Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Field Survey and Occurrence Data Compilation
2.2. Environmental Variables
2.3. Model Processing, Evaluation, and Comprehensive Habitat Suitability Model Building
3. Results
3.1. Model Evaluation and Potential Distribution of Current Suitable Habitats
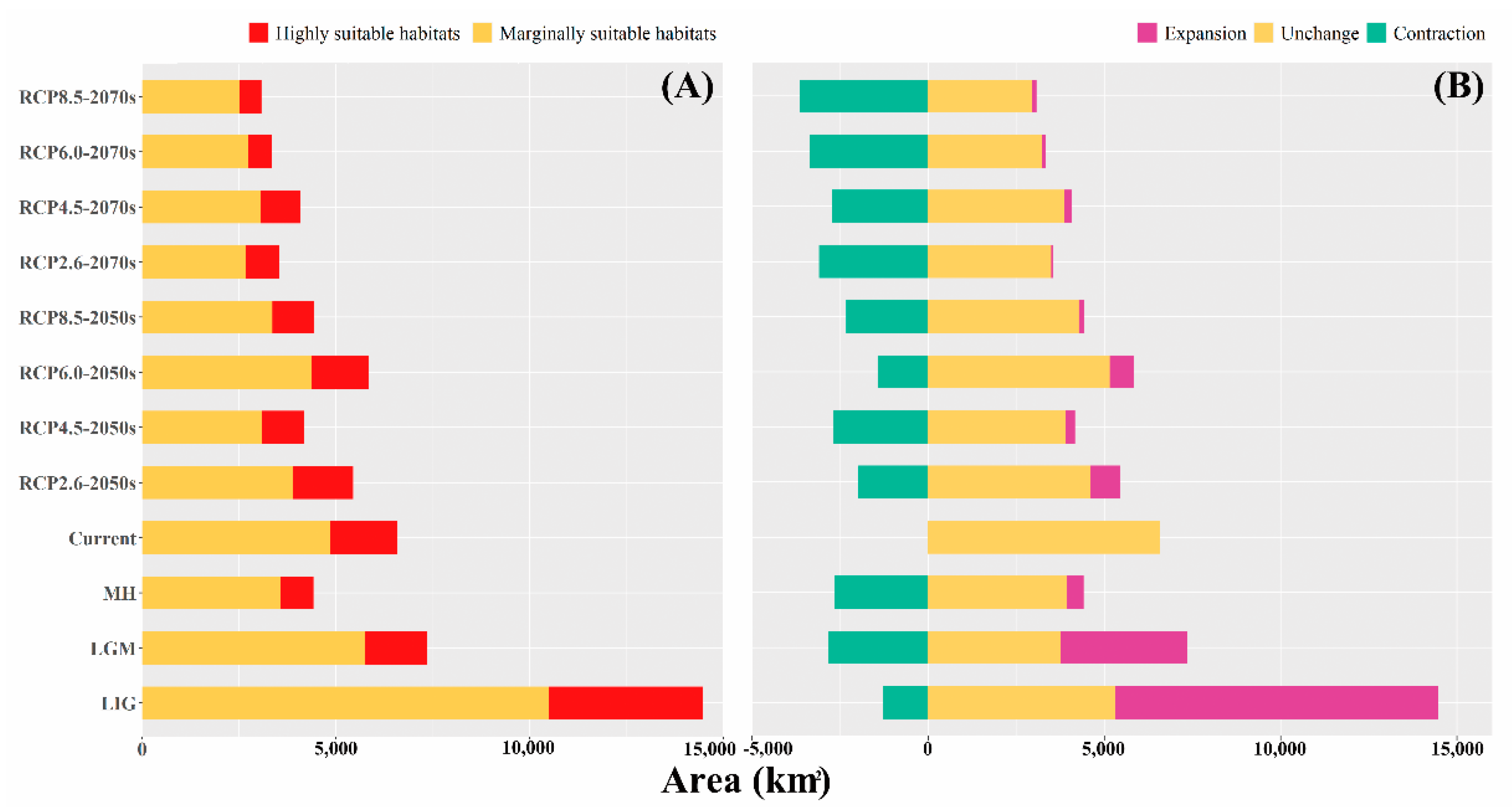
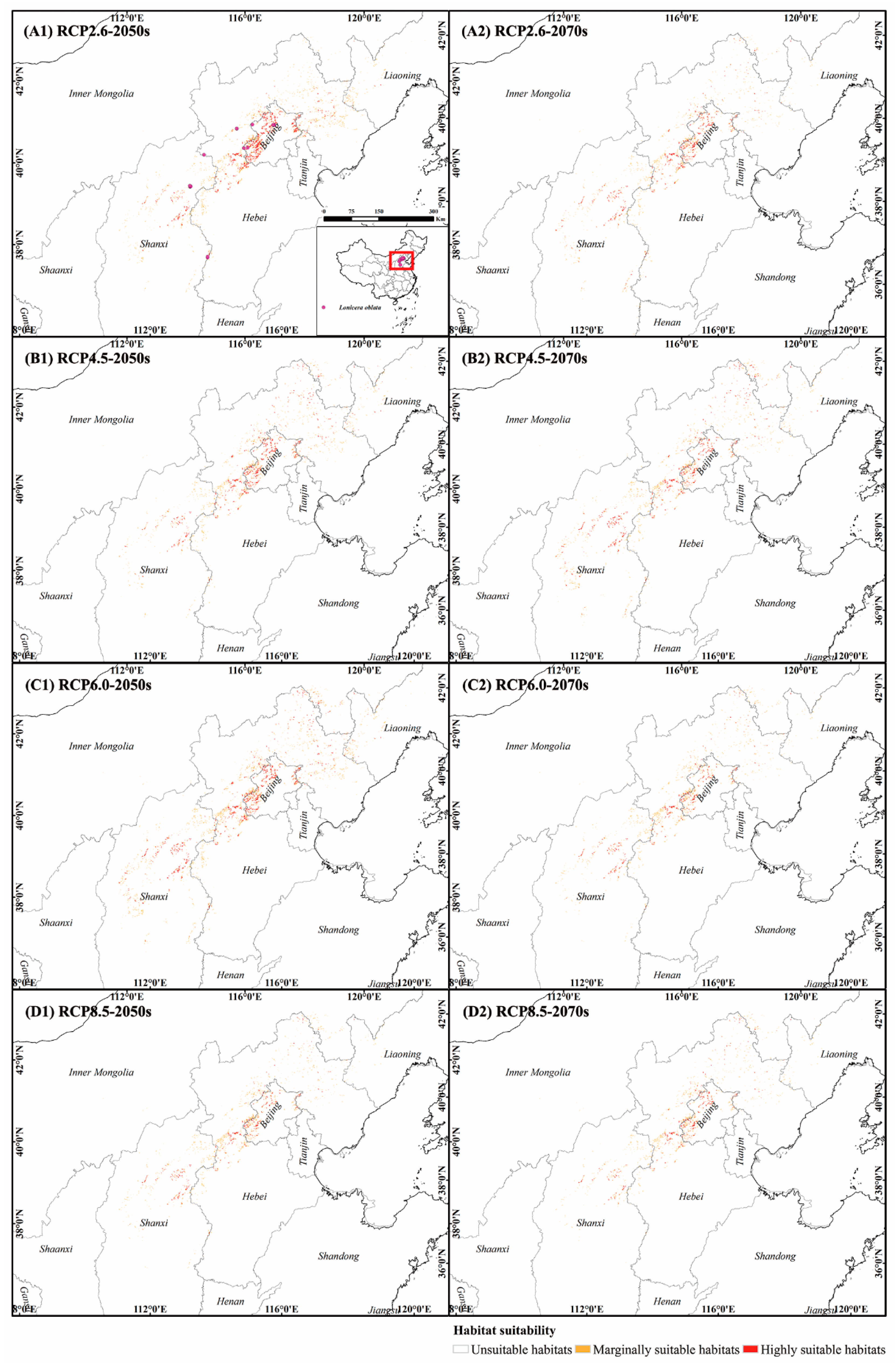
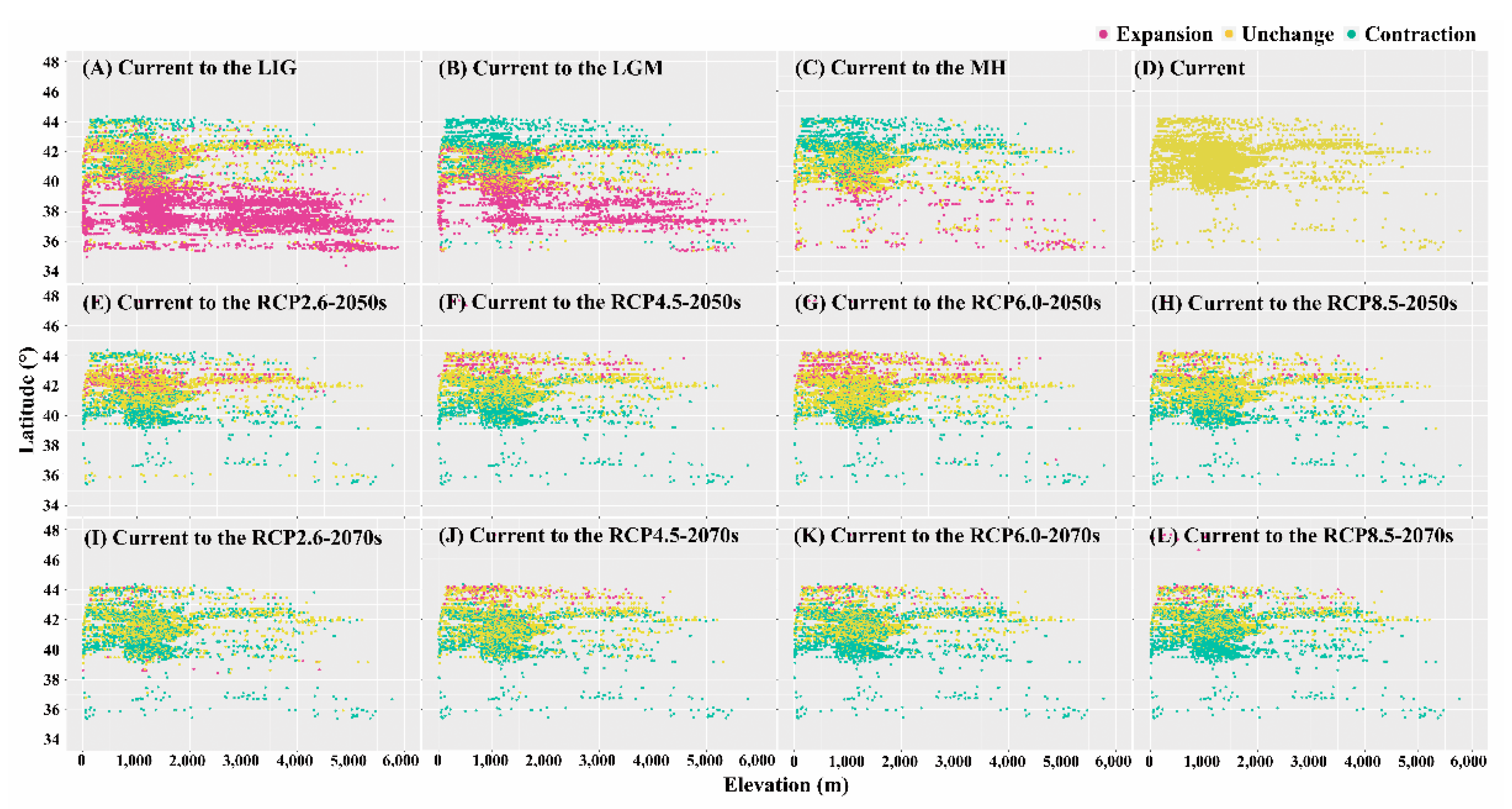
3.2. Dynamics of Suitable Habitat Distribution under Past and Future Scenarios
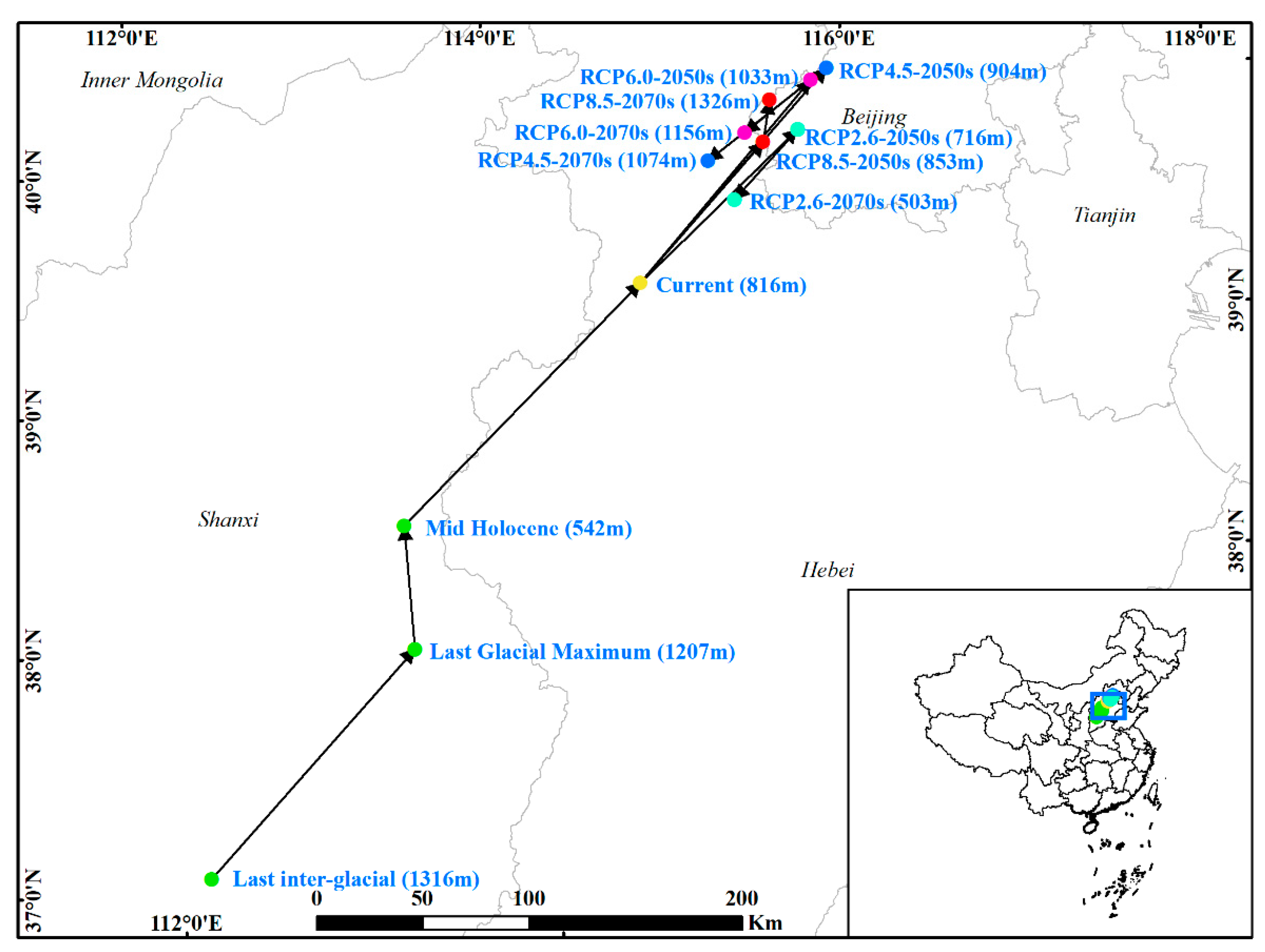
3.3. Shifts of the Core Suitable Habitat Distributions under Climate Change Scenarios
3.4. Contribution of Environmental Variables to Species Distributions
4. Discussion
4.1. Key Environmental Factors Shaping Species Distribution
4.2. Impacts of Climate Change on Species Range Dynamics and Migration Trends
4.3. Stable Climatic Areas, Risks to Species in Climate-Vulnerable Areas, and Conservation Management
4.4. Model Rationality and Drawback
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novacek, M.J. The Biodiversity Crisis: Losing What Counts; The New Press: New York, NY, USA, 2001. [Google Scholar]
- Raup, D.M.; Sepkoski, J.J. Mass extinctions in the marine fossil record. Science 1982, 215, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, D. Extinctions in the fossil record. Philos. Trans. R. Soc. B Biol. Sci. 1994, 344, 11–17. [Google Scholar] [CrossRef]
- Bambach, R.K. Phanerozoic biodiversity mass extinctions. Annu. Rev. Earth Planet. Sci. 2006, 34, 127–155. [Google Scholar] [CrossRef]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.R.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Novacek, M.J.; Cleland, E.E. The current biodiversity extinction event: Scenarios for mitigation and recovery. Proc. Natl. Acad. Sci. USA 2001, 98, 5466–5470. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweigk, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: New York, NY, USA, 2013; Volume 1535. [Google Scholar]
- You, J.; Qin, X.; Ranjitkar, S.; Lougheed, S.C.; Wang, M.; Zhou, W.; Ouyang, D.; Zhou, Y.; Xu, J.; Zhang, W.; et al. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Sci. Rep. 2018, 8, 5879. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, X.; Li, X. Distributional dynamics of a vulnerable species in response to past and future climate change: A window for conservation prospects. PeerJ 2018, 6, e4287. [Google Scholar] [CrossRef]
- Araújo, M.B.; Rahbek, C. How does climate change affect biodiversity? Science 2006, 313, 1396–1397. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, L.J.; Pitman, A.J.; Poulsen, M.; Hughes, L. Where will species go? Incorporating new advances in climate modelling into projections of species distributions. Glob. Chang. Biol. 2007, 13, 1368–1385. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.-C.; Marquet, P.A.; De Ruffray, P.; Brisse, H. A Significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Comes, H.P.; Kadereit, J.W. The effect of Quaternary climatic changes on plant distribution and evolution. Trends Plant Sci. 1998, 3, 432–438. [Google Scholar] [CrossRef]
- Hewitt, G.M. The genetic legacy of the quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Sandel, B.; Arge, L.; Dalsgaard, B.; Davies, R.G.; Gaston, K.J.; Sutherland, W.J.; Svenning, J.-C. The influence of late quaternary climate-change velocity on species endemism. Science 2011, 334, 660–664. [Google Scholar] [CrossRef]
- Dawson, A.G. Ice Age Earth: Late Quaternary Geology and Climate; Routledge: New York, NY, USA, 1992. [Google Scholar]
- Fačkovcová, Z.; Senko, D.; Svitok, M.; Guttová, A. Ecological niche conservatism shapes the distributions of lichens: Geographical segregation does not reflect ecological differentiation. Preslia 2017, 89, 63–85. [Google Scholar] [CrossRef]
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, A.M. The last glacial maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Ohlemüller, R.; Batra, P.; Araújo, M.B. Climate predictors of late quaternary extinctions. Evolution 2010, 64, 2442–2449. [Google Scholar] [CrossRef]
- Davis, M.B. Range shifts and adaptive responses to quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Normand, S.; Ricklefs, R.E.; Skov, F.; Bladt, J.; Tackenberg, O.; Svenning, J.-C. Postglacial migration supplements climate in determining plant species ranges in Europe. Proc. R. Soc. B Boil. Sci. 2011, 278, 3644–3653. [Google Scholar] [CrossRef] [PubMed]
- Svenning, J.-C.; Skov, F. Ice age legacies in the geographical distribution of tree species richness in Europe. Glob. Ecol. Biogeogr. 2007, 16, 234–245. [Google Scholar] [CrossRef]
- Feng, G.; Ma, Z.; Sandel, B.; Mao, L.; Normand, S.; Ordonez, A.; Svenning, J. Species and phylogenetic endemism in angiosperm trees across the Northern Hemisphere are jointly shaped by modern climate and glacial–interglacial climate change. Glob. Ecol. Biogeogr. 2019, 28, 1393–1402. [Google Scholar] [CrossRef]
- Provan, J.; Bennett, K.D. Phylogeographic insights into cryptic glacial refugia. Trends Ecol. Evol. 2008, 23, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Leadley, P.; Pereira, H.M.; Alkemade, R.; Fernandez-Manjarrés, J.F.; Proenca, V.; Scharlemann, J.P.W.; Walpole, M.J. Biodiversity Scenarios: Projections of 21st Century Change in Biodiversity and Associated Ecosystem Services; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2010. [Google Scholar]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Araújo, M.B.; Errea, M.P.; Martínez-Rica, J.P. Exposure of global mountain systems to climate warming during the 21st century. Glob. Environ. Chang. 2007, 17, 420–428. [Google Scholar] [CrossRef]
- Colwell, R.K.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature. 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Broennimann, O.; Thuiller, W.; Hughes, G.; Midgley, G.F.; Alkemade, J.M.R.; Guisan, A. Do geographic distribution, niche property and life form explain plants’ vulnerability to global change? Glob. Chang. Biol. 2006, 12, 1079–1093. [Google Scholar] [CrossRef]
- Boulangeat, I.; Gravel, D.; Thuiller, W. Accounting for dispersal and biotic interactions to disentangle the drivers of species distributions and their abundances. Ecol. Lett. 2012, 15, 584–593. [Google Scholar] [CrossRef]
- Malcolm, J.R.; Liu, C.; Neilson, R.P.; Hansen, L.; Hannah, L. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Kearney, M.R.; Wintle, B.A.; Porter, W.P. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 2010, 3, 203–213. [Google Scholar] [CrossRef]
- Baker, D.J.; Hartley, A.J.; Butchart, S.H.M.; Willis, S.G. Choice of baseline climate data impacts projected species’ responses to climate change. Glob. Chang. Biol. 2016, 22, 2392–2404. [Google Scholar] [CrossRef]
- Busby, J.R. BIOCLIM: A bioclimate analysis and prediction system. Plant Prot. Q. 1991, 6, 8–9. [Google Scholar]
- Stockwell, D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int. J. Geogr. Inf. Sci. 1999, 13, 143–158. [Google Scholar] [CrossRef]
- Bradter, U.; Kunin, W.E.; Altringham, J.D.; Thom, T.J.; Benton, T.G. Identifying appropriate spatial scales of predictors in species distribution models with the random forest algorithm. Methods Ecol. Evol. 2013, 4, 167–174. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning—ICML ’04, Banff, AB, Canada, 4–8 July 2004; Volume 9, pp. 655–662. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Rebelo, H.; Jones, G. Ground validation of presence-only modelling with rare species: A case study on barbastelles Barbastella barbastellus (Chiroptera: Vespertilionidae). J. Appl. Ecol. 2010, 47, 410–420. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Caprifoliaceae. In Flora of China; Yang, Q.E., Landrein, S., Osborne, J., Eds.; Science Press: Beijing, China, 2011; Volume 19, pp. 616–641. [Google Scholar]
- Jiang, W.J.; Wu, J.G.; Ren, J.W.; Bin, Y.B.; Wang, T. The research of characteristics of Lonicera oblata. Chin. Hortic. Abs. 2015, 5. [Google Scholar]
- Liu, Q.R.; Kang, M.Y.; Jiang, Y. Some new recorded plants from Beijing and Hebei (Ⅱ). J. Beijing Norm. Univ. (Nat. Sci.) 2003, 39, 674–676. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R.; Lawrence, D.M.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The community climate system model version 4. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef]
- Beckmann, M.; Václavík, T.; Manceur, A.M.; Šprtová, L.; Von Wehrden, H.; Welk, E.; Cord, A.F. glUV: A global UV-B radiation data set for macroecological studies. Methods Ecol. Evol. 2014, 5, 372–383. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, X.; Liang, S.; Zhou, T.; Huang, K.; Tang, B.; Zhao, W. Time-lag effects of global vegetation responses to climate change. Glob. Chang. Biol. 2015, 21, 3520–3531. [Google Scholar] [CrossRef]
- Shabani, F.; Ahmadi, M.; Peters, K.; Haberle, S.; Champreux, A.; Saltre, F.B.; Bradshaw, C.J.A. Climate-driven shifts in the distribution of koala-browse species from the Last Interglacial to the near future. Ecography 2019, 42, 1587–1599. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Li, M.; He, J.; Zhao, Z.; Lyu, R.; Yao, M.; Cheng, J.; Xie, L. Predictive modelling of the distribution of Clematis sect. Fruticella s. str. under climate change reveals a range expansion during the Last Glacial Maximum. PeerJ 2020, 8, e8729. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, X.; Zhao, Z.; Wei, H.; Gao, B.; Gu, W. Prediction of the potential geographic distribution of the ectomycorrhizal mushroom Tricholoma matsutake under multiple climate change scenarios. Sci. Rep. 2017, 7, srep46221. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, X.; Zhao, Z.; Nawaz, Z. Predicting the impacts of climate change, soils and vegetation types on the geographic distribution of Polyporus umbellatus in China. Sci. Total. Environ. 2019, 648, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Wei, H.Y.; Zhang, Q.Z.; Zhang, X.H.; Zhang, X.Y.; Gu, W. Assessing habitat suitability of parasitic plant cistanche deserticola in Northwest China under future climate scenarios. Forests 2019, 10, 823. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Wei, H.; Ran, Q.; Liu, J.; Zhang, Q.; Gu, W. Potential distribution of Notopterygium incisum Ting ex H. T. Chang and its predicted responses to climate change based on a comprehensive habitat suitability model. Ecol. Evol. 2020, 10, 3004–3016. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Wang, Y.S.; Xie, B.Y.; Wan, F.H.; Xiao, Q.M.; Dai, L.Y. Application of ROC curve analysis in evaluating the performance of alien species' potential distribution models. Biodivers. Sci. 2007, 15, 365–372. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Chahouki, M.A.Z.; Khojasteh, F.; Tavili, A. Distribution of vegetation type according to edaphic properties and topography in Iran. Pol. J. Environ. Stud. 2012, 21, 1071–1077. [Google Scholar]
- Wang, J.; Wang, Y.; Feng, J.; Chen, C.; Chen, J.; Long, T.; Li, J.-Q.; Zang, R.; Li, J. Differential responses to climate and land-use changes in threatened chinese taxus species. Forests 2019, 10, 766. [Google Scholar] [CrossRef]
- Caldwell, M.M. Solar ultraviolet radiation as an ecological factor for alpine plants. Ecol. Monogr. 1968, 38, 243–268. [Google Scholar] [CrossRef]
- Blumthaler, M.; Ambach, W.; Ellinger, R. Increase in solar UV radiation with altitude. J. Photochem. Photobiol. B: Biol. 1997, 39, 130–134. [Google Scholar] [CrossRef]
- Bornman, J.F.; Teramura, A.H. Effects of Ultraviolet-B Radiation on Terrestrial Plants; Young, A.R., Björn, L.O., Moan, J., Nultsch, W., Eds.; Environmental UV Photobiology: Springer, Boston, MA, USA, 1993. [Google Scholar]
- Battaglia, P.R.; Brennan, T.M. Differential effects of short-term exposure to ultraviolet-b radiation upon photosynthesis in cotyledons of a resistant and a susceptible species. Int. J. Plant Sci. 2000, 161, 771–778. [Google Scholar] [CrossRef]
- Albert, K.R.; Mikkelsen, T.N.; Ro-Poulsen, H.; Arndal, M.F.; Michelsen, A. Ambient UV-B radiation reduces PSII performance and net photosynthesis in high Arctic Salix arctica. Environ. Exp. Bot. 2011, 73, 10–18. [Google Scholar] [CrossRef]
- Jansen, M.A.; Bornman, J.F. UV-B radiation: From generic stressor to specific regulator. Physiol. Plant. 2012, 145, 501–504. [Google Scholar] [CrossRef]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Woodwand, F.I. Climate and Plant Distribution; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Zhong, L.; Ma, Y.; Salama, M.S.; Su, Z. Assessment of vegetation dynamics and their response to variations in precipitation and temperature in the Tibetan Plateau. Clim. Chang. 2010, 103, 519–535. [Google Scholar] [CrossRef]
- Chen, M.R. A preliminary study on climate regionalization in China. Sci. Geogr. Sin. 1990, 4. [Google Scholar]
- Lin, L.; He, J.; Xie, L.; Cui, G. Prediction of the suitable area of the Chinese white pines (Pinus subsect. Strobus) under climate changes and implications for their conservation. Forests 2020, 11, 996. [Google Scholar] [CrossRef]
- Feng, J.M. Spatial patterns of species diversity of seed plants in China and their climatic explanation. Biodivers. Sci. 2008, 16, 470. [Google Scholar] [CrossRef]
- Yang, Q.-S.; Xing, Y.; Zhou, Z.-K. Modern geographical distribution of tsuga and its climatic conditions in the Asian monsoon region. Acta Bot. Yunnanica 2009, 31, 389–398. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Loidi, J.; Biurrun, I.; Campos, J.A.; García-Mijangos, I.; Herrera, M. A biogeographical analysis of the European Atlantic lowland heathlands. J. Veg. Sci. 2010, 21, 832–842. [Google Scholar] [CrossRef]
- Ye, X.-Z.; Zhao, G.-H.; Zhang, M.-Z.; Cui, X.-Y.; Fan, H.; Liu, B. Distribution pattern of endangered plant semiliquidambar cathayensis (hamamelidaceae) in response to climate change after the last interglacial period. Forests 2020, 11, 434. [Google Scholar] [CrossRef]
- Ülker, E.D.; Tavşanoğlu, Ç.; Perktaş, U. Ecological niche modelling of pedunculate oak (Quercus robur) supports the ‘expansion–contraction’ model of Pleistocene biogeography. Biol. J. Linn. Soc. 2017, 123, 338–347. [Google Scholar] [CrossRef]
- Li, L.; Abbott, R.J.; Liu, B.; Sun, Y.; Li, L.; Zou, J.; Wang, X.; Miehe, G.; Liu, J. Pliocene intraspecific divergence and Plio-Pleistocene range expansions within Picea likiangensis (Lijiang spruce), a dominant forest tree of the Qinghai-Tibet Plateau. Mol. Ecol. 2013, 22, 5237–5255. [Google Scholar] [CrossRef] [PubMed]
- Navarro Cerrillo, R.M.; Duque-Lazo, J.; Ríos-Gil, N.; Guerrero-Álvarez, J.J.; López-Quintanilla, J.; Palacios-Rodríguez, G. Can habitat prediction models contribute to the restoration and conservation of the threatened tree Abies pinsapo Boiss. in Southern Spain? New For. 2020, 52, 89–112. [Google Scholar] [CrossRef]
- Bertrand, R.; Lenoir, J.; Piedallu, C.; Riofrío-Dillon, G.; de Ruffray, P.; Vidal, C.; Pierrat, J.-C.; Gégout, J.-C. Changes in plant community composition lag behind climate warming in lowland forests. Nature 2011, 479, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-G.; Jin, Y.; Wang, X.-R.; Mao, J.-F.; Li, Y. Predicting impacts of future climate change on the distribution of the widespread conifer platycladus orientalis. PLoS ONE 2015, 10, e0132326. [Google Scholar] [CrossRef]
- Büchi, L.; Vuilleumier, S. Coexistence of specialist and generalist species is shaped by dispersal and environmental factors. Am. Nat. 2014, 183, 612–624. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Klonner, G.; Schütz, M.; Wessely, J.; Willner, W.; Zimmermann, N.E.; Dullinger, S. Range dynamics of mountain plants decrease with elevation. Proc. Natl. Acad. Sci. USA 2018, 115, 1848–1853. [Google Scholar] [CrossRef]
- Araújo, M.B.; Luoto, M. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 2007, 16, 743–753. [Google Scholar] [CrossRef]



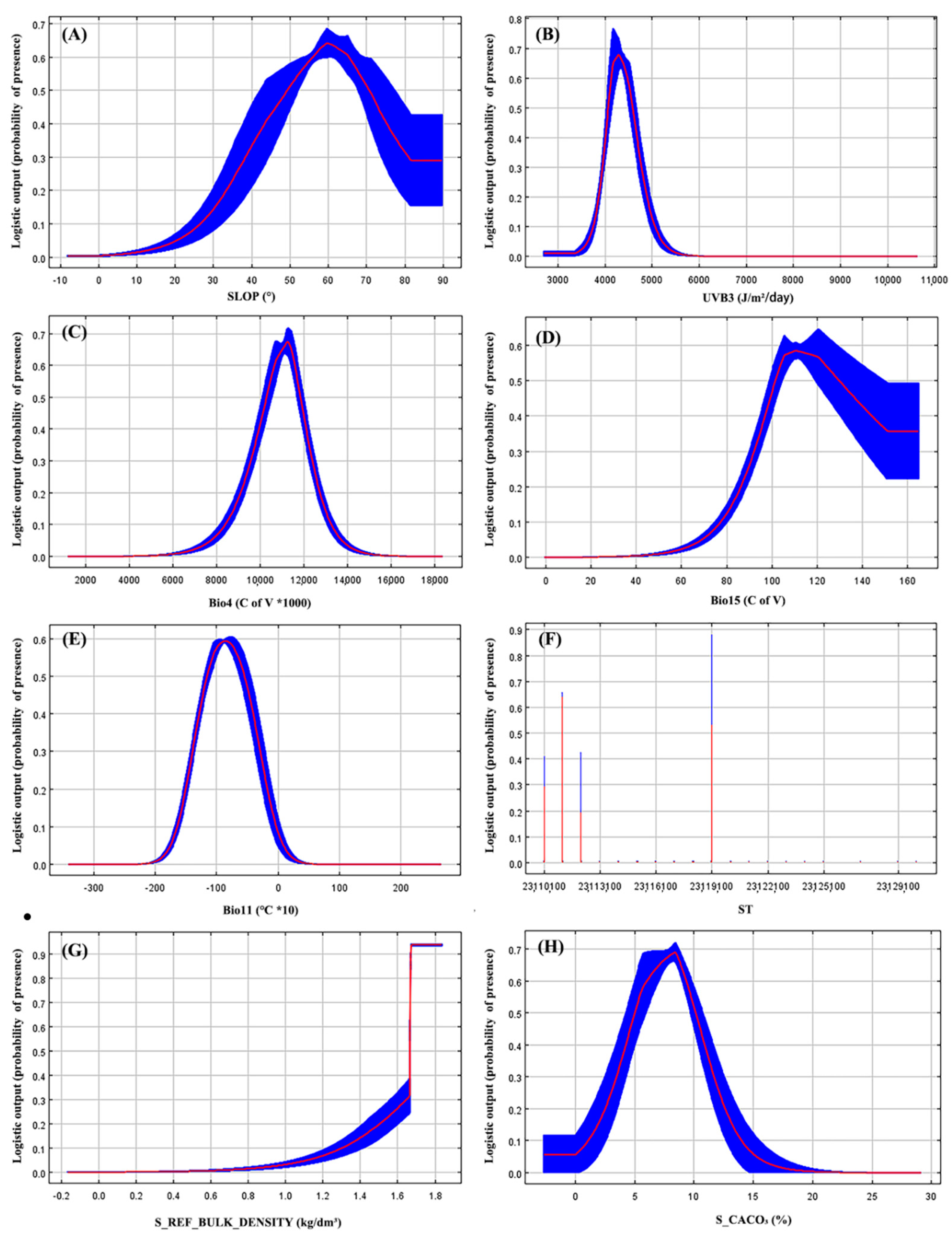
| Environmental Variables | Contribution (%) | Suitable Ranges | Optimal Value | Units |
|---|---|---|---|---|
| Bio4 | 17.1 | 9318.22–12,583.18 | 10,692.94 | * 1000 |
| Bio11 | 8.5 | (−143.65) to (−18.57) | −104.79 | °C * 10 |
| Bio15 | 8.1 | 87.63–164.80 | 120.42 | |
| SLOP | 35.0 | 31.78–89.72 | 65.06 | ° |
| UVB3 | 14.6 | 3900.26–4901.84 | 4170.53 | J/m2/day |
| S_REF_BULK_DENSITY | 9.6 | 1.44–1.84 | 1.84 | kg/dm3 |
| S_CACO3 | 6.1 | 0.91–14.20 | 5.75 | % weight |
| ST | 73.6 | Eluvial brown soil; semi-eluvial cinnamon soil; semi-eluvial gray cinnamon soil; anthropogenic alluvial soil | Anthropogenic alluvial soil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-M.; Shen, X.-L.; Tong, L.; Lei, F.-W.; Mu, X.-Y.; Zhang, Z.-X. Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications. Forests 2021, 12, 125. https://doi.org/10.3390/f12020125
Wu Y-M, Shen X-L, Tong L, Lei F-W, Mu X-Y, Zhang Z-X. Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications. Forests. 2021; 12(2):125. https://doi.org/10.3390/f12020125
Chicago/Turabian StyleWu, Yuan-Mi, Xue-Li Shen, Ling Tong, Feng-Wei Lei, Xian-Yun Mu, and Zhi-Xiang Zhang. 2021. "Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications" Forests 12, no. 2: 125. https://doi.org/10.3390/f12020125
APA StyleWu, Y.-M., Shen, X.-L., Tong, L., Lei, F.-W., Mu, X.-Y., & Zhang, Z.-X. (2021). Impact of Past and Future Climate Change on the Potential Distribution of an Endangered Montane Shrub Lonicera oblata and Its Conservation Implications. Forests, 12(2), 125. https://doi.org/10.3390/f12020125





