Growth Recovery and Phenological Responses of Juvenile Beech (Fagus sylvatica L.) Exposed to Spring Warming and Late Spring Frost
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Collection and Germination of Plants
2.2. Temperature Treatment in the Spring of 2018 and Late Spring Frost in 2019
2.3. Statistical Analysis
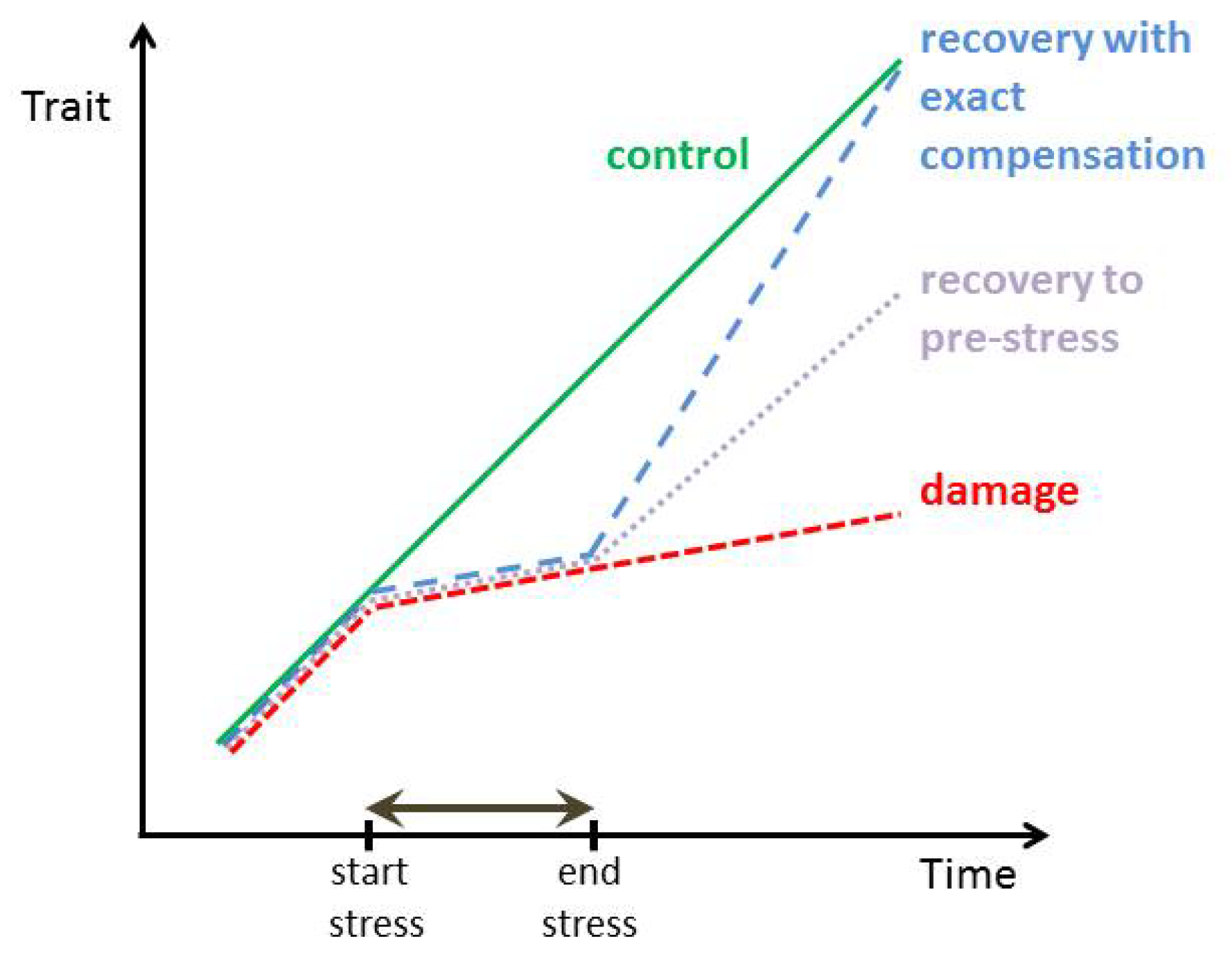
3. Results
3.1. Growth Responses to the Temperature Treatment in the Spring of 2018
3.2. Phenological Responses to the Temperature Treatment in the Spring of 2018
3.3. Damage and Growth Responses to the Late Spring Frost in 2019
3.4. Relative Leaf Chlorophyll Content in 2019
3.5. After Effects: Leaf Senescence in 2019, Bud Burst and Leaf Senescence in 2020
3.6. Differentiation among the Provenances
4. Discussion
4.1. High Temperature Stress in Early Spring
4.2. Effects of a Late Spring Frost
4.3. Population Differentiation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Gillett, N.P.; Stone, D.A.; Stott, P.A.; Nozawa, T.; Karpechko, A.Y.; Hegerl, G.C.; Wehner, M.F.; Jones, P.D. Attribution of polar warming to human influence. Nat. Geosci. 2008, 1, 750–754. [Google Scholar] [CrossRef]
- Tachibana, Y.; Nakamura, T.; Komiya, H.; Takahashi, M. Abrupt evolution of the summer Northern Hemisphere annular mode and its association with blocking. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Francis, J.A.; Vavrus, S.J. Evidence linking Arctic amplification to extreme weather in mid-latitudes. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Belmecheri, S.; Babst, F.; Hudson, A.R.; Betancourt, J.; Trouet, V. Northern Hemisphere Jet Stream Position Indices as Diagnostic Tools for Climate and Ecosystem Dynamics. Earth Interact. 2017, 21, 1–23. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [Green Version]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Shaw, R.G.; Etterson, J.R. Rapid climate change and the rate of adaptation: Insight from experimental quantitative genetics. New Phytol. 2012, 195, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Bresson, C.C.; Kremer, A.; Michalet, R.; Delzon, S. Quantifying phenological plasticity to temperature in two temperate tree species. Funct. Ecol. 2010, 24, 1211–1218. [Google Scholar] [CrossRef]
- Dittmar, C.; Zech, W.; Elling, W. Growth variations of Common beech (Fagus sylvatica L.) under different climatic and environmental conditions in Europe—A dendroecological study. For. Ecol. Manag. 2003, 173, 63–78. [Google Scholar] [CrossRef]
- Thurm, E.A.; Hernandez, L.; Baltensweiler, A.; Ayan, S.; Rasztovits, E.; Bielak, K.; Zlatanov, T.M.; Hladnik, D.; Balic, B.; Freudenschuss, A.; et al. Alternative tree species under climate warming in managed European forests. For. Ecol. Manag. 2018, 430, 485–497. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How much does climate change threaten European forest tree species distributions? Glob. Chang. Biol. 2018, 24, 1150–1163. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech—A review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Kramer, K.; Degen, B.; Buschbom, J.; Hickler, T.; Thuiller, W.; Sykes, M.T.; de Winter, W. Modelling exploration of the future of European beech (Fagus sylvatica L.) under climate change-Range, abundance, genetic diversity and adaptive response. For. Ecol. Manag. 2010, 259, 2213–2222. [Google Scholar] [CrossRef]
- Muller, M.; Kempen, T.; Finkeldey, R.; Gailing, O. Low Population Differentiation but High Phenotypic Plasticity of European Beech in Germany. Forests 2020, 11, 1354. [Google Scholar] [CrossRef]
- Stojnic, S.; Orlovic, S.; Miljkovic, D.; Galic, Z.; Kebert, M.; von Wuehlisch, G. Provenance plasticity of European beech leaf traits under differing environmental conditions at two Serbian common garden sites. Eur. J. For. Res. 2015, 134, 1109–1125. [Google Scholar] [CrossRef]
- Vitasse, Y.; Hoch, G.; Randin, C.F.; Lenz, A.; Kollas, C.; Scheepens, J.F.; Körner, C. Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 2013, 171, 663–678. [Google Scholar] [CrossRef]
- Kramer, K.; Ducousso, A.; Gomory, D.; Hansen, J.K.; Ionita, L.; Liesebach, M.; Lorent, A.; Schuler, S.; Sulkowska, M.; de Vries, S.; et al. Chilling and forcing requirements for foliage bud burst of European beech (Fagus sylvatica L.) differ between provenances and are phenotypically plastic. Agric. For. Meteorol. 2017, 234, 172–181. [Google Scholar] [CrossRef]
- Vitasse, Y.; Porte, A.J.; Kremer, A.; Michalet, R.; Delzon, S. Responses of canopy duration to temperature changes in four temperate tree species: Relative contributions of spring and autumn leaf phenology. Oecologia 2009, 161, 187–198. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstrom, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Zohner, C.M.; Mo, L.D.; Renner, S.S.; Svenning, J.C.; Vitasse, Y.; Benito, B.M.; Ordonez, A.; Baumgarten, F.; Bastin, J.F.; Sebald, V.; et al. Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proc. Natl. Acad. Sci. USA 2020, 117, 12192–12200. [Google Scholar] [CrossRef] [PubMed]
- Puchałka, R.; Koprowski, M.; Gričar, J.; Przybylak, R. Does tree-ring formation follow leaf phenology in Pedunculate oak (Quercus robur L.)? Eur. J. For. Res. 2017, 136, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Sangües-Barreda, G.; Di Filippo, A.; Piovesan, G.; Rozas, V.; Di Fiore, L.; Garcia-Hidalgo, M.; García Cervigón, A.I.; Muñoz-Garachana, D.; Baliva, M.; Olano, J.M. Warmer springs have increased the frequency and extension of late-frost defoliations in southern European beech forests. Sci. Total Environ. 2021, 775, 145860. [Google Scholar] [CrossRef]
- Ningre, F.; Colin, F. Frost damage on the terminal shoot as a risk factor of fork incidence on common beech (Fagus sylvatica L.). Ann. For. Sci. 2007, 64, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Hufkens, K.; Friedl, M.A.; Keenan, T.F.; Sonnentag, O.; Bailey, A.; O’Keefe, J.; Richardson, A.D. Ecological impacts of a widespread frost event following early spring leaf-out. Glob. Chang. Biol. 2012, 18, 2365–2377. [Google Scholar] [CrossRef]
- Dittmar, C.; Fricke, W.; Elling, W. Impact of late frost events on radial growth of common beech (Fagus sylvatica L.) in Southern Germany. Eur. J. For. Res. 2006, 125, 249–259. [Google Scholar] [CrossRef]
- Augspurger, C.K. Frost damage and its cascading negative effects on Aesculus glabra. Plant Ecol. 2011, 212, 1193–1203. [Google Scholar] [CrossRef]
- Vitasse, Y.; Rebetez, M. Unprecedented risk of spring frost damage in Switzerland and Germany in 2017. Clim. Chang. 2018, 149, 233–246. [Google Scholar] [CrossRef]
- Utkina, I.A.; Rubtsov, V.V. Studies of Phenological Forms of Pedunculate Oak. Contemp. Probl. Ecol. 2017, 10, 804–811. [Google Scholar] [CrossRef]
- Dolnicki, A.; Kraj, W. Leaf morphology and the dynamics of frost-hardiness of shoots in two phenological forms of European beech (Fagus sylvatica L.) from Southern Poland. Electronic Journal of Polish Agricultural Universities. 2001. 4. Available online: http://www.ejpau.media.pl/volume4/issue2/forestry/art-01.html (accessed on 27 October 2021).
- Kraj, W.; Sztorc, A. Genetic structure and variability of phenological forms in the European beech (Fagus sylvatica L.). Ann. For. Sci. 2009, 66, 203. [Google Scholar] [CrossRef] [Green Version]
- Kempf, M.; Banach, J.; Skrzyszewska, K. Morphological variability of beech leaves from early and late flushing provenances. Balt. For. 2018, 24, 210–217. [Google Scholar]
- Savolainen, O.; Pyhäjärvi, T.; Knürr, T. Gene Flow and Local Adaptation in Trees. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alia, R.; Gonzalez-Martinez, S.C.; Hanninen, H.; Kremer, A.; Lefevre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change—Evidence from tree populations. Glob. Chang. Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef] [Green Version]
- Kawecki, T.J.; Ebert, D. Conceptual issues in local adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef] [Green Version]
- Frank, A.; Pluess, A.R.; Howe, G.T.; Sperisen, C.; Heiri, C. Quantitative genetic differentiation and phenotypic plasticity of European beech in a heterogeneous landscape: Indications for past climate adaptation. Perspect. Plant Ecol. Evol. Syst. 2017, 26, 1–13. [Google Scholar] [CrossRef]
- Robson, T.M.; Alia, R.; Bozic, G.; Clark, J.; Forsteuter, M.; Gomory, D.; Liesebach, M.; Mertens, P.; Rasztovits, E.; Zitova, M.; et al. The timing of leaf flush in European beech (Fagus sylvatica L.) saplings. In Genetic Resources of European Beech (Fagus Sylvatica L.) for Sustainable Forestry, Proceedings of the COST E52 Final Meeting, Burgos, Spain, 4–6 May 2010; INIA: Madrid, Spain, 2011; Volume 22, pp. 61–80. [Google Scholar]
- Belsky, A.J. Does Herbivory Benefit Plants—A Review of the Evidence. Am. Nat. 1986, 127, 870–892. [Google Scholar] [CrossRef]
- D’Orangeville, L.; Maxwell, J.; Kneeshaw, D.; Pederson, N.; Duchesne, L.; Logan, T.; Houle, D.; Arseneault, D.; Beier, C.M.; Bishop, D.A.; et al. Drought timing and local climate determine the sensitivity of eastern temperate forests to drought. Glob. Chang. Biol. 2018, 24, 2339–2351. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Christensen, R.H.B. Ordinal: Regression Models for Ordinal Data. R Package Version 2015.6–28. 2015. Available online: http://www.cran.r-project.org/package=ordinal/ (accessed on 9 November 2020).
- Chung, H.; Muraoka, H.; Nakamura, M.; Han, S.; Muller, O.; Son, Y. Experimental warming studies on tree species and forest ecosystems: A literature review. J. Plant Res. 2013, 126, 447–460. [Google Scholar] [CrossRef]
- Fu, Y.S.; Campioli, M.; Vitasse, Y.; De Boeck, H.J.; Van den Berge, J.; AbdElgawad, H.; Asard, H.; Piao, S.; Deckmyn, G.; Janssens, I.A. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl. Acad. Sci. USA 2014, 111, 7355–7360. [Google Scholar] [CrossRef] [Green Version]
- Di Iorio, A.; Giacomuzzi, V.; Chiatante, D. Acclimation of fine root respiration to soil warming involves starch deposition in very fine and fine roots: A case study in Fagus sylvatica saplings. Physiol. Plant. 2016, 156, 294–310. [Google Scholar] [CrossRef]
- Martinez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Saxe, H.; Cannell, M.G.R.; Johnsen, B.; Ryan, M.G.; Vourlitis, G. Tree and forest functioning in response to global warming. New Phytol. 2001, 149, 369–399. [Google Scholar] [CrossRef]
- Frank, A.; Heiri, C.; Kupferschmid, A.D. Growth and quality of Fagus sylvatica saplings depend on seed source, site, and browsing intensity. Ecosphere 2019, 10, e02580. [Google Scholar] [CrossRef] [Green Version]
- Wiley, E.; Casper, B.B.; Helliker, B.R. Recovery following defoliation involves shifts in allocation that favour storage and reproduction over radial growth in black oak. J. Ecol. 2017, 105, 412–424. [Google Scholar] [CrossRef]
- Zohner, C.M.; Rockinger, A.; Renner, S.S. Increased autumn productivity permits temperate trees to compensate for spring frost damage. New Phytol. 2019, 221, 789–795. [Google Scholar] [CrossRef]
- Pagter, M.; Andersen, U.B.; Andersen, L. Winter warming delays dormancy release, advances budburst, alters carbohydrate metabolism and reduces yield in a temperate shrub. AoB Plants 2015, 7, plv024. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, E.; Cuneo, I.F.; Luedeling, E.; Alvarado, L.; Farias, D.; Saa, S. Starch and hexoses concentrations as physiological markers in dormancy progression of sweet cherry twigs. Trees 2019, 33, 1187–1201. [Google Scholar] [CrossRef]
- Augspurger, C.K. Spring 2007 warmth and frost: Phenology, damage and refoliation in a temperate deciduous forest. Funct. Ecol. 2009, 23, 1031–1039. [Google Scholar] [CrossRef]
- Principe, A.; van der Maaten, E.; van der Maaten-Theunissen, M.; Struwe, T.; Wilmking, M.; Kreyling, J. Low resistance but high resilience in growth of a major deciduous forest tree (Fagus sylvatica L.) in response to late spring frost in southern Germany. Trees 2017, 31, 743–751. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Bresson, C.C.; Michalet, R.; Kremer, A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res. 2009, 39, 1259–1269. [Google Scholar] [CrossRef] [Green Version]
- Vitasse, Y.; Basler, D. What role for photoperiod in the bud burst phenology of European beech. Eur. J. For. Res. 2013, 132, 851–864. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for Climate Change: Forestry and Assisted Migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Gomory, D.; Krajmerova, D.; Hrivnak, M.; Longauer, R. Assisted migration vs. close-to-nature forestry: What are the prospects for tree populations under climate change? Cent. Eur. For. J. 2020, 66, 63–70. [Google Scholar] [CrossRef]
- Vitasse, Y.; Bottero, A.; Cailleret, M.; Bigler, C.; Fonti, P.; Gessler, A.; Levesque, M.; Rohner, B.; Weber, P.; Rigling, A.; et al. Contrasting resistance and resilience to extreme drought and late spring frost in five major European tree species. Glob. Chang. Biol. 2019, 25, 3781–3792. [Google Scholar] [CrossRef] [PubMed]
- Dolschak, K.; Gartner, K.; Berger, T.W. The impact of rising temperatures on water balance and phenology of European beech (Fagus sylvatica L.) stands. Modeling Earth Syst. Environ. 2019, 5, 1347–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Country/Region/City | Provenance Name | Origin (Climate) at Home Site | Abb. | Latitude | Longitude | Altitude (m) |
|---|---|---|---|---|---|---|
| Belgium, Flanders, Brussels | Sonian | Local (Atlantic) | Fs_l | 50.755274 | 4.418704 | 132 |
| Belgium, Flanders, Wetteren | Warande | Local (Atlantic) | Fw_l | 51.007612 | 3.904923 | 9 |
| Belgium, Flanders, As | Caelenberg | Local (Atlantic) | Fc_l | 51.028484 | 5.608106 | 81 |
| Belgium, Flanders, Voeren | Roodbos | Local (continental) | Fr_l | 50.742425 | 5.854472 | 273 |
| Belgium, Flanders, Halle | Hallerbos | Local (Atlantic) | Fh_l | 50.7036 | 4.283928 | 138 |
| Belgium, Flanders, Halle | Hallerbos | Non-local: German * | Fh_nl | 50.7036 | 4.283928 | 138 |
| Belgium, Flanders, Brakel | Brakelbos | Non-local: German * | Fb_nl | 50.770969 | 3.720924 | 124 |
| Spain, Pyrenees | Linas De Broto | Local (alpine) | S | 42.630049 | −0.169068 | 1270 |
| Belgium, Walloon region | Rurbusch | Local (continental) | W | 50.506035 | 6.163101 | 604 |
| The Netherlands, Nunspeet | Elspeterbosch | Local (Atlantic) | N | 52.292467 | 5.817916 | 57 |
| Abb. | n° mpl | n° 2017 | n°t c/w | n° 2019 | % frost |
|---|---|---|---|---|---|
| Fs_l | 4 | 40 | 20/20 | 39 | 18 |
| Fw_l | 2 | 20 | 10/10 | 18 | 6 |
| Fc_l | 3 | 30 | 15/15 | 26 | 38 |
| Fr_l | 5 | 49 | 24/25 | 48 | 46 |
| Fh_l | 1 | 10 | 5/5 | 10 | 10 |
| Fh_nl | 3 | 30 | 15/15 | 29 | 31 |
| Fb_nl | 3 | 25 | 13/12 | 23 | 65 |
| S | 6 | 60 | 30/30 | 54 | 59 |
| W | 2 | 19 | 9/10 | 18 | 39 |
| N | 3 | 28 | 14/14 | 25 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vander Mijnsbrugge, K.; Malanguis, J.M.; Moreels, S.; Lauwers, A.; Thomaes, A.; De Keersmaeker, L.; Vandekerkhove, K. Growth Recovery and Phenological Responses of Juvenile Beech (Fagus sylvatica L.) Exposed to Spring Warming and Late Spring Frost. Forests 2021, 12, 1604. https://doi.org/10.3390/f12111604
Vander Mijnsbrugge K, Malanguis JM, Moreels S, Lauwers A, Thomaes A, De Keersmaeker L, Vandekerkhove K. Growth Recovery and Phenological Responses of Juvenile Beech (Fagus sylvatica L.) Exposed to Spring Warming and Late Spring Frost. Forests. 2021; 12(11):1604. https://doi.org/10.3390/f12111604
Chicago/Turabian StyleVander Mijnsbrugge, Kristine, Jessa May Malanguis, Stefaan Moreels, Amy Lauwers, Arno Thomaes, Luc De Keersmaeker, and Kris Vandekerkhove. 2021. "Growth Recovery and Phenological Responses of Juvenile Beech (Fagus sylvatica L.) Exposed to Spring Warming and Late Spring Frost" Forests 12, no. 11: 1604. https://doi.org/10.3390/f12111604
APA StyleVander Mijnsbrugge, K., Malanguis, J. M., Moreels, S., Lauwers, A., Thomaes, A., De Keersmaeker, L., & Vandekerkhove, K. (2021). Growth Recovery and Phenological Responses of Juvenile Beech (Fagus sylvatica L.) Exposed to Spring Warming and Late Spring Frost. Forests, 12(11), 1604. https://doi.org/10.3390/f12111604






