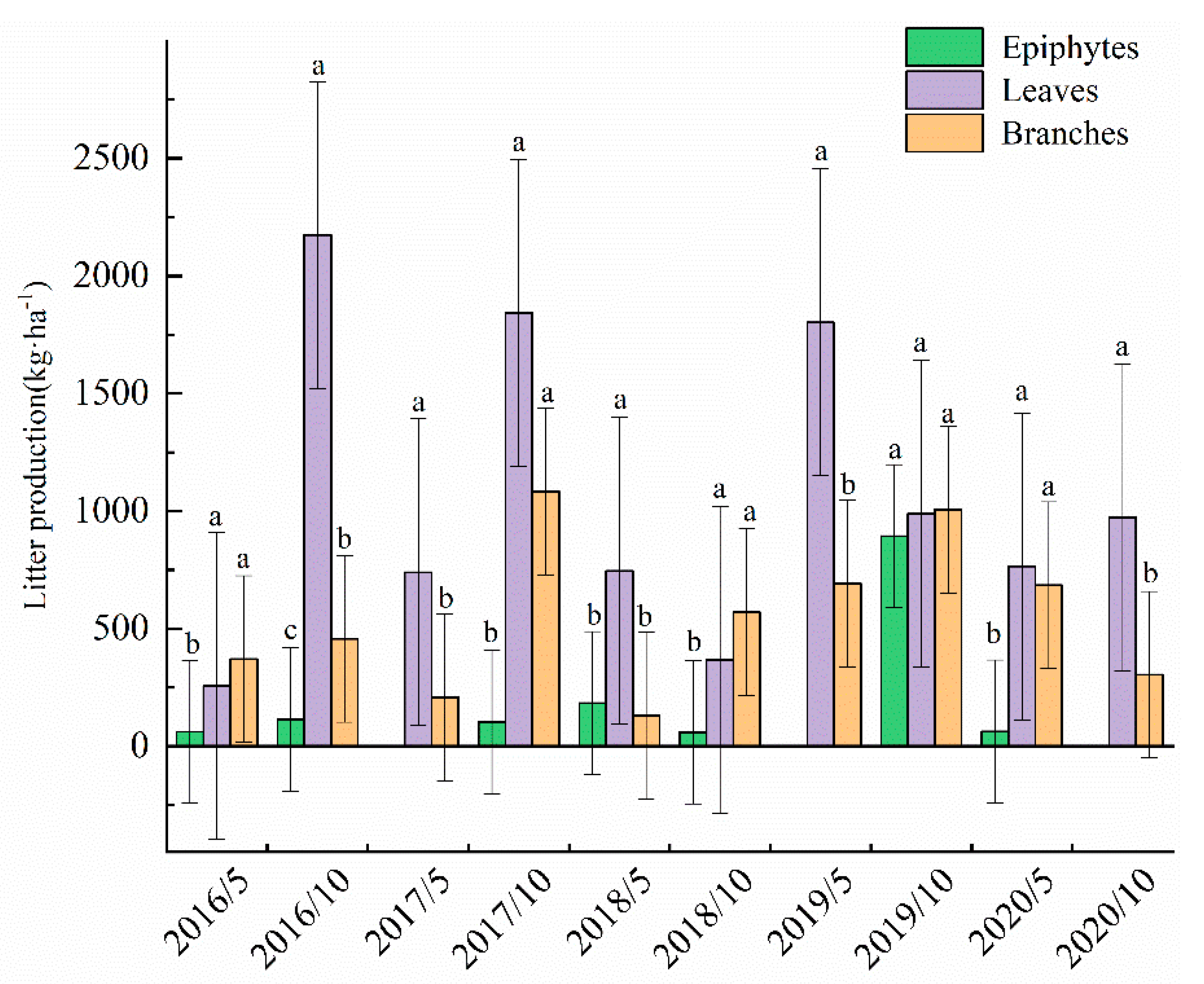
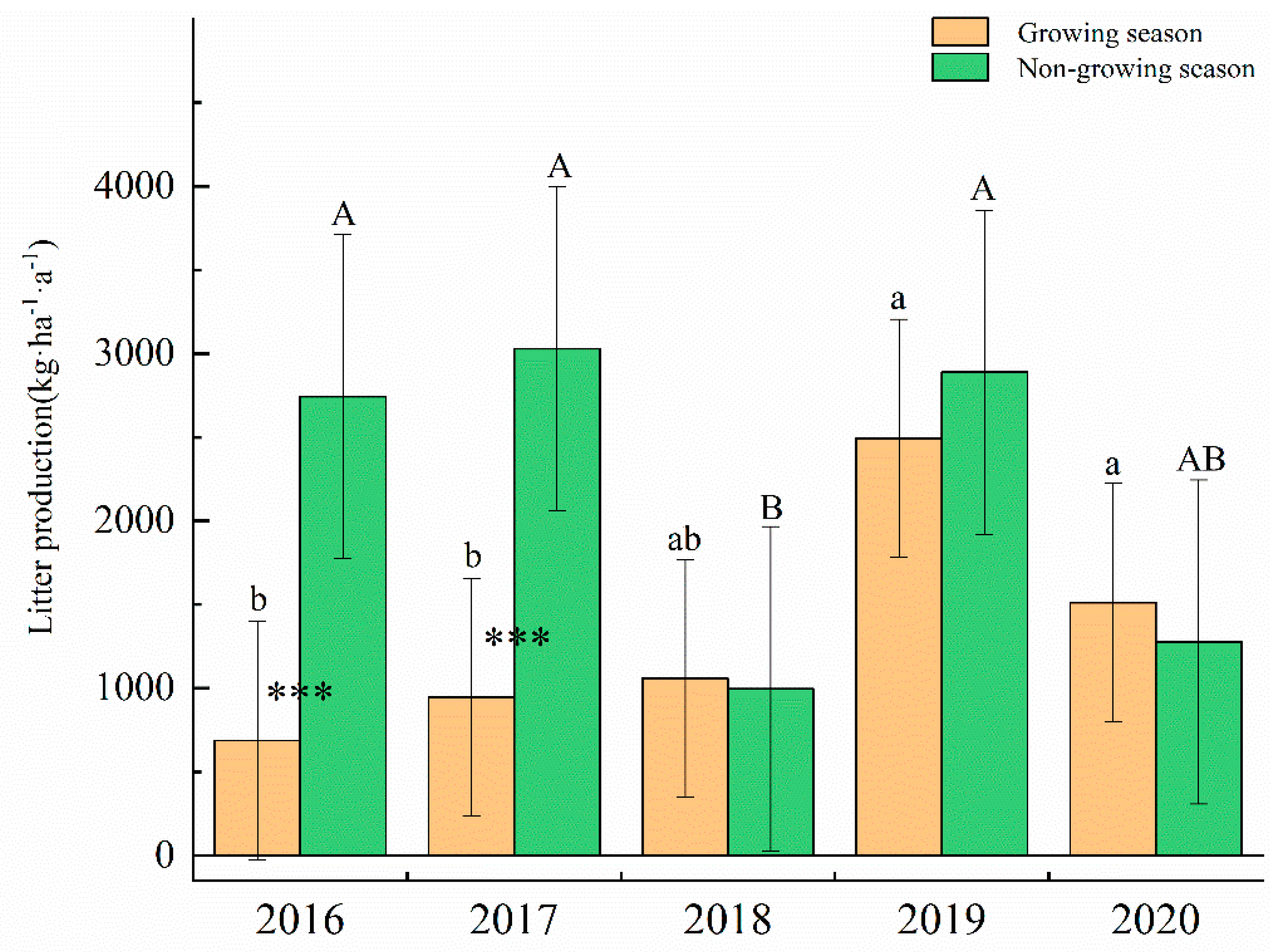
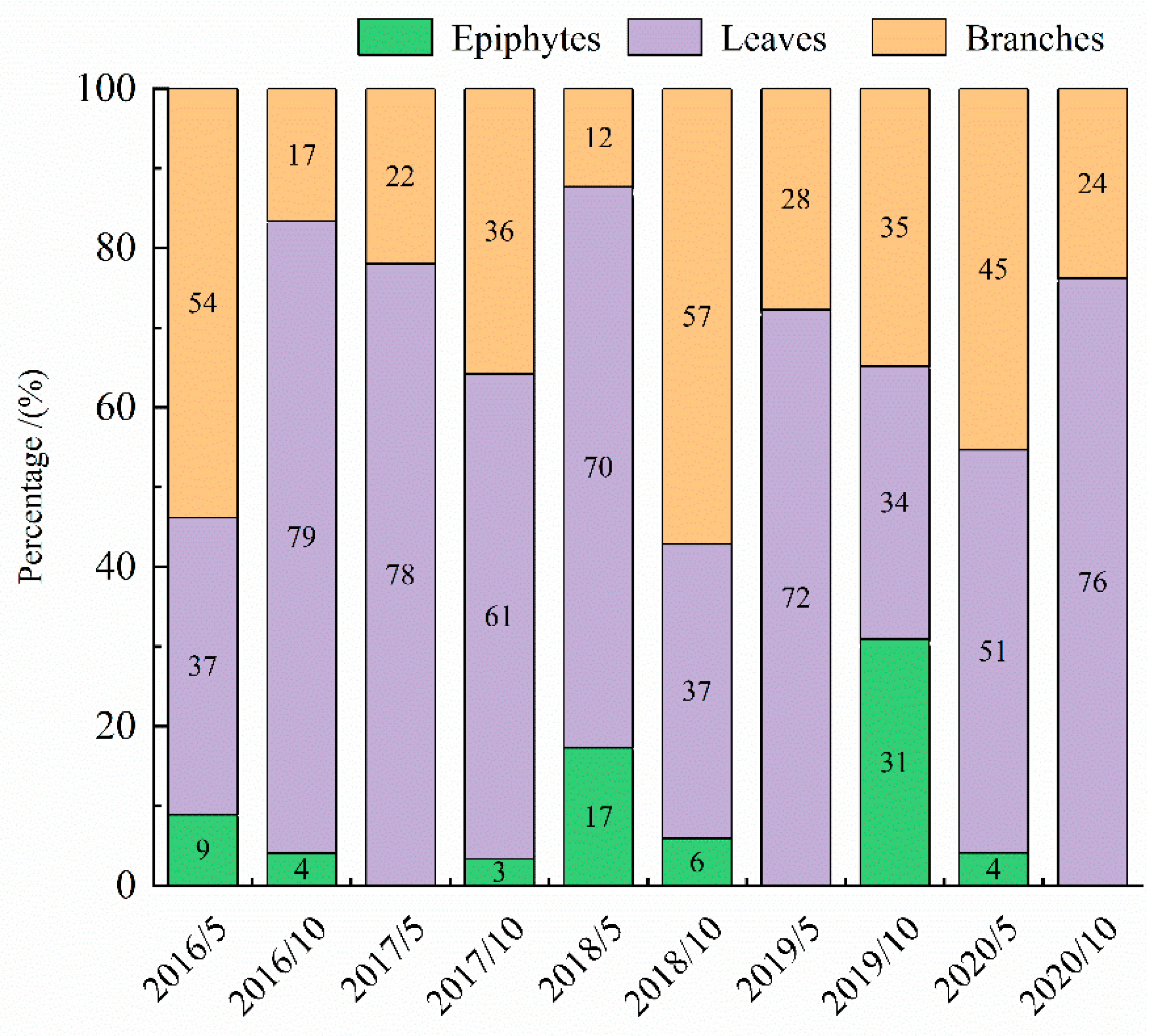
3.1. Litter Input and Composition
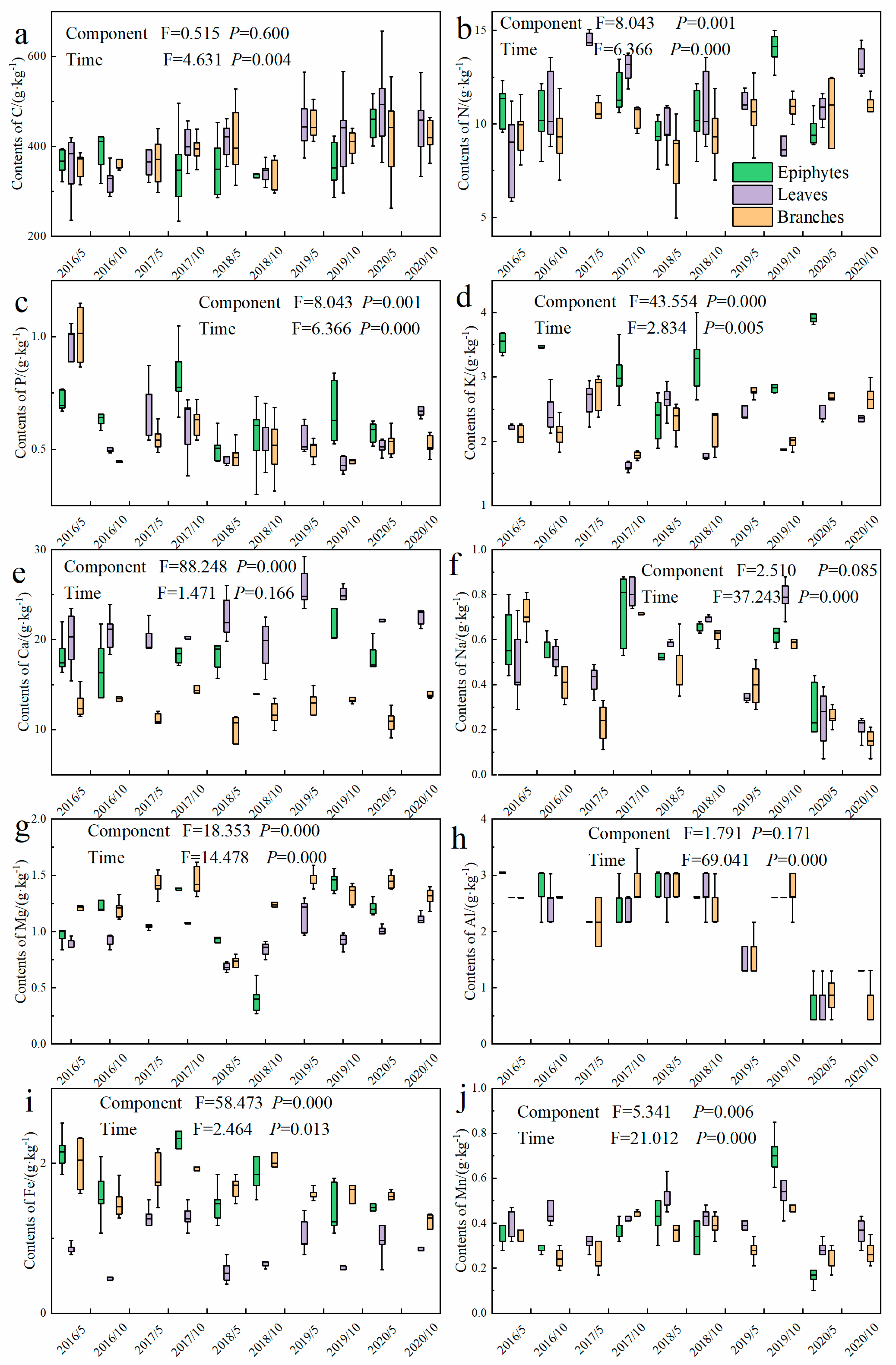
3.2. Element Contents of Litter
3.3. Element Return Amount of Litter
4. Discussion
4.1. Litter Input Production
4.2. Element Contents and Returned Amounts of Litterfall
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, G.L.; Li, J.Q.; Zuo, M. Study on litter input dynamics and nutrient return of Pinus yunnanensis forest in Mopanshan, Central Yunnan Plateau. Ecol. Environ. Sci. 2019, 28, 2158–2164. [Google Scholar]
- Li, Q.; Zhou, D.W.; Chen, X.Y. Accumulation and decomposition of above-ground litter and its role in terrestrial ecosystems. Acta Ecol. Sin. 2014, 34, 3807–3819. [Google Scholar]
- Cole, D.M.; Rapp, M. Elemental cycling in forest ecosystems. In Dynamic Properties of Forest Ecosystems; Reichle, D.E., Ed.; Cambridge University Press: Cambridge, UK, 1981; Volume 23, pp. 341–409. [Google Scholar]
- Wang, X.D.; Zhu, W.Z.; Chen, G.W. Progress of observation experiment and research on alpine ecosystem of Gongga Mountain. J. Mt. Sci. 2006, 05, 612–619. [Google Scholar]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, W.; Jiang, X.; Zakari, S.; Lu, E.; Singh, A.K.; Yang, B.; Liu, W. Conversion of primary tropical rainforest into rubber plantation degrades the hydrological functions of forest litter: Insights from experimental study. Catena 2021, 200, 105172. [Google Scholar] [CrossRef]
- Fan, Z.P.; Wang, Q.; Li, F.Y. Seasonal dynamics and driving factors of soil organic carbon in different forest types in the mountainous area of eastern Liaoning Province. J. Ecol. 2018, 37, 3220–3230. [Google Scholar]
- Qing, Q.Q.; Wang, H.Y. Spatial variability of forest litter nutrients and its influencing factors. J. Ecol. 2020, 39, 1318–1329. [Google Scholar]
- Zhou, G.; Guan, L.; Wei, X.; Zhang, D.; Zhang, Q.; Yan, J.; Wen, D.; Liu, J.; Liu, S.; Huang, Z.; et al. Litterfall production along successional and altitudinal gradients of subtropical monsoon evergreen broadleaved forests in Guangdong, China. Plant Ecol. 2007, 188, 77–89. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F.I.; Cavieres, L.A. Distinguishing local from global climate influences in the variation of carbon status with altitude in a tree line species. Glob. Ecol. Biogeogr. 2011, 20, 307–318. [Google Scholar] [CrossRef]
- Millard, P.; Sommerkorn, M.; Grelet, G.A. Environmental change and carbon limitationgan in trees: A biochemical, ecophysiological and ecosystem appraisal. New Phytol. 2007, 175, 11–28. [Google Scholar] [CrossRef]
- Wang, J.P.; Wu, Y.H. Effects of phosphorus bioavailability on mountain ecosystem. Acta. Ecol. Sin. 2016, 36, 1204–1214. [Google Scholar]
- He, X.H. Nickel—Essential metal element for plants. Bioscience 1993, 3, 45. [Google Scholar]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Chen, Y.; Zhang, J.; Liu, Y. Litter stoichiometric traits have stronger impact on humification than environment conditions in an alpine treeline ecotone. Plant Soil 2020, 453, 545–560. [Google Scholar] [CrossRef]
- Kaspari, M. The seventh macronutrient: How sodium shortfall ramifies through populations, food webs and ecosystems. Ecol. Lett. 2020, 23, 1153–1168. [Google Scholar] [CrossRef]
- Paudel, E.; Dossa, G.G.; Xu, J.; Harrison, R.D. Litterfall and nutrient return along a disturbance gradient in a tropical montane forest. For. Ecol. Manag. 2015, 353, 97–106. [Google Scholar] [CrossRef]
- Lukumbuzya, T.K.; Fyles, J.W.; Cote, B. Effects of base-cation fertilization on litter decomposition in a sugar maple forest in southern Quebec. Can. J. For. Res. 1994, 24, 447–452. [Google Scholar] [CrossRef]
- Ahirwal, J.; Saha, P.; Nath, A.; Nath, A.J.; Deb, S.; Sahoo, U.K. Forests litter dynamics and environmental patterns in the Indian Himalayan region. For. Ecol. Manag. 2021, 499, 119612. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Y.; Liu, Y.; Heděnec, P.; Peng, Y.; Xu, Z.; Tan, B.; Zhang, L.; Guo, L.; Wang, L.; et al. Effects of Litter Quality Diminish and Effects of Vegetation Type Develop During Litter Decomposition of Two Shrub Species in an Alpine Treeline Ecotone. Ecosystems 2021, 24, 197–210. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Zhou, Y.; Zheng, H.; Xu, Z.; Tan, B.; You, C.; Zhang, L.; Li, H.; Guo, L.; et al. Litter chemical traits strongly drove the carbon fractions loss during decomposition across an alpine treeline ecotone. Sci. Total Environ. 2021, 753, 142287. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Zhou, Y.; Xu, Z.; Tan, B.; You, C.; Zhang, L.; Li, H.; Zheng, H.; Guo, L.; et al. Environmental conditions and litter nutrients are key determinants of soluble C, N, and P release during litter mixture decomposition. Soil Tillage Res. 2021, 209, 104928. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; He, R.; Chen, Y.; Yang, L.; Zheng, H.; Li, H.; Xiao, J.; Liu, Y. Impacts of soil fauna on lignin and cellulose degradation in litter decomposition across an alpine forest-tundra ecotone. Eur. J. Soil Biol. 2018, 87, 53–60. [Google Scholar] [CrossRef]
- Kaspari, M.; Garcia, M.N.; Harms, K.E.; Santana, M.; Wright, S.J.; Yavitt, J.B. Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol. Lett. 2008, 11, 35–43. [Google Scholar] [CrossRef]
- Luciana, S.; Valentini, C.M.A.; Pinto, O.B.P. Seasonal and interannual litter dynamics of a tropical semideciduous forest of the southern Amazon Basin, Brazil. J. Geophys. Res. Biogeosci. 2008, 113, G4. [Google Scholar]
- Kubota, Y.; Narikawa, A.; Shimatani, K. Litter dynamics and its effects on the survival of Castanopsis sieboldii seedlings in a subtropical forest in southern Japan. Ecol. Res. 2007, 22, 792–801. [Google Scholar] [CrossRef]
- Zhu, J.; He, X.; Wu, F.; Yang, W.; Tan, B. Decomposition of Abies faxoniana litter varies with ferrze-thaw stages and altitudes in subalpine forests of southwest China. Scand. J. For. Res. 2012, 27, 586–596. [Google Scholar] [CrossRef]
- Fanin, N.; Fromin, N.; Buatois, B.; Hättenschwiler, S. An experimental test of the hypothesis of non-homeostatic consumer stoichiometry in a plant litter-microbe system. Ecol. Lett. 2013, 16, 764–772. [Google Scholar] [CrossRef]
- Zeng, X.; Fu, C.K.; Yang, J.P. Restitution dynamics of K and Na of litter in a primary forest of Sequoia chinensis and Abies minjiang. J. Ecol. 2020, 39, 1426–1435. [Google Scholar]
- Weltzin, J.F.; Keller, J.K.; Bridgham, S.D.; Pastor, J.; Allen, P.B.; Chen, J. Litter controls plant community composition in a northern fen. Oikos 2005, 110, 537–546. [Google Scholar] [CrossRef]
- Pen, G.Q.; Cui, X.; Wu, C.C.; Yang, D.M. Study on litter quantity and seasonal dynamic variation of Abies fargesii Forest at Different Altitudes in Minjiang River. Shaanxi For. Sci. Technol. 2011, 4, 1–4. [Google Scholar]
- Yang, J.P.; Liao, R.; Yang, W.Q.; Tan, B.; Fu, C.K.; Zhang, Y.; Wu, F.Z. Litter yield and dynamics of dark coniferous forest in alpine canyon region. Chin. J. Appl. Environ. Biol. 2017, 23, 103–107. [Google Scholar]
- Li, W.; Luo, J.; Cheng, G.W.; Song, M.Q. Characteristics of litterfall in Abies emei forest of Gongga Mountain. J. Mt. Sci. 2003, 21, 287–292. [Google Scholar]
- Gairola, S.; Rawal, R.S.; Dhar, U. Patterns of litterfall and return of nutrients across anthropogenic disturbance gradients in three subalpine forests of west Himalaya, India. J. For. Res. 2009, 14, 73–80. [Google Scholar] [CrossRef]
- Yang, Y.S.; Chen, Y.X.; Chen, G.S.; Guo, J.F.; Zheng, Y.M. Comparison of litter decomposition and nutrient dynamics of Chinese fir plantation in Fujian. For. Sci. 2004, 40, 2–10. [Google Scholar]
- Seta, T.; Demissew, S.; Woldu, Z. Litterfall dynamics in boter-becho borest moist evergreen montane forest of Southwestern Ethiopia. J. Ecol. Nat. Environ. 2018, 10, 13–21. [Google Scholar]
- Qi, M.Z.; Wang, K.Y. Dynamics of litter yield and nutrient return in the subalpine timberline ecotone of western Sichuan. J. Ecol. 2010, 29, 434–438. [Google Scholar]
- Chave, J.; Navarrete, D.; Almeida, S.; Álvarez, E.; Aragão, L.E.; Bonal, D.; Châtelet, P.; Silva-Espejo, J.E.; Goret, J.Y.; Hildebrand, P.V.; et al. Regional and seasonal patterns of litterfall in tropical South America. Biogeosciences 2017, 7, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.C.; Yuan, W.P.; Dong, W.J.; Liu, S. Seasonal patterns of litterfall in forest ecosystem worldwide. Ecol. Complex. 2014, 14, 884–892. [Google Scholar] [CrossRef]
- Cui, B.H.; Jiang, Y.; Chen, S.W.; Jin, M.R.; Hu, K.B.; Wang, S.Q. Dynamics of litter yield and nutrient return in subalpine coniferous forest and coniferous and broad-leaved mixed forest in western Sichuan. Sichuan For. Sci. Technol. 2012, 33, 16–20. [Google Scholar]
- Fu, C.K. Effects of Forest Gap on Litter Production and Element Return in Alpine Forest. Ph.D. Thesis, Sichuan Agricultural University, Chengdu, China, 2018. [Google Scholar]
- Vasconcelos, H.L.; Luizão, F.J. Litter production and litter nutrient concentrations in a fragmented Amazonian land. Ecol. Appl. 2014, 14, 884–892. [Google Scholar] [CrossRef]
- Samuelsonlisa, J.; Stokestom, A.; Butnorjohn, R. Eco system carbon stocks in Pinus palustris forests. Can. J. For. Res. 2014, 44, 476–486. [Google Scholar] [CrossRef]
- González-Rodríguez, H.; Domínguez-Gómez, T.G.; Cantú-Silva, I.; Gómez-Meza, M.V.; Ramírez-Lozano, R.G.; Pando-Moreno, M.; Fernández, C.J. Litterfall deposition and leaf litter nutrient return in different locations at Northeastern Mexico. Plant Ecol. 2011, 212, 1747–1757. [Google Scholar] [CrossRef]
- Yang, H.; Yin, C.Y.; Zhen, D.H.; Tang, B.; Zhao, W.Q.; Li, N.; Pu, X.Z.; Liu, Q. Stoichiometric characteristics of leaf C, N and P in growing and non-growing seasons of Picea spruce and Abies in subalpine coniferous forests of western Sichuan. Chin. J. Appl. Environ. Biol. 2017, 23, 1089–1095. [Google Scholar]
- Pan, Y.; Bai, H.T.; Li, H. Effects of cultivation area, harvest season and plant age on the composition and antibacterial activity of essential oil from rosemary. Acta Bot. Sin. 1998, 22, 566–570. [Google Scholar]
- Fu, C.; Yang, W.; Tan, B.; Xu, Z.; Zhang, Y.; Yang, J.; Ni, X.; Wu, F. Seasonal dynamics of litterfall in a subalpine spruce-fir forest on the eastern Ti-betan Plateau: Allometric scaling relationships based on one year of observations. Forests 2017, 8, 314. [Google Scholar] [CrossRef] [Green Version]
- Neumann, M.; Ukonmaanaho, L.; Johnson, J.; Benham, S.; Vesterdal, L.; Novotný, R.; Verstraeten, A.; Lundin, L.; Thimonier, A.; Michopoulos, P.; et al. Quantifying carbon and nutrient input from litterfall in European forests using field observations and modeling. Glob. Biogeochem. Cycles 2018, 32, 784–798. [Google Scholar] [CrossRef]
- Wang, J.L.; Tao, L.; Lu, Z.W. Characteristics of litterfall in spruce forest in Nyingchi, Tibet. Chin. J. Plant Ecol. 1998, 22, 566–570. [Google Scholar]
- Liu, W.F.; Fan, H.B.; Shen, F.F.; Huang, R.Z.; Yuan, Y.H.; Li, Y.Y.; Liao, Y.C. Study on litter decomposition in eucalyptus plantation with continuous age series. J. Soil Water Conserv. 2010, 24, 132–136. [Google Scholar]
- Osono, T.; Takeda, H. Organic chemical and nutrient dynamics in decomposing beech leaf litter in relation to fungal ingrowth and succession during 3-year decomposition processes in a cool temperate deciduous forest in Japan. Ecol. Res. 2001, 16, 649–670. [Google Scholar] [CrossRef]
- Asigbaase, M.; Dawoe, E.; Lomax, B.H.; Sjogersten, S. Temporal changes in litterfall and potential nutrient return in cocoa agroforestry systems under organic and conventional management, Ghana. Heliyon 2021, 7, e08051. [Google Scholar] [CrossRef] [PubMed]
| Litter Amount | Air Temperature | Precipitation | |||
|---|---|---|---|---|---|
| R | p | R | p | ||
| Seasonal | Epiphytes | 0.264 | 0.461 | 0.132 | 0.717 |
| Leaves | 0.213 | 0.555 | 0.253 | 0.480 | |
| Branches | −0.163 | 0.653 | −0.235 | 0.513 | |
| Total | 0.430 | 0.215 | −0.460 | 0.843 | |
| Annual | Epiphytes | −0.112 | 0.857 | −0.442 | 0.457 |
| Leaves | −0.449 | 0.448 | −0.034 | 0.956 | |
| Branches | −0.277 | 0.652 | −0.556 | 0.331 | |
| Total | −0.123 | 0.181 | −0.812 | 0.095 | |
| Element | Element Returned Amount (kg·ha−1·a−1) | ||||
|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | |
| C | 1179.63 b | 1569.14 ab | 744.80 b | 2275.12 a | 1243.31 ab |
| N | 30.55 d | 49.00 b | 19.80 d | 59.00 a | 32.40 c |
| P | 2.04 ab | 2.50 a | 1.03 c | 2.81 a | 1.61 b |
| K | 1.94 ± 0.96 a | 0.91 ± 0.69 b | 1.26 ± 1.85 ab | 2.00 ± 1.40 a | 1.46 ± 0.89 ab |
| Ca | 18.88 ± 12.50 a | 7.04 ±5.78 b | 9.85 ± 16.07 ab | 15.58 ± 15.48 ab | 10.26 ± 8.32 ab |
| Na | 0.31 ± 0.44 c | 0.51 ± 0.59 b | 0.23 ± 0.16 c | 0.58 ± 0.22 a | 0.13 ± 0.09 c |
| Mg | 1.20 ± 0.65 a | 0.33 ± 0.26 b | 0.49 ± 0.70 b | 0.95 ± 0.63 a | 0.66 ± 0.40 ab |
| Al | 1.58 ± 2.15 ab | 1.99 ± 1.86 a | 1.03 ± 0.77 ab | 2.29 ± 0.79 a | 0.55 ± 0.55 b |
| Fe | 1.37 ± 0.86 a | 0.45 ± 0.43 b | 0.41 ± 0.38 b | 0.98 ± 0.58 a | 0.63 ± 0.41 ab |
| Mn | 0.48 ± 0.26 a | 0.17 ± 0.14 b | 0.22 ± 0.36 b | 0.26 ± 0.23b | 0.16 ± 0.13 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Zhang, Y.; Wang, L.; Zhou, Y.; Chen, Y.; He, S.; Zhang, J.; Liu, Y. Litterfall and Element Return in an Abies faxoniana Forest in Tibet—A Five-Year Study. Forests 2021, 12, 1577. https://doi.org/10.3390/f12111577
Wu W, Zhang Y, Wang L, Zhou Y, Chen Y, He S, Zhang J, Liu Y. Litterfall and Element Return in an Abies faxoniana Forest in Tibet—A Five-Year Study. Forests. 2021; 12(11):1577. https://doi.org/10.3390/f12111577
Chicago/Turabian StyleWu, Weiting, Yabei Zhang, Lifeng Wang, Yu Zhou, Yamei Chen, Shuqin He, Jian Zhang, and Yang Liu. 2021. "Litterfall and Element Return in an Abies faxoniana Forest in Tibet—A Five-Year Study" Forests 12, no. 11: 1577. https://doi.org/10.3390/f12111577
APA StyleWu, W., Zhang, Y., Wang, L., Zhou, Y., Chen, Y., He, S., Zhang, J., & Liu, Y. (2021). Litterfall and Element Return in an Abies faxoniana Forest in Tibet—A Five-Year Study. Forests, 12(11), 1577. https://doi.org/10.3390/f12111577






