Abstract
Adaptive forest management (AFM) is an urgent need because of the uncertainty regarding how changes in the climate will affect the structure, composition and function of forests during the next decades. Current research initiatives for the long-term monitoring of impacts of silviculture are scattered and not integrated into research networks, with the consequent losses of opportunities and capacity for action. To increase the scientific and practical impacts of these experiences, it is necessary to establish logical frameworks that harmonize the information and help us to define the most appropriate treatments. In this context, a number of research groups in Spain have produced research achievements and know-how during the last decades that can allow for the improvement in AFM. These groups address the issue of AFM from different fields, such as ecophysiology, ecohydrology and forest ecology, thus resulting in valuable but dispersed expertise. The main objective of this work is to introduce a comprehensive strategy aimed to study the implementation of AFM in Spain. As a first step, a network of 34 experimental sites managed by 14 different research groups is proposed and justified. As a second step, the most important AFM impacts on Mediterranean pines, as one of the most extended natural and planted forest types in Spain, are presented. Finally, open questions dealing with key aspects when attempting to implement an AFM framework are discussed. This study is expected to contribute to better outlining the procedures and steps needed to implement regional frameworks for AFM.
1. Introduction
1.1. Forests and Climate Change
The current and future consequences of climate change directly affect fundamental climate variables, such as temperature, precipitation, and other components of the water and energy balances (cloudiness, albedo, evapotranspiration, soil moisture, runoff, etc.) [1,2,3,4], thus influencing water availability, aridity and key plant functional traits [5,6]. If these biophysical variables are considered at the biome scale, it would then be possible to forecast the evolution of such variables through climate scenarios [7]. For instance, models show that the warm and dry North African phyto-climates are expected to dominate in some transitional and temperate zones of the Mediterranean Basin [8].
Despite the high certainty regarding the changes in environmental regions, the transposition of these changes into impacts on the structure and functions of forest ecosystems is, however, not straightforward [2,9]. This is mainly due to: (a) the inherent uncertainty in the climate regionalization process, which makes it difficult to determine the change in a local climate affecting a particular forest stand; (b) the lack of linearity in the forest responses to changes in climate, including more-extreme climatic events, such as extreme droughts or heat waves, making it necessary to translate these variables into others with greater physiological significance; (c) the high variability in forest responses to extreme climatic values, compared to the means commonly reported and used in models [10,11] and (d) the differential responses that different tree taxa, provenances and genotypes can exhibit in the face of climatic variability, as well as the plasticity within populations according to their size and life stage. Thus, the use of relatively simple climate scenarios, with a reduced number of variables, is unlikely to produce an accurate picture of the changes in a particular forest ecosystem [2]. This lack of realism in such projections of forest responses is especially relevant in drought-prone territories with high biogeoclimatic variability, such as the Iberian Peninsula [12].
Direct observations [1,13,14,15] or process-based models [16,17] clearly demonstrate the impacts that climate change might have on forest ecosystems through changes in the disturbance regimes [18]. Spain is not an exception; in the case of forest fires, for instance, the change in the risk indices, the longer duration of the fire season [19], their increasing frequency and intensity [20,21] and the increasing area affected are metrics that show how recent changes in the fire regime may affect forest resilience [22]. Additionally, the extreme droughts registered in the last years and their interaction with other agents, such as bark beetles or defoliating insects, have affected some forests very negatively [23,24,25,26,27,28]. The incidence of forest pests and diseases is also affected by climate change [25,29,30], either due to more favorable conditions for the life cycle of pathogens or an increase in magnitude underwater- or heat-stress conditions [31,32].
1.2. The Need for Adaptive Forest Management
The impact of climate and global changes on forest ecosystem goods and services, such as productivity, timber, water resources, habitat for wildlife, or biodiversity, is a well-known fact that has gone far beyond research through international policy actions [33]. Many governments and institutions have already started to develop strategies of adaptive forest management (AFM) in order to preserve or improve forest ecosystem services in the context of climate change (e.g., US Forest Service, 2008–2010).
AFM aims to adapt forests to the new environmental conditions determined by future changes in climate or, specifically, to improve forest resilience to changing disturbance regimes [18]. The nature and extent of the observed and/or projected impacts on different types of forests and biomes may differ, requiring the development of local adaptive management strategies. This has led to the development of contrasting concepts, such as reactive versus proactive adaptive silviculture [2] or anticipating and mitigating risks as opposed to promoting forest resilience [18]. These contrasting terms correspond to the extremes of a continuum ranging from little or no apparent forest damage to the presence of very-severe impacts, such as tree dieback and mortality across hundreds of hectares [14]. Within the AFM framework adopted by Millar et al. [34] and translated into management options in Janowiak et al. [35] and Nagel et al. [36], this continuum is represented by several silvicultural strategies, depending on both the magnitude of the expected impacts and the changes in forest structure and functioning: (a) no actions, encompassing intrinsic forest ecosystem responses to changes under the absence of forest management; (b) resistance treatments, such as actions improving the defense of forests against changes and disturbances, focused on limiting the impacts on ecosystem structure and functioning; (c) resilience treatments, such as actions that allow for a certain degree of change but also allow the return to previous or reference conditions after a particular disturbance; and (d) transition treatments, such as actions that intentionally accommodate and facilitate change and allow forest ecosystems to respond adaptively to changing and new conditions.
The increase in forest cover during recent decades through the colonization of abandoned agricultural land, reforestation and forest encroachment into former croplands and grasslands has not been accompanied by active forest management in most Spanish forests [37,38,39]. As a result, the vulnerability of current mature forests to disturbance has increased, especially for those growing under Mediterranean conditions [40,41,42,43]. Within the continuum of management strategies that can be posed, a form of AFM that consists of silviculture practices oriented towards the reduction of stand evapotranspiration, such as tree density reduction or understory treatments, may improve forest adaptation to climate change through the watering effect on the remaining trees [44,45]. These practices can be also congruent with promoting more fire-resilient forests at the landscape level [46,47], reducing carbon loss and stimulating resistance to drought and pests [48].
Forest management has traditionally been focused on objectives, such as biomass production or soil protection, based on “static” historical climatic values. This is not necessarily adequate in the current changing climatic context, with the validity of such an approach depending on specific vegetation-climate interactions [9,34,49]. As a consequence, it seems that AFM‘s objectives should be based on a range of new criteria, which must be related to the responses of forests to recent and coming changes. In this respect, field experiments and modeling approaches providing long-term data are essential for a correct diagnosis when facing the issue of climate change in a more-holistic way [43,48,49,50].
1.3. The Need of Coordinating Efforts among Research Groups Addressing AFM in Spain
The project ALTER-net (http://www.alter-net.info/, last accesed on 18 December 2021) was the first one in creating a European coordinated network (LTER-Europe) aimed at putting together long-term series of environmental variables. Further efforts were carried out through the eLTER-H2020 project (https://www.lter-europe.net/elter, last accesed on 18 December 2021), where the LTER-Europe network collaborated with other European networks to increment experimental sites and the range of environmental and social issues analyzed. Spain is participating through 31 experimental sites (https://deims.org/; last accessed on 19 August 2021). In addition, within the European ICP-forests program [51], the Spanish network of forest sites consists of 674 experimental locations with (1) standard monitoring level I (N = 620 plots) in order to gain insight into the geographic and temporal variations in forest condition (e.g., tree defoliation), and/or (2) intensive monitoring level II (N = 54 plots), which are selected forest ecosystems aiming to clarify cause-effect relationships.
Apart from the European initiatives where Spanish experimental sites are participating, the 4th National Forest Inventory [52] has more than 90,000 permanent plots which are a very valuable tool for assessing the ongoing forest responses to climate change in terms of forest productivity, structure, composition and biodiversity (see for instance Astigarraga et al., 2020 [53]). There are, however, important aspects that are hardly addressed by forest inventory data and require long-term experiments providing, for example, quantitative information about forest management effects on key variables and processes [49].
The four most important disturbances affecting the Mediterranean basin can be categorized as droughts, pests, wildfires and windstorms [48]. An improved evaluation of direct drought impacts and how AFM can modulate them requires a better understanding of water-related processes (see key messages from EU-COSTS actions, such as FORMAN, ECHOES and CLIMO, https://www.cost.eu/, last accesed on 18 December 2021), the identification of traits involved in plant adaptive strategies under drought and the transposition of plant responses into structural changes at the ecosystem level. As stated in Tramblay et al. (2020) [54], this holistic approach may be possible by accounting for (a) water balance processes within the soil-plant-atmosphere continuum, which allows the soil water available to plants to be quantified, (b) plant functional responses to drought through key phenological, morphological and physiological traits that represent the diversity of short- and long-term plant drought strategies, and (c) ecosystem-level adjustments of plant density/species composition to accurately partition surface runoff, transpiration and evaporation. Within this water-based approach, AFM can address forest responses to wildfires, pests and windstorms and thus potential trade-offs could be further evaluated [48].
The variety and extension of forested areas in Spain, the differences in climates, species, management and socio-economic aspects among its regions, have led several research groups to separately address the issue of adaptive management through disciplines, such as plant physiology, ecohydrology, land restoration, ecological modeling or biogeochemistry. Complementary to the European initiatives and the National Forest Inventory, these groups have deployed their own experimental sites and datasets and have produced results so far that could allow for a holistic assessment of AFM criteria with regard to climate change, especially for water-limited regions where impacts are already noticeable and specific actions should be promoted. The challenge remained to coordinate these efforts to create and promote a coherent experimental network.
1.4. Objectives
The main aim of this work is to demonstrate how different experimental sites, monitoring schemes and results regarding AFM may be engaged into a national network (Silvadapt.net) in order to define a practical framework and roadmap of adaptive silviculture in Spain, where active management is specially called to improve forest adaptation to climate change in arid and semi-arid regions [48]. In the first section, we describe existing experimental sites that are proposed to be part of Silvadapt.net. The second section highlights how the results from selected sites (Mediterranean pine forests, one of the most representative forest types in Spain) can be scaled-out in terms of AFM, in order to show the potential when integrating them into a collaborative network. The last section focuses on open questions and the next steps we believe need to be further addressed.
2. Implementing Adaptive Forest Management in Spain
2.1. Framing Different Climates and Forest Types into a Nation-Wide Approach of AFM
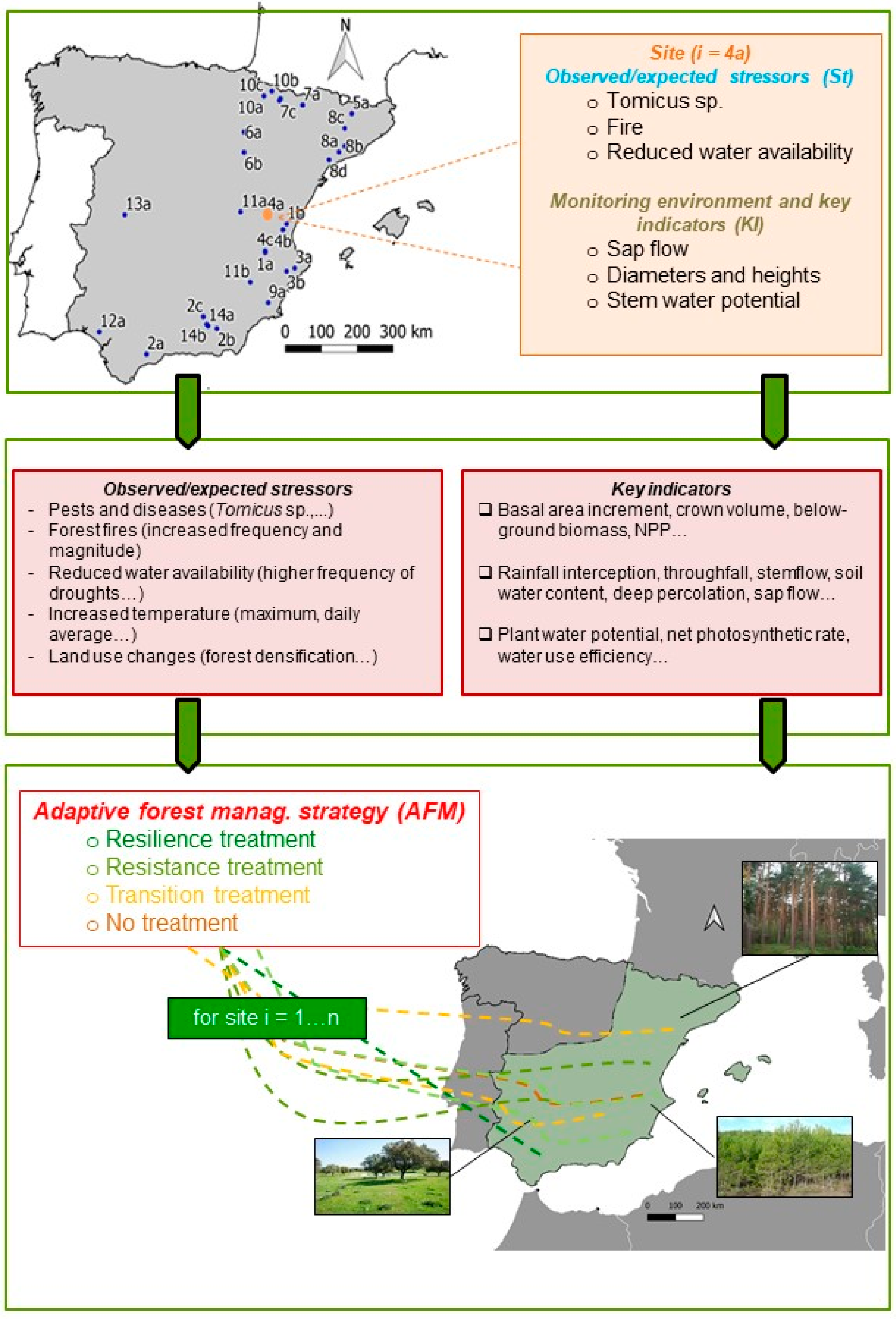
A total of 34 experimental sites, located across a wide geographic and altitudinal range (36.716–42.813° N, −6.468–1.802° W, 140–1800 m a.s.l.) and managed by 14 Spanish research groups, have been identified to be gathered in a nation-wide AFM network (SilvAdapt.net) (Table 1, Figure 1, Appendix A).

Table 1.
Description of the experimental sites selected, including: (a) the pool of overstory tree species; (b) the forest treatments assayed: NT: no actions, T: vegetation/soil treatments (including different degrees of intervention for a particular study site); and (c) the monitored variables: A: climate, B: forest structure, growth or survival, C: hydrology, D: physiology, E: dendrochronology, F: biogeochemistry, G: biodiversity, H: soil processes. In most of the experimental sites, there are also measurements of soil properties, but they are not shown for simplicity. The location of sites (1a–14b) is presented in Figure 1 “Forest treat.” Refers to management actions that are tested in the experimental sites. C: control; Thinning information: Lth: low thinning intensity treatment; Mth: moderate thinning intensity treatment; Hth: high thinning intensity treatment. See Appendix A for more detailed information about the measurements carried out within the experimental sites.
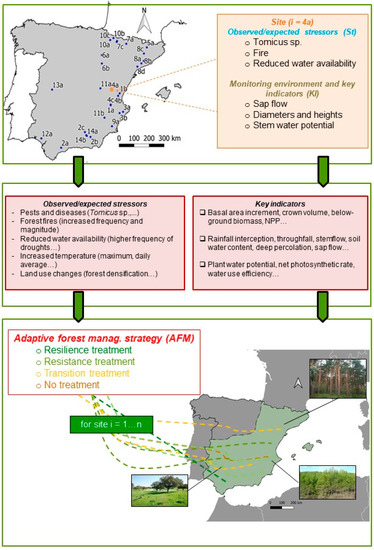
Figure 1.
Schematic overview of the AFM framework proposed for Spanish forests. The map of Spain shows the distribution of the 34 experimental sites. Each site is very specific regarding the impacts observed, the processes and indicators being monitored and the methodological and research approach used, thus limiting the extrapolation and usefulness of these site-based results. Integrating the sites in a network can provide the foundations to better analyze, understand and extend the results towards a region-wide framework for AMF. See Table 1 and Appendix A for a detailed description of the measurements taken at every experimental site.
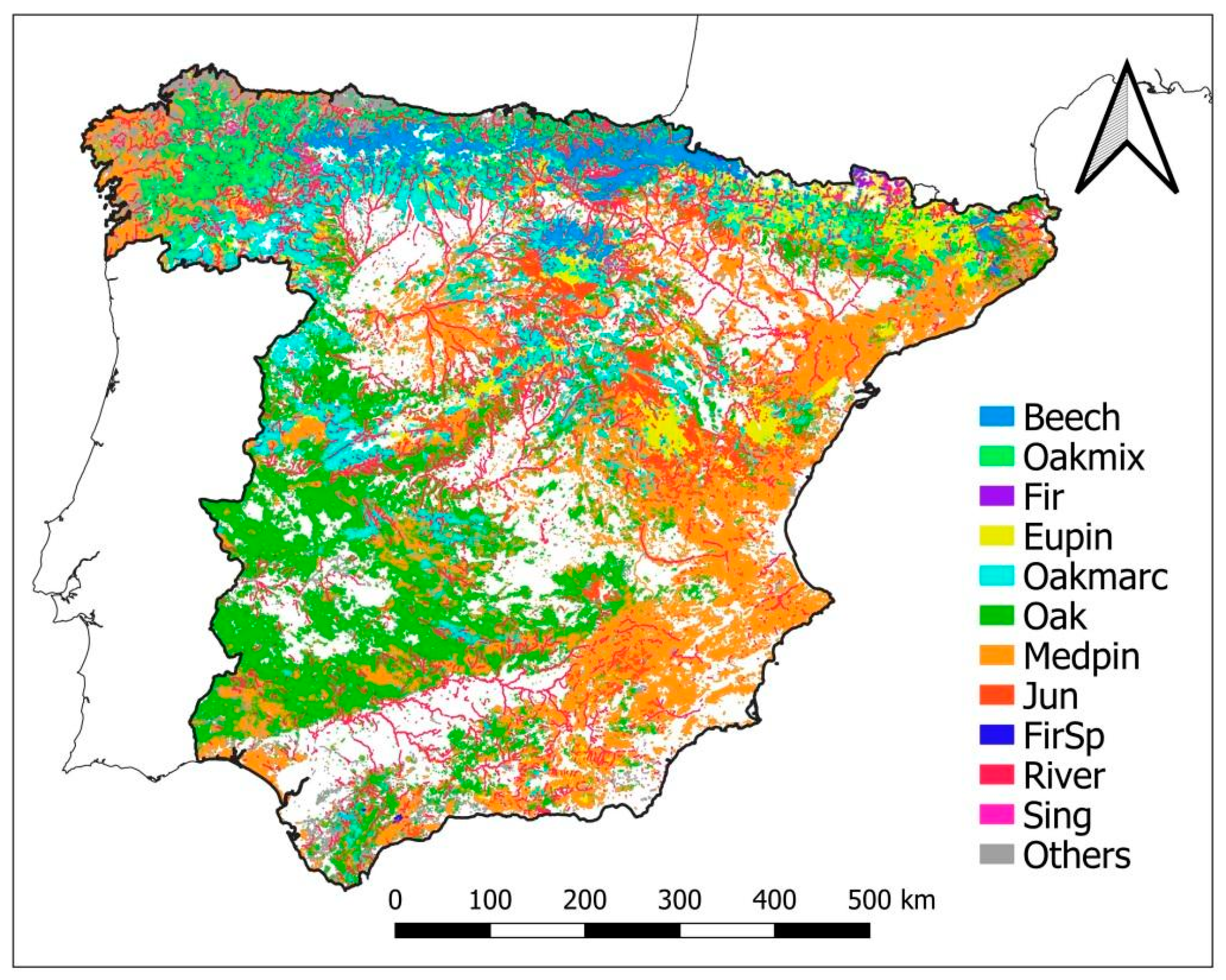
The bioclimatic and forest typology representativeness of the Network were described according to the bioclimatic system of Allué (1990) [55] and the forest typologies to Blanco et al. (1997) [56], respectively (Table 2, Figure 2 and Figure 3). Allué’s (1990) [55] classification considers four general climatic types subdivided into eighteen different subtypes based on temperature and rainfall characteristics where forests can appear. The Mediterranean climatic type has, for example, eight related subtypes. In respect to the forest types, a first characterization was made through the Spanish Forest Map at a 1:50,000 resolution (MFE50, https://www.miteco.gob.es/es/biodiversidad/servicios/banco-datos-naturaleza/informacion-disponible/mfe50.aspx, last accesed on 18 December 2021). Then forests were grouped as follows: (a) European beech forests, (b) Oak and mixed broad-leaved species forests, (c) Silver fir forests, (d) Eurosiberian pine forests, I Marcescent oak forests, (f) Oak and cork oak forests, (g) Mediterranean pine forests, (h) Juniper forests, (i) Spanish fir forests, (j) Riparian forests, (k) Forests of birch, hollies, hazel, linden and aspen, and (l) Other forests and scrublands not included in the previous categories (Blanco et al., 1997) [56]. In contrast to classes (a) to (k), a very high number of small forest patches together with scrubland and disperse woodlands fall within the last category (l), as most of them are characterized by degraded environmental conditions (recurrent forest fires, extreme climatic conditions, rocky soils, etc.). Given that the digital MFE50 map is only available at the province level, the data were upscaled at the national level by aggregating the province data (Figure 2).

Table 2.
Total area (×1000 ha) of each forest type according to the climatic types and subtypes observed in Spain following Allué (1990) [56]. Relative percentage of total forested area for each forest type is also indicated. European beech forests (Beech), Oak and mixed broad-leaved species forests (Oakmix), Silver fir forests (Fir), Eurosiberian pine forests (Eupin), Marcescent oak forests (Oakmarc), Oak and cork oak forests (Oak), Mediterranean pine forests (Medpin), Juniper forests (Jun), Forests of birch, hollies, hazel, linden and aspen trees (Sing), Spanish fir forests (FirSp), Riparian forests (River), and other forests not included in the other categories (Others).
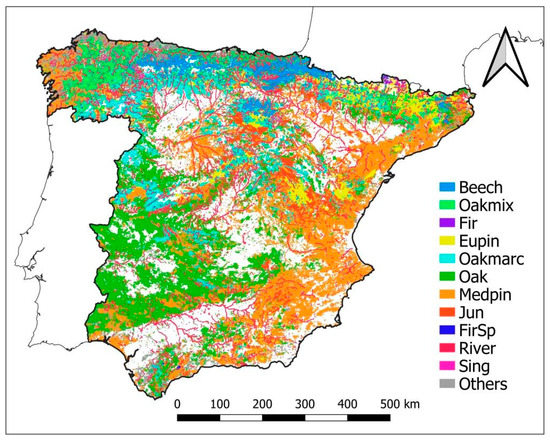
Figure 2.
Classification of forests in Spain according to Blanco et al. (1997) based on the Spanish Forest Map at 1:50,000 (MF50): European beech forests (Beech), Oak and mixed broad-leaved species forests (Oakmix), Silver fir forests (Fir), Eurosiberian pine forests (Eupin), Marcescent oak forests (Oakmarc), Oak and cork oak forests (Oak), Mediterranean pine forests (Medpin), Juniper forests (Jun), Spanish fir forests (FirSp), River forests (River), Forests of birch, hollies, hazel, linden and aspen trees (Sing) and other forests non included in the other categories (Others).
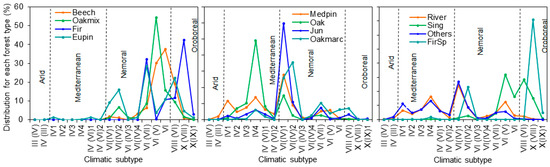
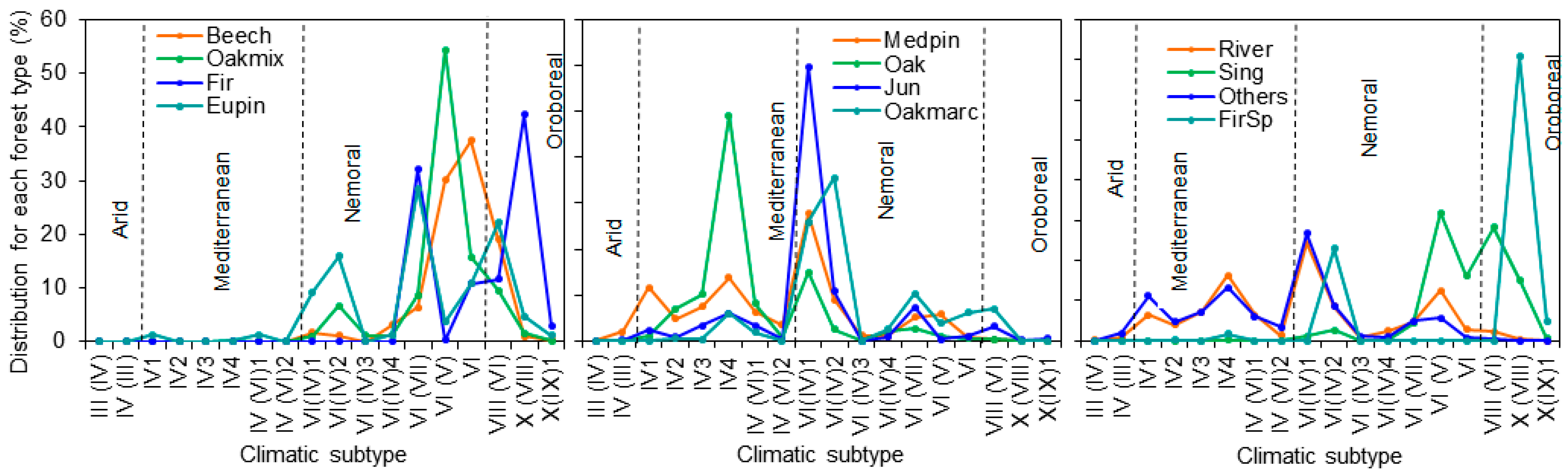
Figure 3.
Relative distribution of every forest type (% of total for each type) in the climatic subtypes presented in Spain according to Allué (1990) [55]: European beech forests (Beech), Oak and mixed broad-leaved species forests (Oakmix), Silver fir forests (Fir), Eurosiberian pine forests (Eupin), Marcescent oak forests (Oakmarc), Oak and cork oak forests (Oak), Mediterranean pine forests (Medpin), Juniper forests (Jun), Spanish fir forests (FirSp), Riparian forests (River), Forests of birch, hollies, hazel, linden and aspen trees (Sing) and other forests non-included in the other categories (Others).
The result of crossing all relevant climates subtypes and forest types, that provides a nationwide distribution, is presented in Table 2 and Figure 3. Without considering forest type (l), oak and cork oak stands are the most common type in Spain (28.5 %), followed by Mediterranean pines (22.6%), Eurosiberian pines (10.0%) and marcescent oaks (6.3%). The rest of the forest types show percentages lower than 5%. The climatic range is quite different depending on the forest type, led by the differences in natural distribution but also by their anthropic use. It is worth mentioning the particular cases of Mediterranean pines, that are present across all climatic subtypes due to extensive reforestation of degraded lands aimed at mitigating erosion issues (Figure 3), and that of the oak forests, only absent in the aridest climatic subtypes, due to their ecological plasticity. In contrast, the distribution of the rest of the forest types is more clearly related to their specific ecological requirements (Figure 3).
According to this classification, about 65% of the experimental sites (22 sites) correspond to mono-specific stands of Mediterranean pines (most of them with Aleppo pine, Pinus halepensis Mill., as the dominant species), of which 11 correspond to Arid or Mediterranean conditions. Oak and Eurosiberian pine forests are equally common in the network, with a relative distribution of 14.7%. Experimental sites with oak stands fall within intermediate Mediterranean or Nemoral conditions, while those with Eurosiberian pines exhibit conditions that range from Mediterranean to Oroboreal. The rest of the experimental sites consist of beech and marcescent oaks stands whose presence is lower than 3%. Regarding forest management practices, most experimental sites characterized by Arid or Mediterranean conditions include non-active vs. active measurements, while “no actions” are commonly found under more humid conditions with no clear impact of stressors. In this respect, thinning is the most frequent action tested, and this action has the main objective of improving the growth status of remaining trees through increases in water and nutrient inputs. In addition, in other sites also characterized by arid or Mediterranean conditions where wildfires occurred, there are restoration treatments focused on preventing soil erosion but also recovering plant cover. Soil treatments for improving soil performance or the introduction of saplings under forest canopy are also tested actions but to a lesser extent (Table 1).
2.2. Observed Effects of AFM in the Network: The Case of Thinning Intensity in Mediterranean Pines
The observations taken at the different experimental sites in SilvAdapt.net are expected to allow for a comprehensive analysis of the role of forest management to adapt Spanish forests to new climatic conditions, especially the occurrence of more intense and recurrent drought events.
The categorization of the specific management actions and silvicultural practices into broad forest adaptation concepts and strategies is not straightforward due to the high degree of uncertainty when meeting the desired outcome in a changing environment [35,48]. This requires an iterative approach to “learn by doing”. With the primary aim of showing the potential benefits of combining different approaches, experimental designs and measurements into a common framework, we describe and try to categorize here the selected action of thinning intensity and its observed impacts in the Mediterranean pines as one of the forest types suffering the worst impacts of recent drought episodes in Spain (Appendix B summarizes the environmental conditions, vegetation, experimental design and AFM effects observed in the experimental sites with Mediterranean pines).
Regarding the AFM strategy of no intervention, observations from the different experimental sites will especially allow improving our understanding of the quantitative thresholds of environmental factors and physiological traits when explaining the complex dieback and mortality processes [57,58], but also identifying which conditions (e.g., environmental and stand structure) advice for this strategy to be successful in maintaining the current forest conditions in the future.
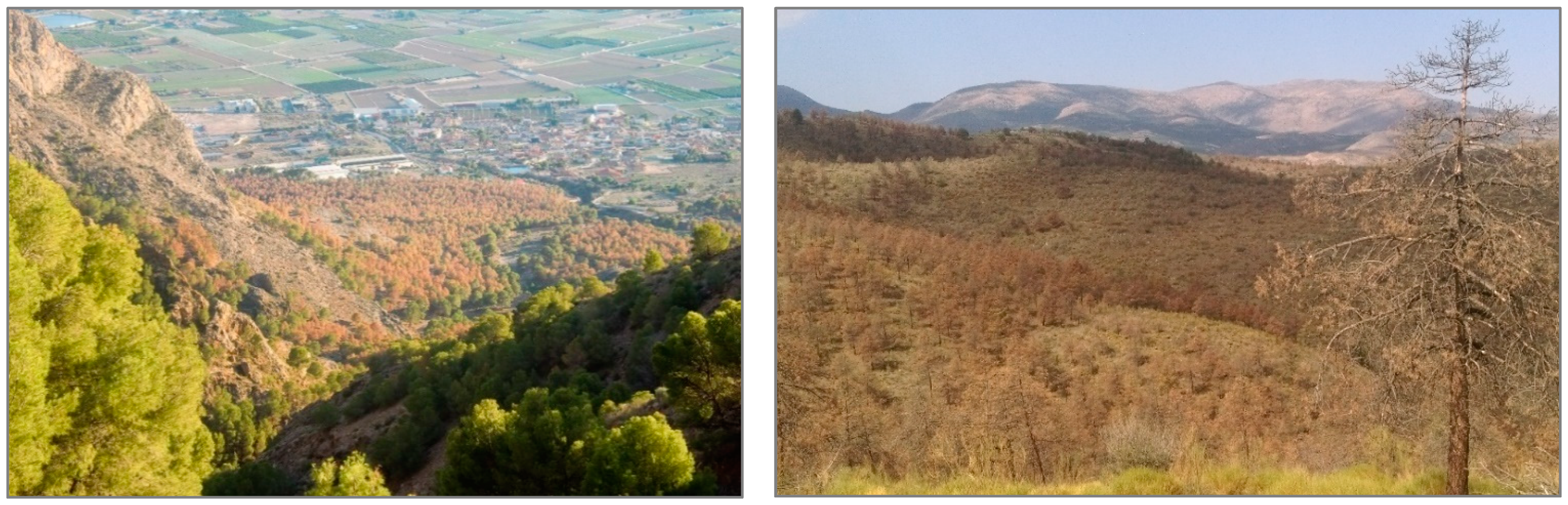
In the last decade, the most extended dieback processes have taken place mostly in mature natural forests and plantations of P. halepensis Mill., P. pinaster Aiton and P. sylvestris L. located in the south, southeast and east Spain. In the Calderona site (Valencia province, site 1b in Figure 1 and Table 1), the impacts of the extreme drought (annual precipitation <100 mm) that occurred during 2014 caused 25% of tree mortality in those places with the poorest soil conditions (red sandstones from Buntsandstein facies), pointing out the importance of local soil properties variability when explaining this phenomenon [59]. Tree mortality ranged from 10 to 20% in other pine plantations in Alicante province [25], while the effects of this extreme drought were very negative and tree mortality almost reached 100% in the Orihuela site (annual precipitation in 2014 was 60 mm, and several months with temperatures 1–3 °C higher than normal, 4b in Figure 1 and Table 1) [25]. In this site, the lowest mean tree water potential reached −8 MPa, and xylem cavitation and bark beetle infections were observed in most of the trees. The short-term effects of this drought period in other pine plantations in Granada, Murcia and Almería (south and southeast Spain), such as those in “Sierra de Baza” (site 2b in Figure 1 and Table 1), caused isolated trees to die, but dieback and mortality processes were more generally extended from 2016 on (affecting 1916 ha in the area) (Figure 4, right), highlighting the cumulative effect of drought and growing conditions on such processes [58,60,61]. In contrast, no evidence of dieback and tree mortality processes related to recent drought episodes have been observed in other experimental sites, such as the mature plantations from La Hunde and Tarazona (sites 1a and 6a in Figure 1 and Table 1) or naturally regenerated stands from La Calderona and Montmell (sites 1b and 8b in Figure 1 and Table 1). Within this context (different responses to drought across the country), it is possible to determine to what extent different AFM actions may improve forest health and vigor by addressing specific processes (Figure 4, left).
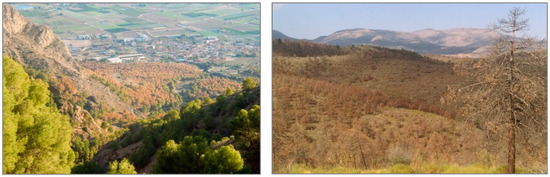
Figure 4.
Episodes of pine stand mortality in Spain. (Left): Early impacts of the 2014 drought at the Orihuela site (23 m a.s.l., Alicante province, Eastern Spain) and the positive firebreak effects on the survival of the border trees where tree competence was partly removed. Photography taken by Ángela Botella. (Right): Extended mortality episode of P. pinaster in afforestation lands of Sierra de Baza, Collado del Fraile site, 2016 (850 m a.s.l., Granada province, South-Eastern Spain). Photography taken by Francisco J. Ruiz-Gómez.
The experimental sites where tree density reduction was assayed allow for the evaluation of active AFM strategies, such as resistance and resilience in terms of optimum tree density reduction and the timing for thinning out again [45,48]. Thinning has clearly improved secondary tree growth in the sites at both the short- and the mid-term, and has reduced its sensitivity to climate [39,62]. As demonstrated in several studies, thinning could enhance tree growth by decreasing tree competition for water and nutrients, increasing the photosynthetic rates, and improving water use and carbon uptake [37,39,48].
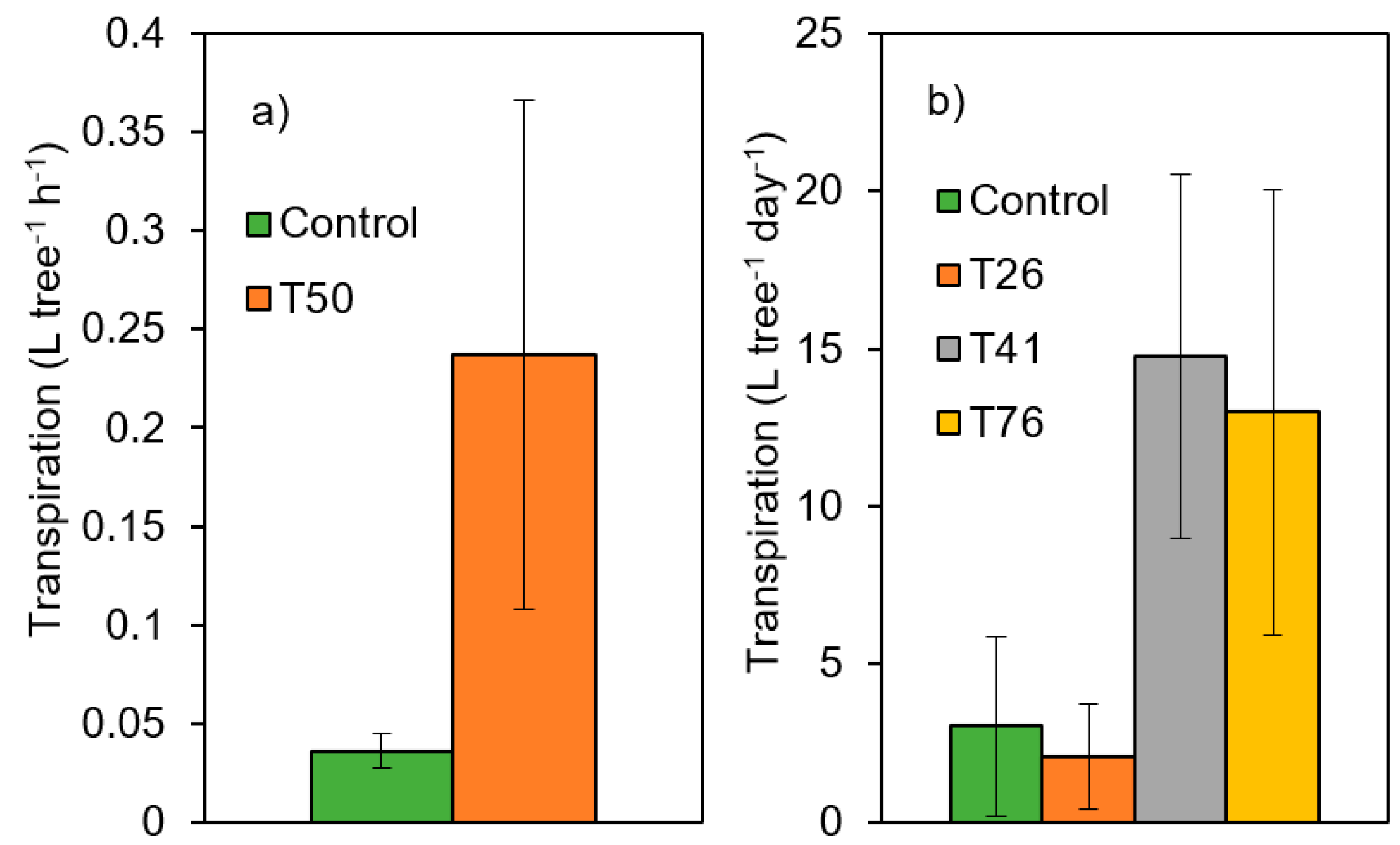
Observations from the experimental sites show better tree physiological performance (higher tree transpiration) several years after thinning when at least 40% of the basal area is removed (Figure 5, statistical comparisons are presented in the reference cited in the figure caption, while the unpublished data indicate significant differences between the control and T50 treatments). By the same, growth calculations within the experimental sites allow for inter-site comparisons of resistance, recovery and resilience indices. For instance, while in La Hunde and Tarazona sites (mature Aleppo pine plantations), resistance was enhanced by both moderate and heavy thinning treatments [62], resistance and resilience indices were both improved by a heavy thinning treatment (>60% of basal area removed) in mature plantations of P. sylvestris located in Southern Spain [39].
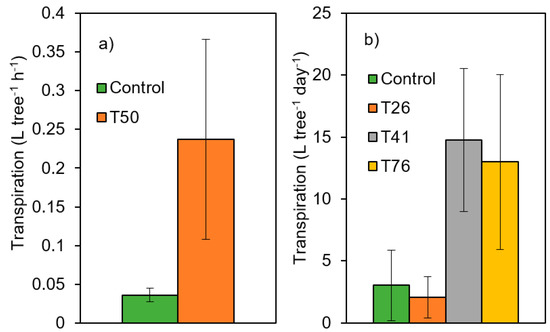
Figure 5.
Thinning impacts on transpiration of pine trees growing under Mediterranean conditions for the 4th year after thinning intervention. C: Control, T: treatments (numbers indicate the % of basal area removed). (a) Forest stand of Aleppo pine regenerated after a wildfire occurred in 1992 (12,280 trees·ha−1) in La Calderona (Valencia, 39°42′ N, 0°27′ W, 790 m a.s.l., site 1b in Figure 1 and Table 1); measurements are the means ± standard deviations; (b) Mature planted stand of Aleppo pine (1500 trees·ha−1) in La Hunde (Valencia, 39°05′ N and 1°12′ W, 950 m a.s.l., site 1a in Figure 1 and Table 1); measurements are the means ± standard deviations for the 10th year after thinning intervention. Unpublished data for the Calderona Site. Data from Molina et al. (2021) [45] for la Hunde site.
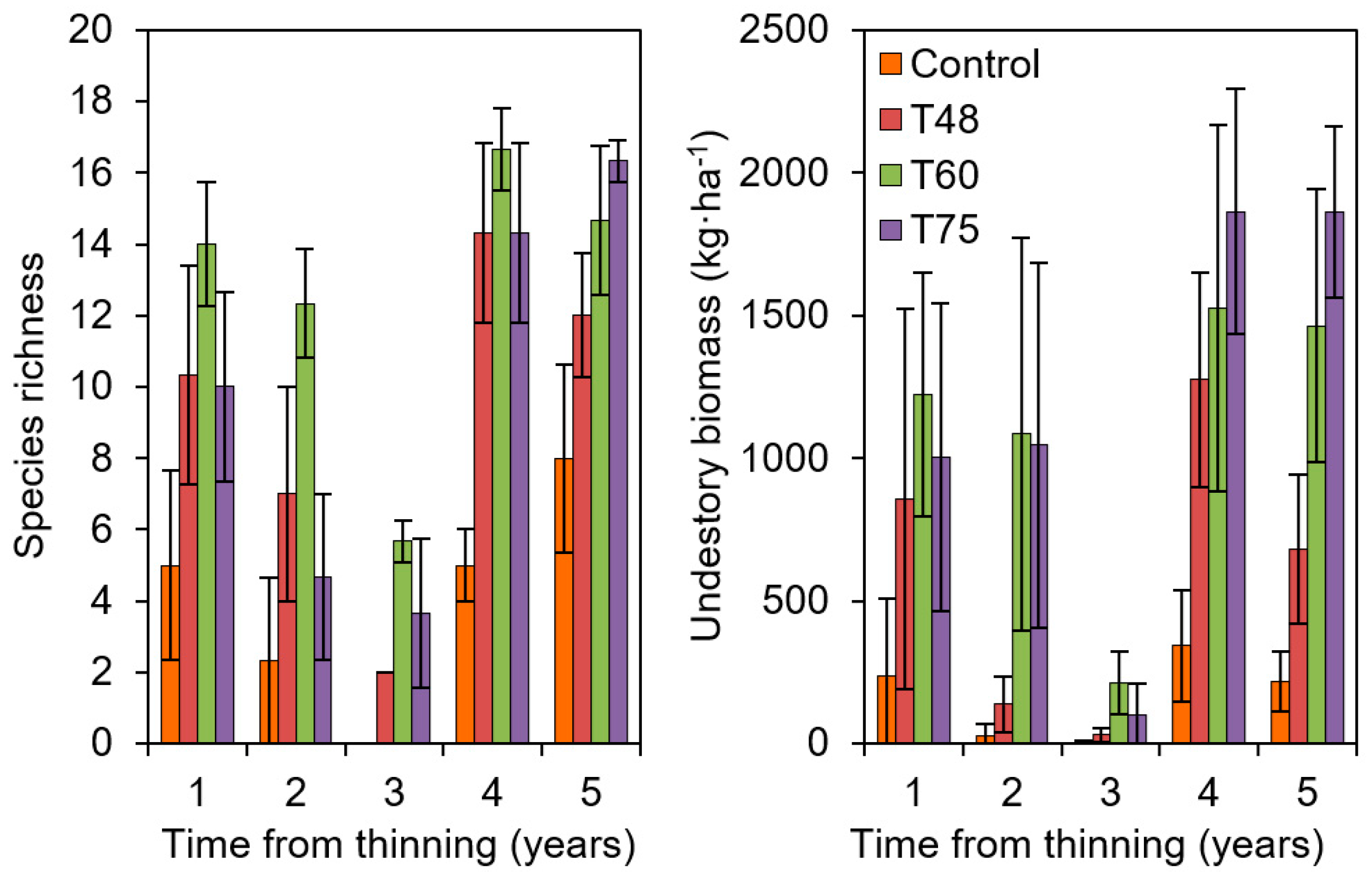
The main objective of a resistance treatment is focused on preserving mature pine structures into a future of warmer, drier growing conditions. In the case of a resilience one, this will be moving beyond pine species as the dominant species to others with expected increased habitat suitability or promoting higher genetic diversity [36,48]. In the Aleppo pine plantations, growth indices showed improved and durable response by intermediate thinning intensity, but this was not the case in the mature plantations of P. sylvestris located in Southern Spain [39], where thinning may be insufficient to maintain the current forest structure in the future. In these sites, the impacts of drought are already noticeable in terms of dieback and tree mortality processes, and resilience or transition strategies of AFM may be more appropriate. Apart from the differences that may arise from the physiological strategies between pine species when facing extreme drought episodes [58], we hypothesize that, under these limited environmental conditions, the margin for improvement by a thinning treatment at intermediate intensity (such as those silvicultural practices following standard procedures for timber production in terms of intensity and timing) may be too low, and thus more proactive strategies (resilience and/or transition) would be expected to show better results when managing forested areas at the long-term. In this sense, high-intensity thinning treatments promoting high understory diversity and ground cover may represent an option, as the results obtained during the 5th year after intervention in the Altiplano del Conejo site indicated (Figure 6) (south Spain, Granada province, site 14a in Figure 1 and Table 1).
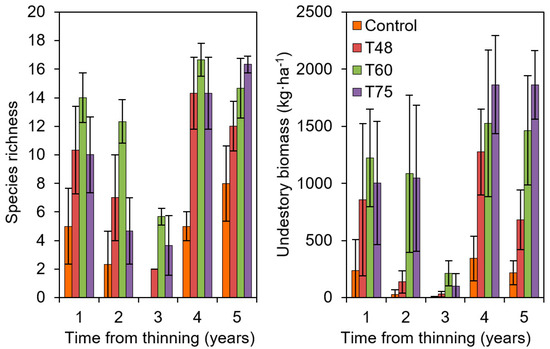
Figure 6.
Thinning impacts on plant understory (species richness and total dry biomass) of plantations of Aleppo pine located in Altiplano del Conejo (Granada, 37°26′ N and 3°5′ W, 1100 m a.s.l., site 14a in Figure 1 and Table 1); C: Control (1500 trees·ha−1), T: treatments (numbers indicate the % of basal area removed). Data from Jiménez et al. (2015) [63].
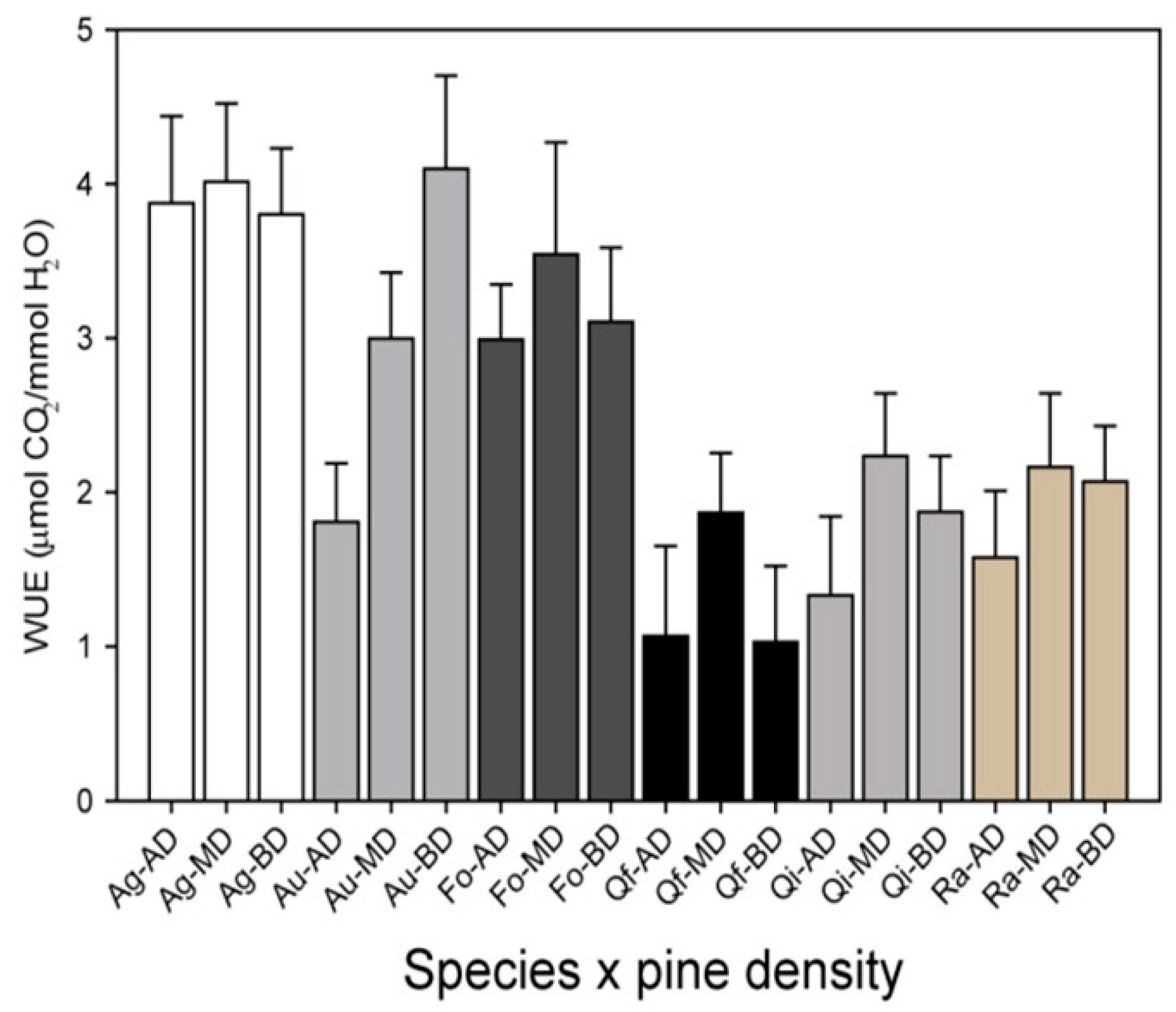
These treatments are thus expected to promote also changes in the overstory species and structural diversity, by enhancing species richness and tree diameter/age variability (i.e., moving from even-age to uneven-age in the cases of mature plantations with high tree density). In addition, other actions where thinning is combined with planting under canopy cover (Figure 7) may be helpful to further evaluate and compare resilience and transition strategies when meeting the desired outcome. In this sense, experimental data characterizing seedling responses, such as growth, survival, or water use efficiency, can be combined with overstory measures to assess the effects of these types of actions at the ecosystem level, thus addressing how overstory-understory interactions may change over time. In any case, a good characterization of site quality is essential to design proper AFM strategies and actions for a particular area, as well as vulnerability assessments of the current species to the expected impacts of climate change [35,36].
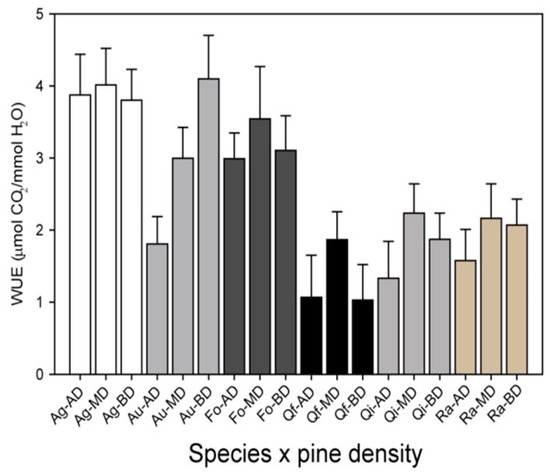
Figure 7.
Water use efficiency (WUE) for several seedlings of species planted under different pine cover densities in mature Aleppo pine plantations located in Valencia province (eastern Spain, La Hunde site, site 1a in Figure 1 and Table 1). Species: Ag = Acer granatense Boiss, Au = Arbutus unedo L., Fo = Fraxinus ornus L., Qf = Quercus faginea Lam, Qi = Quercus ilex L. ssp. Ballota (Desf.), Ra = Rhamnus alaternus L. Pine densities: AD, high density (1067 ± 141 trees ha−1); MD, medium density (344 ± 19 trees ha−1); and BD, low density (165 ± 25 trees ha−1). Data from Granados et al. (2019) [64].
2.3. Upscaling Site Observations into a More Meaningful Framework. Open Questions and Next Steps
In this work we have focused on the potential benefits of integrating experimental sites with different designs, measurements, results and approaches for the case of Mediterranean pines growing under a variety of climates, but how would these or different AFM strategies work beyond these sites? It is especially important to firstly focus on the forest types where the climatic impacts are already significantly affecting forest functions (e.g., water-limited ecosystems), and the worst climatic scenarios are expected in the future [8]. As shown in the previous section, the current treatments implemented in the SilvAdapt.net network can be assimilated into any of the four strategies proposed by Millar et al. (2007) [34] and assessed in terms of key functional tree/stand traits and processes, such as growth, forest-water relationships, tree vigor, etc. Ideally, all AFM strategies should be replicated for at least the most representative forest types and climates, an approach that is being implemented in the USA where the four AFM strategies are simultaneously considered at every new experimental site [36]. Alternatively, process-based models and satellite data acquisition and data management [65,66] are tools that can be used for those forest type-climate combinations where no observations are available [50]. Thanks to the existing datasets from the experimental sites for a particular forest type and climate, the calibrated and validated process-based models can be used to estimate AFM effects under new climatic conditions, while remote sensing can serve to obtain metrics concerning forest structure, growth and environmental data. These remote sensing products may also be used to validate the models. However, this modeling approach would be insufficient for forest types where experimental data are not available. In this sense, complementary experimental sites with active AFM actions for mixed broadleaved forests, marcescent oaks and specific threatened forest types (such as riparian or Spanish fir forests) are desirable to improve the framework proposed.
In addition, this work has focused on showing the potential benefits of the proposed network as thinning as the main action to improve the forest responses to droughts. It is important not only to consider other types of actions that may be designed in order to fulfill this objective (such as considering different provenances with differential responses to soil water limitation) but also other actions required for the achievement of other possible objectives. Thus, if the general objective is to improve forest resilience to drought impacts but also to other important disturbances, the effects of the different actions should be assessed as a whole, and thus to recognize and assess the possible trade-offs [48]. An example could be improving the resilience of a certain area with extended tree mortality processes, but also large areas of connected forest stands. Under these circumstances, for instance, the incorporation of new tree species into the forest structure could be greatly promoted by improving understory development, but the risk of both surface and crown fires may also increase (better performance against drought vs. worse performance against wildfire ignition and propagation). Another example could be to increase forest resistance in a healthy stand by thinning at an intermediate intensity, but carbon sequestration may be reduced (better tree performance against drought vs. lower carbon sequestration capacity of the forest).
Another important point to be achieved would be to frame experimental data into comparable and normalized units for a consistent evaluation of climate change impacts and forest management interactions [51]. The key idea is to identify and obtain or estimate specific indicators describing key processes, variables and indices, such as Net Primary Production (NPP), leaf area index, biodiversity, water use efficiency or stand transpiration. For example, NPP can directly be assessed through the mensuration of classic forest metrics, such as diameter at breast height (DBH, i.e., diameter at 1.30 m), stand density and height and leaf area index; while indirect estimates for these metrics can be obtained through spectral vegetation indices, such as NDVI, EVI and SAVI [67,68] if field observations are not available.
Granting data accessibility once the results are integrated for the different adaptation options would also be desirable in order to disseminate the most important results for stakeholders. Finally, implementing an AFM framework in Spain should also allow the quantification of mid- and long- terms effects of forest management (>15 years from thinning). This is especially critical when studying the direct effects of vegetation reduction on tree/forest-water, but also the indirect expected effects, such as those regarding forest fire continuity or tree resistance to pests and diseases.
3. Conclusions
The main objective of this work was to describe the steps and the potential benefits that arise from a progressive implementation of a framework that integrates and coordinates research groups working on the adaptation of Spanish forests to the effects of climate change to the bigger aim of creating a useful tool for practitioners and policy-makers. The natural and aggravated limitation of water availability and the related die-off and mortality processes force us to focus on and join efforts in arid and semi-arid regions. This requires (a) experimental data that go beyond common forest inventory measurements, (b) the quantification of the effects of different management scenarios on key ecohydrological variables and related processes, and (c) to inform about innovative silviculture approaches more aligned with adaptive strategies. This should lead to the development of technical AFM guidelines for at least the most threatened forest types growing in arid and semi-arid regions. Future efforts need to be carried out in order to promote adaptive silviculture for other climatic and forests contexts according to the common issues observed through experimental data.
Author Contributions
Conceptualization, A.J.M., A.D.d.C.; riting—original draft, A.J.M.; Supervision and writing—original draft: A.J.M., A.D.d.C. and R.M.N.-C..; Writing—review & editing, all the authors. Funding acquisition, A.D.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
A.J. Molina is beneficiary of an “APOSTD” fellowship (APOSTD/2019/111) funded by the Generalitat Valenciana. M. Moreno-de las Heras is beneficiary of a Serra Hunter fellowship (UB-LE-9055) funded by the Generalitat de Catalunya. F.J. Ruiz-Gómez is supported by a postdoctoral fellowship of the Junta de Andalucía (Sevilla, Spain), and the European Social Fund 2014–2020 Program (DOC_0055). The authors received national and international funding through the following projects: SILVADAPT.NET (RED2018-102719-T), ESPECTRAMED (CGL2017-86161-R), Life-FOREST CO2 (LIFE14 CCM/ES/001271), ALTERACLIM (CGL2015-69773-C2-1-P), INERTIA (PID2019-111332RB-C22-BDV), CEHYRFO-MED (CGL2017-86839-C3-2-R), DEHESACLIM (IB16185), RESILIENTFORESTS (LIFE17 CCA/ES/000063), Rhysotto (PID2019-106583RB-I00), AGL2017-83828-C2-2-R, RTI2018-096884-B-C31, ESPAS (CGL2015-65569-R), and caRRRascal (RTI2018-095037-B-I00).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available for research upon request.
Acknowledgments
We thank the financial support from the “Ministerio de Ciencia e Innovación -Redes de Investigación 2018, Programa Estatal de Generación de Conocimiento y Fortalecimiento Científico y Tecnológico del Sistema de I + D + I”.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Variables Measured in the Experimental Sites
Climate:
Sixty-two percent of the experimental sites are monitoring standard meteorological variables following the World Meteorological Organization recommendations. They include rainfall (mm), temperature (°C) and relative humidity (%) with a time resolution from 5 to 60 min. Furthermore, additional variables of particular research interest are also measured in some experimental sites, such as those to calculate evapotranspiration by the FAO-Penman-Monteith method. The rest of the sites obtain this type of information from the nearest official meteorological stations.
Forest structure, growth and survival:
Fifty-seven percent of the sites are periodically measuring variables characterizing stand structure, such as diameter at breast height (DBH, cm), basal area increment (cm2), tree height (m), forest cover (%) and tree density (trees·ha−1). Leaf Area Index (LAI, m2·m−2) is monitored in 36% of the sites. Tree survival measurements are carried in the experimental sites where impacts of forest fires or understory competition are evaluated.
Water cycle:
Hydrological measurements are those which are better represented in the network due to the important role of forest-water relations in most of the sites; they are mainly carried out at both the tree and the stand scales with automatic sensors describing rainfall partitioning (mm), tree or stand transpiration (l·tree−1 or mm) and soil water content for different depths (%) or extended to the whole soil profile (mm). Soil recharge (mm) through manual lysimeters is also evaluated in some experimental sites where the effects of forest or soil treatments are tested vs. unmanaged conditions. In addition, a limited number of experimental sites measure stream discharges at catchment outlet and piezometer levels to characterize watershed runoff (mm) and water table dynamics (cm), respectively. In addition, an eddy-correlation tower is placed above the forest canopy of one particular experimental site to estimate water and carbon cycles at the stand scale.
Plant physiology:
Tree transpiration (l·h−1) is estimated in 10 experimental sites by automatic sap flow sensors measuring heat pulse velocity (cm·h−1) in different points within tree sapwood depth (using different combinations of thermocouples and heaters). Some of the experimental sites with tree transpiration measurements also carry out measurements of leaf water potential (Mpa), leaf canopy conductance (mmol/m2·s) and gas exchange between leave and atmosphere (mmol/m2·s, CO2 and water).
Dendrochronology:
Dendrochronological surveys are normally conducted in eight experimental sites, while this type of data has been collected in a total of 15 experimental sites. These measurements are carried out to primary study the role of climatic and soil water conditions (especially for evaluating the effect of water deficit on tree growth responses), but also the effect of tree competence on growth dynamics. Measurements are normally carried at two perpendicular axes around the tree trunk, and tree growth indices, such as the annual basal area increment (BAI, cm2) are derived from ring width data (cm).
Biogeochemistry:
Nutrient flows have been studied by stoichiometric analysis of litterfall, collected during the seasons of higher fall, for several years depending on the experimental site. Nutrient immobilization and release from litter have been studied following the litterbag technique with experimental durations from 1 to 5 years. In addition, nutrient flows in atmospheric deposition, throughfall and soil solution have been studied with chemical analysis of water samples following several sampling techniques.
Soil characteristics and processes:
Soil measurements are mainly carried out to characterize physical soil properties, such as texture, porosity, organic matter content, or soil water holding capacity. In addition, measurements are also carried out to describe soil respiration, enzymatic/biological activity in the experimental sites where nutrient flows are studied.
Appendix B. Collection of Cards Summarizing Vegetation Description, Experimental Approach and Main AFM Impacts for Some Sites with Mediterranean Pines within the Silvadapt.Net Network

Figure A1.
Spanish black pine natural forest in Cuenca Mountains.
Figure A1.
Spanish black pine natural forest in Cuenca Mountains.

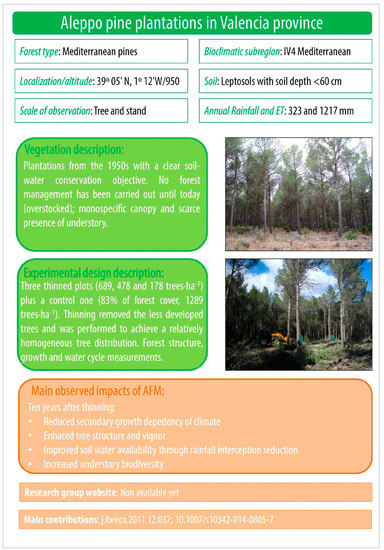
Figure A2.
Aleppo pine plantations in Valencia province.
Figure A2.
Aleppo pine plantations in Valencia province.


Figure A3.
Aleppo pine plantations in Granada province.
Figure A3.
Aleppo pine plantations in Granada province.

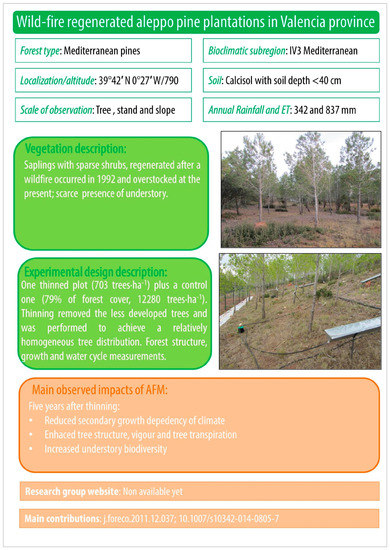
Figure A4.
Wild-fire regenerated aleppo pine plantations in Valencia province.
Figure A4.
Wild-fire regenerated aleppo pine plantations in Valencia province.
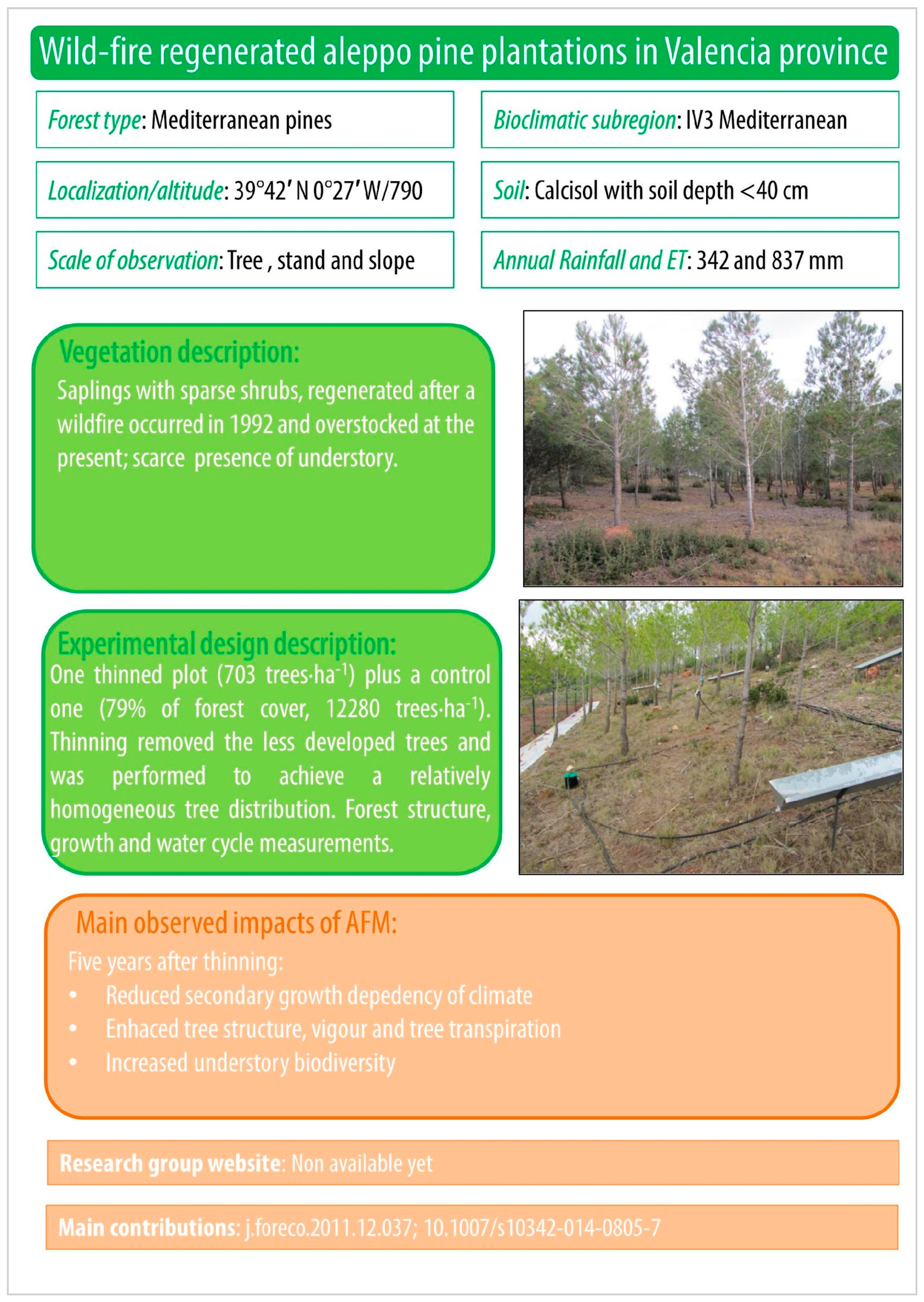

Figure A5.
Aleppo pine plantations in Zaragoza province.
Figure A5.
Aleppo pine plantations in Zaragoza province.

References
- Vayreda, J.; Martínez-Vilalta, J.; Gracia, M.; Retana, J. Recent climate changes interact with stand structure and management to determine changes in tree carbon stocks in Spanish forests. Glob. Chang. Biol. 2012, 18, 1028–1041. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.; Lasch, P.; Eggers, J.; der Maaten-Theunissen, M. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- Amblar-Francés, M.P.; Casado Calle, M.J.; Pastor Saavedra, M.A.; Ramos Calzado, P.; Rodríguez Camino, E. Guía de Escenarios Regionalizados de Cambio Climático Sobre España a Partir de los Resultados del IPCC-AR5; AEMET: Madrid, Spain, 2017. [Google Scholar]
- Serrano-Notivoli, R.; Beguería, S.; Saz, M.Á.; de Luis, M. Recent trends reveal decreasing intensity of daily precipitation in Spain. Int. J. Climatol. 2018, 38, 4211–4224. [Google Scholar] [CrossRef]
- Anderegg, W.R. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytol. 2015, 205, 1008–1014. [Google Scholar] [CrossRef]
- Mastrotheodoros, T.; Pappas, C.; Molnar, P.; Burlando, P.; Keenan, T.F.; Gentine, P.; Gough, C.M.; Fatichi, S. Linking plant functional trait plasticity and the large increase in forest water use efficiency. J. Geophys. Res. Biogeosci. 2017, 122, 2393–2408. [Google Scholar] [CrossRef]
- Metzger, M.J.; Bunce, R.G.H.; Leemans, R.; Viner, D. Projected environmental shifts under climate change: European trends and regional impacts. Environ. Conserv. 2018, 35, 64–75. [Google Scholar] [CrossRef]
- Soteriades, A.D.; Murray-Rust, D.; Trabucco, A.; Metzger, M.J. Understanding global climate change scenarios through bioclimate stratification. Environ. Res. Lett. 2017, 12, 084002. [Google Scholar] [CrossRef]
- Puettmann, K.J. Silvicultural challenges and options in the context of global change: “Simple” fixes and opportunities for new management approaches. J. For. 2011, 109, 321–331. [Google Scholar]
- Keenan, T.; Maria Serra, J.; Lloret, F.; Ninyerola, M.; Sabate, S. Predicting the future of forests in the Mediterranean under climate change, with niche-and process-based models: CO2 matters! Glob. Chang. Biol. 2011, 17, 565–579. [Google Scholar] [CrossRef]
- Reyer, C.P.; Leuzinger, S.; Rammig, A.; Wolf, A.; Bartholomeus, R.P.; Bonfante, A.; de Lorenzi, F.; Dury, M.; Gloning, P.; Jaoudé, R.A.; et al. A plant’s perspective of extremes: Terrestrial plant responses to changing climatic variability. Glob. Chang. Biol. 2013, 19, 75–89. [Google Scholar] [CrossRef]
- Roselló, E. Biogeoclimatic Classification of Peninsular and Balearic Spain; Ministerio de Agricultura, Pesca y Alimentacion: Madrid, Spain, 1997. [Google Scholar]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; De Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef]
- Seidl, R.; Spies, T.A.; Peterson, D.L.; Stephens, S.L.; Hicke, J.A. Searching for resilience: Addressing the impacts of changing disturbance regimes on forest ecosystem services. J. Appl. Ecol. 2016, 53, 120–129. [Google Scholar] [CrossRef]
- Moriondo, M.; Good, P.; Durao, R.; Bindi, M.; Giannakopoulos, C.; Corte-Real, J. Potential impact of climate change on fire risk in the Mediterranean area. Clim. Res. 2006, 31, 85–95. [Google Scholar] [CrossRef]
- de la Cueva, A.V.; Quintana, J.R.; Cañellas, I. Fire activity projections in the SRES A2 and B2 climatic scenarios in peninsular Spain. Int. J. Wildland Fire 2012, 21, 653–665. [Google Scholar] [CrossRef]
- Vázquez, A.; Climent, J.M.; Casais, L.; Nieto, J.R.Q. Current and future estimates for the fire frequency and the fire rotation period in the main woodland types of peninsular Spain: A case-study approach. For. Syst. 2015, 24, 10. [Google Scholar] [CrossRef]
- Doblas-Miranda, E.; Alonso, R.; Arnan, X.; Bermejo, V.; Brotons, L.; De las Heras, J.; Estiarte, M.; Hódar, J.A.; Llorens, P.; Lloret, F.; et al. A review of the combination among global change factors in forests, shrublands and pastures of the Mediterranean Region: Beyond drought effects. Glob. Planet. Chang. 2017, 148, 42–54. [Google Scholar] [CrossRef]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe drought effects on Mediterranean woody flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar]
- Pasho, E.; Camarero, J.J.; de Luis, M.; Vicente-Serrano, S.M. Impacts of drought at different time scales on forest growth across a wide climatic gradient in north-eastern Spain. Agric. For. Meteorol. 2011, 151, 1800–1811. [Google Scholar] [CrossRef]
- de la Serrana, R.G.; Vilagrosa, A.; Alloza, J.A. Pine mortality in southeast Spain after an extreme dry and warm year: Interactions among drought stress, carbohydrates and bark beetle attack. Trees 2015, 29, 1791–1804. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Cantero, A.; Sánchez-Salguero, R.; Sánchez-Miranda, A.; Granda, E.; Serra-Maluquer, X.; Ibáñez, R. Forest growth responses to drought at short- and long-term scales in Spain: Squeezing the stress memory from tree rings. Front. Ecol. Evol. 2018, 6, 9. [Google Scholar] [CrossRef]
- Peña-Gallardo, M.; Vicente-Serrano, S.; Camarero, J.J.; Gazol, A.; Sánchez-Salguero, R.; Domínguez-Castro, F.; el Kenawy, A.; Beguería-Portugés, S.; Gutiérrez, E.; de Luis, M.; et al. Drought sensitiveness on forest growth in peninsular Spain and the Balearic Islands. Forests 2018, 9, 524. [Google Scholar] [CrossRef]
- Morcillo, L.; Gallego, D.; González, E.; Vilagrosa, A. Forest decline triggered by phloem parasitism-related biotic factors in Aleppo pine (Pinus halepensis). Forests 2019, 10, 608. [Google Scholar] [CrossRef]
- De Dios, V.R.; Fischer, C.; Colinas, C. Climate change effects on Mediterranean forests and preventive measures. New For. 2007, 33, 29–40. [Google Scholar] [CrossRef]
- Prieto-Recio, C.; Martín-García, J.; Bravo, F.; Diez, J.J. Unravelling the associations between climate, soil properties and forest management in Pinus pinaster decline in the Iberian Peninsula. For. Ecol. Manag. 2015, 356, 74–83. [Google Scholar] [CrossRef]
- Pautasso, M.; Dehnen-Schmutz, K.; Holdenrieder, O.; Pietravalle, S.; Salama, N.; Jeger, M.J.; Lange, E.; Hehl-Lange, S. Plant health and global change–some implications for landscape management. Biol. Rev. 2010, 85, 729–755. [Google Scholar] [CrossRef] [PubMed]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; .Worrall, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- MEA. Millennium Ecosystem Assessment: Ecosystems and Human Well-Being 5; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Millar, C.I.; Stephenson, N.L.; Stephens, S.L. Climate change and forests of the future: Managing in the face of uncertainty. Ecol. Appl. 2007, 17, 2145–2151. [Google Scholar] [CrossRef]
- Janowiak, M.K.; Swanston, C.W.; Nagel, L.M.; Brandt, L.A.; Butler, P.R.; Handler, S.D.; Shannon, P.D.; Iverson, L.R.; Matthews, S.N.; Prasad, A.; et al. A practical approach for translating climate change adaptation principles into forest management actions. J. For. 2014, 112, 424–433. [Google Scholar] [CrossRef]
- Nagel, L.M.; Palik, B.J.; Battaglia, M.A.; D’Amato, A.W.; Guldin, J.M.; Swanston, C.W.; Janowiak, M.K.; Powers, M.P.; Joyce, L.A.; Millar, C.I.; et al. Adaptive silviculture for climate change: A national experiment in manager-scientist partnerships to apply an adaptation framework. J. For. 2017, 115, 167–178. [Google Scholar] [CrossRef]
- del Campo, A.D.; Fernandes, T.J.; Molina, A.J. Hydrology-Oriented (adaptive) silviculture in a semiarid pine plantation: How much can be modified the water cycle through forest management? Eur. J. For. Res. 2014, 133, 879–894. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Sánchez-Salguero, R.; Herrera, R.; Ruiz, C.C.; Moreno-Rojas, J.M.; Manzanedo, R.D.; López-Quintanilla, J. Contrasting growth and water use efficiency after thinning in mixed Abies pinsapo–Pinus pinaster–Pinus sylvestris forests. J. For. Sci. 2016, 62, 53–64. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Sánchez-Salguero, R.; Rodriguez, C.; Lazo, J.D.; Moreno-Rojas, J.M.; Palacios-Rodriguez, G.; Camarero, J.J. Is thinning an alternative when trees could die in response to drought? The case of planted Pinus nigra and P. sylvestris stands in southern Spain. For. Ecol. Manag. 2019, 433, 313–324. [Google Scholar] [CrossRef]
- Felicísimo, A.M.; Muñoz, J.; Mateo, R.G.; Villalba, C.J. Vulnerabilidad de la flora y vegetación españolas ante el cambio climático. Ecosistemas 2012, 21, 1–6. [Google Scholar] [CrossRef]
- Fernández-Cancio, Á.; Sánchez Salguero, R.; Gil Hernández, P.M.; Manrique Menéndez, E.; Fernández Fernández, R.; Navarro Cerrillo, R.M. Efectos del cambio climático sobre la distribución de los alcornocales españoles: Una aproximación fitoclimática para la futura gestión. Ecosistemas 2012, 21, 50–62. [Google Scholar] [CrossRef]
- García-Güemes, C.; Calama, R. La Práctica de la Selvicultura Para la Adaptación al Cambio Climático. Los Bosques y la Biodiversidad Frente al Cambio Climático: Impactos, Vulnerabilidad y Adaptación en España, 12; Miteco: Madrid, Spain, 2014. [Google Scholar]
- Ruiz-Benito, P.; Herrero, A.; Zavala, M.Á. Vulnerabilidad de los bosques Ibéricos frente al Cambio Climático: Evaluación mediante modelos. Ecosistemas 2014, 22, 21–28. [Google Scholar] [CrossRef]
- Tsamir, M.; Gottlieb, S.; Preisler, Y.; Rotenberg, E.; Tatarinov, F.; Yakir, D.; Tague, C.; Klein, T. Stand density effects on carbon and water fluxes in a semi-arid forest, from leaf to stand-scale. For. Ecol. Manag. 2019, 453, 117573. [Google Scholar] [CrossRef]
- Molina, A.J.; González-Sanchis, M.; Biel, C.; del Campo, A.D. Ecohydrological turnover in overstocked Aleppo pine plantations: Does the effect of thinning, in relation to water, persist at the mid-term? For. Ecol. Manag. 2021, 483, 118781. [Google Scholar] [CrossRef]
- Agee, J.K.; Skinner, C.N. Basic principles of forest fuel reduction treatments. For. Ecol. Manag. 2005, 211, 83–96. [Google Scholar] [CrossRef]
- Forestry Commission. Building Wildfire Resilience into Forest Management Planning. Building Wildfire Resilience into Forest Management Planning; Forestry Commission: Edinburgh, UK, 2014. [Google Scholar]
- Vilà-Cabrera, A.; Coll, L.; Martínez-Vilalta, J.; Retana, J. Forest management for adaptation to climate change in the Mediterranean basin: A synthesis of evidence. For. Ecol. Manag. 2018, 407, 16–22. [Google Scholar] [CrossRef]
- Pretzsch, H.; del Río, M.; Biber, P.; Arcangeli, C.; Bielak, K.; Brang, P.; Dudzinska, M.; Forrester, D.I.; Klädtke, J.; Kohnle, U.; et al. Maintenance of long-term experiments for unique insights into forest growth dynamics and trends: Review and perspectives. Eur. J. For. Res. 2019, 138, 165–185. [Google Scholar] [CrossRef]
- Ruiz-Benito, P.; Vacchiano, G.; Lines, E.R.; Reyer, C.P.; Ratcliffe, S.; Morin, X.; Hartig, F.; Mäkelä, A.; Yousefpour, R.; Chaves, J.E.; et al. Available and missing data to model impact of climate change on European forests. Ecol. Model. 2020, 416, 108870. [Google Scholar] [CrossRef]
- Michel, A.; Seidling, W. Forest Condition in Europe: 2016 Technical Report of ICP Forests: Report under the UNECE Convention on Long-Range Transboundary Air Pollution (CLRTAP); International Cooperative Programme on Assessment and Monitoring of Air Pollution Effects on Forests: Vienna, Austria, 2016; ISBN 978-3-902762-65-8. [Google Scholar]
- Alberdi, I.; Sandoval, V.; Condes, S.; Cañellas, I.; Vallejo, R. El Inventario Forestal Nacional español, una herramienta para el conocimiento, la gestión y la conservación de los ecosistemas forestales arbolados. Ecosistemas 2016, 25, 88–97. [Google Scholar] [CrossRef]
- Astigarraga, J.; Andivia, E.; Zavala, M.A.; Gazol, A.; Cruz-Alonso, V.; Vicente-Serrano, S.M.; Ruiz-Benito, P. Evidence of non-stationary relationships between climate and forest responses: Increased sensitivity to climate change in Iberian forests. Glob. Chang. Biol. 2020, 26, 5063–5076. [Google Scholar] [CrossRef] [PubMed]
- Tramblay, Y.; Koutroulis, A.; Samaniego, L.; Vicente-Serrano, S.M.; Volaire, F.; Boone, A.; Le Page, M.; Llasat, M.C.; Albergel, C.; Burak, S.; et al. Challenges for drought assessment in the Mediterranean region under future climate scenarios. Earth-Sci. Rev. 2020, 210, 103348. [Google Scholar] [CrossRef]
- Allué Andrade, J.L. Atlas Fitoclimático de España Taxonomías, 1st ed.; Instituto Nacional de Investigaciones Agrarias: Madrid, Spain, 1990. [Google Scholar]
- Blanco, E.; Casado, M.A.; Costa, M.; Escribano, R.; García, M.; Génova, M.; Regato, P. Los Bosques ibéricos. Una Interpretación Geobotánica; Planeta: Barcelona, Spain, 1997; p. 598. [Google Scholar]
- Hartmann, H.; Moura, C.F.; Anderegg, W.R.; Ruehr, N.K.; Salmon, Y.; Allen, C.D.; Arndt, S.K.; Breshears, D.D.; Davi, H.; Galbraith, D.; et al. Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 2018, 218, 15–28. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Gómez-Sanz, V. Site-Scale ecological marginality: Evaluation model and application to a case study. Ecol. Model. 2019, 408, 108739. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Rodriguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J.J. Cumulative drought stress leads to a loss of growth resilience and explains higher mortality in planted than in naturally regenerated Pinus pinaster stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef]
- Bose, A.K.; Gessler, A.; Bolte, A.; Bottero, A.; Buras, A.; Cailleret, M.; Camarero, J.J.; Haeni, M.; Hereş, A.; Hevia, A.; et al. Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Glob. Chang. Biol. 2020, 26, 4521–4537. [Google Scholar] [CrossRef]
- Manrique-Alba, À.; Beguería, S.; Molina, A.J.; González-Sanchis, M.; Tomàs-Burguera, M.; Del Campo, A.D.; Colangelo, M.; Camarero, J.J. Long-Term thinning effects on tree growth, drought response and water use efficiency at two Aleppo pine plantations in Spain. Sci. Total Environ. 2020, 728, 138536. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.N.; Spotswood, E.N.; Cañadas, E.M.; Navarro, F.B. Stand management to reduce fire risk promotes understorey plant diversity and biomass in a semi-arid Pinus halepensis plantation. Appl. Veg. Sci. 2015, 18, 467–480. [Google Scholar] [CrossRef]
- Granados, M.E.; Chirino Miranda, E.; Gandía Navalón, C.; Vallejo Calzada, V.R.; Vilagrosa Carmona, A. Effect of light and soil moisture on physiological variables in six Mediterranean forest species planted under a pine forest canopy. Revista Chapingo Serie Ciencias Forestales y del Ambiente 2019, 25, 461–476. [Google Scholar] [CrossRef]
- Eastaugh, C.S.; Pötzelsberger, E.; Hasenauer, H. Assessing the impacts of climate change and nitrogen deposition on Norway spruce (Picea abies L. Karst) growth in Austria with BIOME-BGC. Tree Physiol. 2011, 31, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Masek, J.G.; Goward, S.N.; Kennedy, R.E.; Cohen, W.B.; Moisen, G.G.; Schleeweis, K.; Huang, C. United States forest disturbance trends observed using Landsat time series. Ecosystems 2013, 16, 1087–1104. [Google Scholar] [CrossRef]
- Moreno-de las Heras, M.; Bochet, E.; Monleón, V.; Espigares, T.; Nicolau, J.M.; Molina, M.J.; García-Fayos, P. Aridity induces nonlinear effects of human disturbance on precipitation-use efficiency of Iberian woodlands. Ecosystems 2018, 21, 1295–1305. [Google Scholar] [CrossRef]
- Turner, D.P.; Cohen, W.B.; Kennedy, R.E.; Fassnacht, K.S.; Briggs, J.M. Relationships between leaf area index and Landsat TM spectral vegetation indices across three temperate zone sites. Remote Sens. Environ. 1999, 70, 52–68. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).