Recent Warming-Induced Tree Growth Enhancement at the Tibetan Treeline and the Link to Improved Water-Use Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Conditions and Field Sampling
2.2. Tree-Ring Width Methods
2.3. Tree-Ring δ13C Methods
2.4. Climate Variables
2.5. Growth–Climate Relationships
3. Results
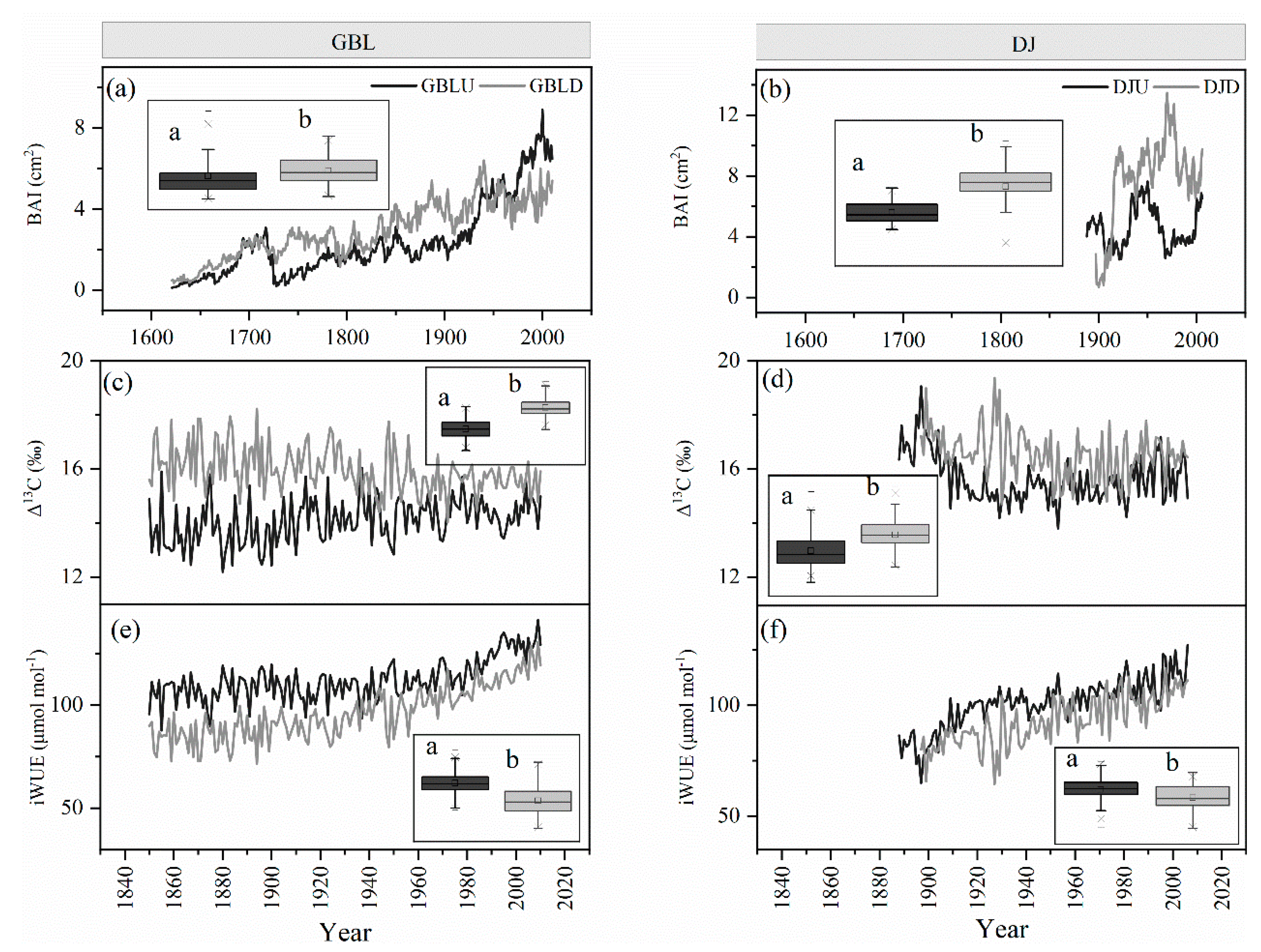
3.1. Long-Term Trend of Tree Growth
3.2. Cellulose Stable Isotopes
3.3. Tree Growth–Climate Relationships
3.4. Tree-Ring ∆ 13C Responses to Climate
3.5. Factors Regulating the Variation in BAI and iWUE
4. Discussion
4.1. Climate Effects on the BAI and the Tree-Ring Isotopes
4.2. Enhanced Tree Growth Occurred in Treelines but Not in Lower-Elevation Forests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, M.A.; Buckley, T.N.; Turnbull, T.L. Diminishing CO2-driven gains in water-use efficiency of global forests. Nat. Clim. Change 2020, 10, 466–471. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Sardans, J.; Chevallier, F.; Ciais, P.; Obersteiner, M.; Vicca, S.; Canadell, J.G.; Bastos, A.; Friedlingstein, P.; Sitch, S.; et al. Global trends in carbon sinks and their relationships with CO2 and temperature. Nat. Clim. Change 2019, 9, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Peters, W.; van der Velde, I.R.; van Schaik, E.; Miller, J.B.; Ciais, P.; Duarte, H.F.; van der Laan-Luijkx, I.T.; van der Molen, M.K.; Scholze, M.; Schaefer, K.; et al. Increased water-use efficiency and reduced CO2 uptake by plants during droughts at a continental scale. Nat. Geosci. 2018, 11, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Allen, C.D.; Marshall, L. Growth, carbon-isotope discrimination, and drought-associated mortality across a Pinus ponderosa elevational transect. Glob. Chang. Biol. 2010, 16, 399–415. [Google Scholar] [CrossRef]
- Panthi, S.; Fan, Z.-X.; van der Sleen, P.; Zuidema, P.A. Long-term physiological and growth responses of Himalayan fir to environmental change are mediated by mean climate. Glob. Chang. Biol. 2020, 26, 1778–1794. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.C.; Ballantyne, A.P.; Cooper, L.A.; Sala, A. Limited evidence for CO2 -related growth enhancement in northern Rocky Mountain lodgepole pine populations across climate gradients. Glob. Chang. Biol. 2018, 24, 3922–3937. [Google Scholar] [CrossRef]
- Penuelas, J.; Hunt, J.M.; Ogaya, R.; Jump, A.S. Twentieth century changes of tree-ring delta C-13 at the southern range-edge of Fagus sylvatica: Increasing water-use efficiency does not avoid the growth decline induced by warming at low altitudes. Glob. Chang. Biol. 2008, 14, 1076–1088. [Google Scholar] [CrossRef] [Green Version]
- Quadri, P.; Silva, L.C.R.; Zavaleta, E.S. Climate-induced reversal of tree growth patterns at a tropical treeline. Sci. Adv. 2021, 7, eabb7572. [Google Scholar] [CrossRef]
- Andreu-Hayles, L.; Planells, O.; Gutierrez, E.; Muntan, E.; Helle, G.; Anchukaitis, K.J.; Schleser, G.H. Long tree-ring chronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests. Glob. Chang. Biol. 2011, 17, 2095–2112. [Google Scholar] [CrossRef]
- Gómez-Guerrero, A.; Silva, L.C.R.; Barrera-Reyes, M.; Kishchuk, B.; Velazquez-Martinez, A.; Martinez-Trinidad, T.; Plascencia-Escalante, F.O.; Horwath, W.R. Growth decline and divergent tree ring isotopic composition (13C and 18O) contradict predictions of CO2 stimulation in high altitudinal forests. Glob. Chang. Biol. 2013, 19, 1748–1758. [Google Scholar] [CrossRef]
- Wu, G.J.; Liu, X.H.; Chen, T.; Xu, G.B.; Wang, W.Z.; Zeng, X.M.; Zhang, X.W. Elevation-dependent variations of tree growth and intrinsic water-use efficiency in Schrenk spruce (Picea schrenkiana) in the western Tianshan Mountains, China. Front. Plant Sci. 2015, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Zhu, H.F.; Liu, X.H.; Liang, E.Y.; Griessinger, J.; Wu, G.J.; Li, X.X.; Brauning, A. Does increasing intrinsic water use efficiency (iWUE) stimulate tree growth at natural alpine timberline on the southeastern Tibetan Plateau? Glob. Planet Chang. 2017, 148, 217–226. [Google Scholar] [CrossRef]
- Silva, L.C.R.; Sun, G.; Zhu-Barker, X.; Liang, Q.L.; Wu, N.; Horwath, W.R. Tree growth acceleration and expansion of alpine forests: The synergistic effect of atmospheric and edaphic change. Sci. Adv. 2016, 2, e1501302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Z.H.; Liu, H.Y.; Wu, X.C.; Hao, Q. Climate-driven speedup of alpine treeline forest growth in the Tianshan Mountains, Northwestern China. Glob. Chang. Biol. 2015, 21, 816–826. [Google Scholar] [CrossRef]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Gloor, M.; Ziv, G. Tree demography dominates long-term growth trends inferred from tree rings. Glob. Chang. Biol. 2017, 23, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu Rev. Plant. Phys. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Chang. Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Sigdel, S.R.; Wang, Y.F.; Camarero, J.J.; Zhu, H.F.; Liang, E.Y.; Penuelas, J. Moisture-mediated responsiveness of treeline shifts to global warming in the Himalayas. Glob. Chang. Biol. 2018, 24, 5549–5559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, R.L.; Kozinn, W.P. Pneumonia and Bacteremia Associated with Hemophilus-Influenzae Serotype-D. J. Clin. Microbiol. 1983, 18, 730–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Leavitt, S.W. Tree-ring C-H-O isotope variability and sampling. Sci. Total Environ. 2010, 408, 5244–5253. [Google Scholar] [CrossRef]
- Loader, N.J.; Robertson, I.; Barker, A.C.; Switsur, V.R.; Waterhouse, J.S. An improved technique for the batch processing of small wholewood samples to alpha-cellulose. Chem Geol. 1997, 136, 313–317. [Google Scholar] [CrossRef]
- Laumer, W.; Andreu, L.; Helle, G.; Schleser, G.H.; Wieloch, T.; Wissel, H. A novel approach for the homogenization of cellulose to use micro-amounts for stable isotope analyses. Rapid Commun. Mass Spectrom. 2009, 23, 1934–1940. [Google Scholar] [CrossRef]
- Berninger, F.; Sonninen, E.; Aalto, T.; Lloyd, J. Modeling C-13 discrimination in tree rings. Glob. Biogeochem. Cycles 2000, 14, 213–223. [Google Scholar] [CrossRef]
- Lefcheck, J.S. PIECEWISESEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Brownlee, A.H.; Sullivan, P.F.; Csank, A.Z.; Sveinbjornsson, B.; Ellison, S.B.Z. Drought-induced stomatal closure probably cannot explain divergent white spruce growth in the Brooks Range, Alaska, USA. Ecology 2016, 97, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Paradigm shift in plant growth control. Curr. Opin. Plant. Biol. 2015, 25, 107–114. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Oleary, M.H.; Berry, J.A. On the Relationship between Carbon Isotope Discrimination and the Inter-Cellular Carbon-Dioxide Concentration in Leaves. Aust J. Plant Physiol 1982, 9, 121–137. [Google Scholar]
- Lautner, S. Wood Formation Under Drought Stress and Salinity; Springer: Berlin/Heidelberg, Germany, 2013; Volume 20, pp. 187–202. [Google Scholar]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhausser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; Lopez, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Wolf, A.; Arango-Velez, A.; Choat, B.; Chmura, D.J.; Jansen, S.; Kolb, T.; Li, S.; Meinzer, F.C.; Pita, P.; et al. Woody plants optimise stomatal behaviour relative to hydraulic risk. Ecol. Lett. 2018, 21, 968–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabon, A.; Fernandez-de-Una, L.; Gea-Izquierdo, G.; Meinzer, F.C.; Woodruff, D.R.; Martinez-Vilalta, J.; De Caceres, M. Water potential control of turgor-driven tracheid enlargement in Scots pine at its xeric distribution edge. New Phytol. 2020, 225, 209–221. [Google Scholar] [CrossRef]
- Peters, R.L.; Steppe, K.; Cuny, H.E.; De Pauw, D.J.W.; Frank, D.C.; Schaub, M.; Rathgeber, C.B.K.; Cabon, A.; Fonti, P. Turgor-a limiting factor for radial growth in mature conifers along an elevational gradient. New Phytol. 2021, 229, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, R.; Belmecheri, S.; Ollinger, S.V.; Asbjornsen, H.; Jennings, K.; Xiao, J.; Stocker, B.D.; Martin, M.; Hollinger, D.Y.; Bracho-Garrillo, R.; et al. Disentangling the role of photosynthesis and stomatal conductance on rising forest water-use efficiency. Proc. Natl. Acad. Sci. USA 2019, 116, 16909–16914. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, W.; Xu, G.; Zeng, X.; Wu, G.; Zhang, X.; Qin, D. Tree growth and intrinsic water-use efficiency of inland riparian forests in northwestern China: Evaluation via delta C-13 and delta O-18 analysis of tree rings. Tree Physiol. 2014, 34, 966–980. [Google Scholar] [CrossRef] [Green Version]
- Mathias, J.M.; Thomas, R.B. Global tree intrinsic water use efficiency is enhanced by increased atmospheric CO(2) and modulated by climate and plant functional types. Proc. Natl. Acad. Sci. USA 2021, 118, e2014286118. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, L.; Voelker, S.; Xu, G.; Zeng, X.; Zhang, X.; Zhang, L.; Sun, W.; Zhang, Q.; Wu, G.; et al. Warming and CO2 enrichment modified the ecophysiological responses of Dahurian larch and Mongolia pine during the past century in the permafrost of northeastern China. Tree Physiol. 2019, 39, 88–103. [Google Scholar] [CrossRef]
- Timofeeva, G.; Treydte, K.; Bugmann, H.; Rigling, A.; Schaub, M.; Siegwolf, R.; Saurer, M. Long-term effects of drought on tree-ring growth and carbon isotope variability in Scots pine in a dry environment. Tree Physiol. 2017, 37, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; McDowell, N.G.; Liu, X.H.; Xu, G.B.; Wu, G.J.; Zeng, X.M.; Wang, G.X. Contrasting growth responses of Qilian juniper (Sabina przewalskii) and Qinghai spruce (Picea crassifolia) to CO2 fertilization despite common water-use efficiency increases at the northeastern Qinghai-Tibetan plateau. Tree Physiol. 2021, 41, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Yang, B.; Boucher, E.; Rossi, S. How did climate and CO2 concentration affect intrinsic water-use efficiency and tree growth in a semi-arid region of China? Trees 2021, 35, 769–781. [Google Scholar] [CrossRef]
- Hartl-Meier, C.; Zang, C.; Dittmar, C.; Esper, J.; Gottlein, A.; Rothe, A. Vulnerability of Norway spruce to climate change in mountain forests of the European Alps. Clim Res. 2014, 60, 119–132. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstrom, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Salzer, M.W.; Hughes, M.K.; Bunn, A.G.; Kipfmueller, K.F. Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. USA 2009, 106, 20348–20353. [Google Scholar] [CrossRef] [Green Version]
- Giammarchi, F.; Vacchiano, G.; Bertagnolli, A.; Ventura, M.; Panzacchi, P.; Cherubini, P.; Tonon, G. Effects of the lack of forest management on spatiotemporal dynamics of a subalpine Pinus cembra forest. Scand. J. For. Res. 2017, 32, 142–153. [Google Scholar] [CrossRef]
- McLane, S.C.; Daniels, L.D.; Aitken, S.N. Climate impacts on lodgepole pine (Pinus contorta) radial growth in a provenance experiment. For. Ecol. Manag. 2011, 262, 115–123. [Google Scholar] [CrossRef]
- Pu, X.; Wang, X.; Lyu, L. Tree-Ring Isotopes Provide Clues for Sink Limitation on Treeline Formation on the Tibetan Plateau. Atmosphere 2021, 12, 540. [Google Scholar] [CrossRef]
- Lavergne, A.; Voelker, S.; Csank, A.; Graven, H.; de Boer, H.J.; Daux, V.; Robertson, I.; Dorado-Linan, I.; Martinez-Sancho, E.; Battipaglia, G.; et al. Historical changes in the stomatal limitation of photosynthesis: Empirical support for an optimality principle. New Phytol. 2020, 225, 2484–2497. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Prentice, I.C.; Davis, T.W.; Keenan, T.F.; Wright, I.J.; Peng, C. Photosynthetic responses to altitude: An explanation based on optimality principles. New Phytol. 2017, 213, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Bresson, C.C.; Kowalski, A.S.; Kremer, A.; Delzon, S. Evidence of altitudinal increase in photosynthetic capacity: Gas exchange measurements at ambient and constant CO2 partial pressures. Ann. For. Sci. 2009, 66, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Siegwolf, R.T.; Durka, W.; Korner, C. Phylogenetically balanced evidence for structural and carbon isotope responses in plants along elevational gradients. Oecologia 2010, 162, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Tortosa, D.; Castro, J.; Villar-Salvador, P.; Vinegla, B.; Matias, L.; Michelsen, A.; de Casas, R.R.; Querejeta, J.I. The “isohydric trap”: A proposed feedback between water shortage, stomatal regulation, and nutrient acquisition drives differential growth and survival of European pines under climatic dryness. Glob. Chang. Biol. 2018, 24, 4069–4083. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Liang, E.R.; Wang, Y.F.; Babst, F.; Camarero, J.J. Mountain treelines climb slowly despite rapid climate warming. Glob. Ecol. Biogeogr. 2021, 30, 305–315. [Google Scholar] [CrossRef]
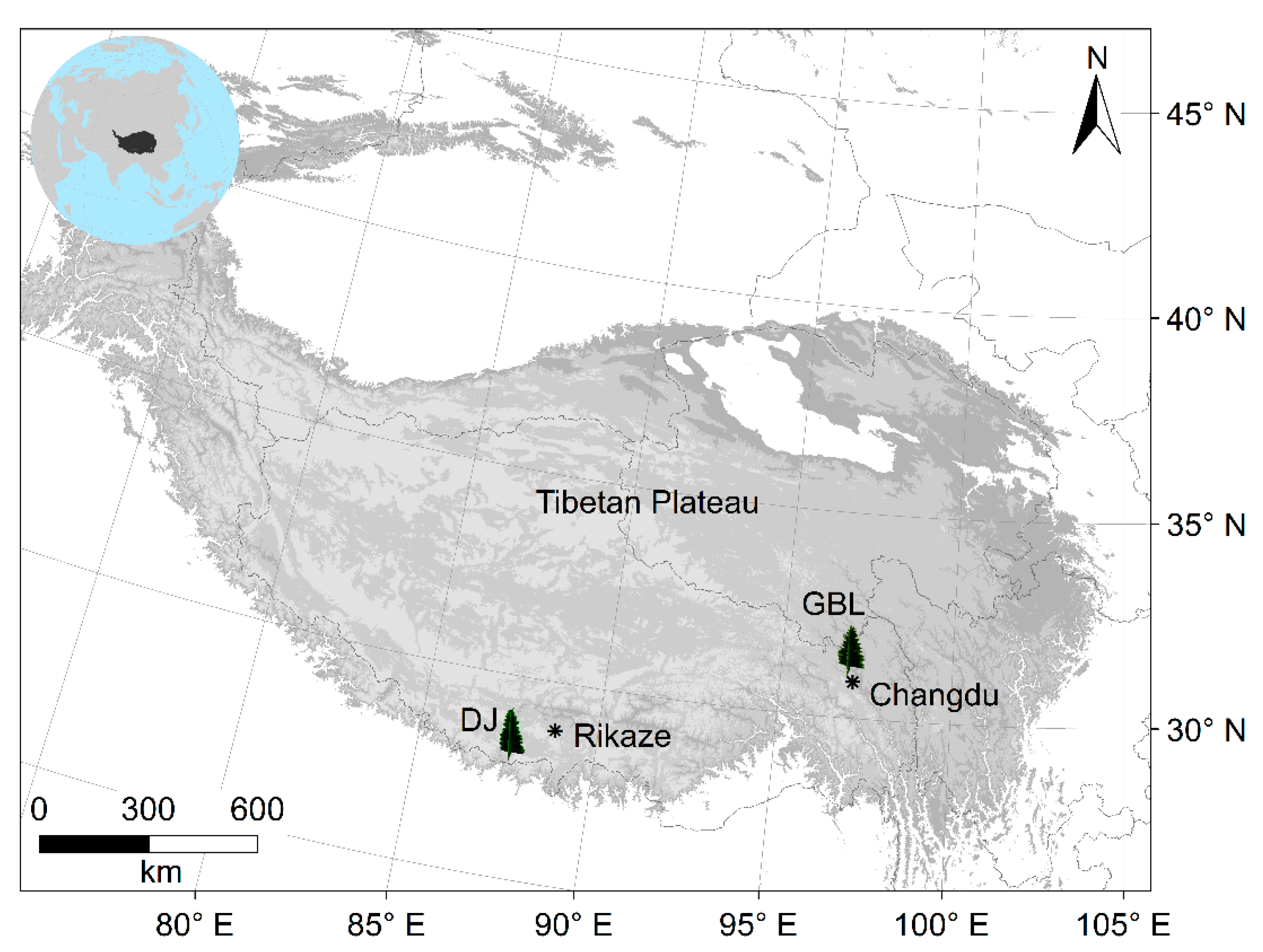




| Site | Species | Latitude (°N) | Longitude (°E) | Altitude (m) | Cores/Trees | TRW Time Span | Isotope Data Time Span |
|---|---|---|---|---|---|---|---|
| GBLU | PIBA | 31 | 96.97 | 4557 | 29/29 | 1773–2010 | 1850–2010 |
| GBLD | PIBA | 31 | 96.96 | 4455 | 33/33 | 1621–2010 | 1850–2010 |
| DJU | ABSP | 27.837 | 87.47 | 3920 | 19/19 | 1780–2006 | 1888–2006 |
| DJD | ABSP | 27.84 | 87.46 | 3410 | 13/13 | 1893–2006 | 1897–2006 |
| Site | Response Variable | Predictor Variable | β | S.E. | Critical Value | p-Value |
|---|---|---|---|---|---|---|
| Treeline | BAI | Tgrs | 0.6537 | 0.1938 | 6.7469 | <0.001 |
| BAI | VPD | −0.4339 | 0.1961 | −5.4778 | <0.001 | |
| BAI | iWUE | 0.3659 | 0.0128 | 4.4411 | <0.001 | |
| iWUE | Tgrs | 0.6215 | 1.2916 | 6.1758 | <0.001 | |
| iWUE | VPD | −0.3059 | 1.5057 | −3.2257 | <0.01 | |
| iWUE | Ca | 0.3443 | 0.0488 | 4.0213 | <0.001 | |
| Lower-elevation | BAI | Tgrs | −0.9092 | 0.3387 | −9.0619 | <0.001 |
| BAI | VPD | 0.4058 | 0.3524 | 4.8106 | <0.001 | |
| BAI | iWUE | −0.0258 | 0.0262 | −0.2948 | 0.769 | |
| iWUE | Tgrs | 0.4557 | 1.19 | 4.3074 | <0.001 | |
| iWUE | VPD | −0.0412 | 1.3873 | −0.4137 | 0.6803 | |
| iWUE | Ca | 0.4094 | 0.045 | 4.5477 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, X.; Wang, X.; Lyu, L. Recent Warming-Induced Tree Growth Enhancement at the Tibetan Treeline and the Link to Improved Water-Use Efficiency. Forests 2021, 12, 1702. https://doi.org/10.3390/f12121702
Pu X, Wang X, Lyu L. Recent Warming-Induced Tree Growth Enhancement at the Tibetan Treeline and the Link to Improved Water-Use Efficiency. Forests. 2021; 12(12):1702. https://doi.org/10.3390/f12121702
Chicago/Turabian StylePu, Xing, Xiaochun Wang, and Lixin Lyu. 2021. "Recent Warming-Induced Tree Growth Enhancement at the Tibetan Treeline and the Link to Improved Water-Use Efficiency" Forests 12, no. 12: 1702. https://doi.org/10.3390/f12121702
APA StylePu, X., Wang, X., & Lyu, L. (2021). Recent Warming-Induced Tree Growth Enhancement at the Tibetan Treeline and the Link to Improved Water-Use Efficiency. Forests, 12(12), 1702. https://doi.org/10.3390/f12121702






