Climate Change Effects on Trophic Interactions of Bark Beetles in Inner Alpine Scots Pine Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Sites
2.2. Insect Sampling
2.3. Insect Classification and Mortality Assessment
2.4. Resin Collection
2.5. Resin Duct Density
2.6. Analyses
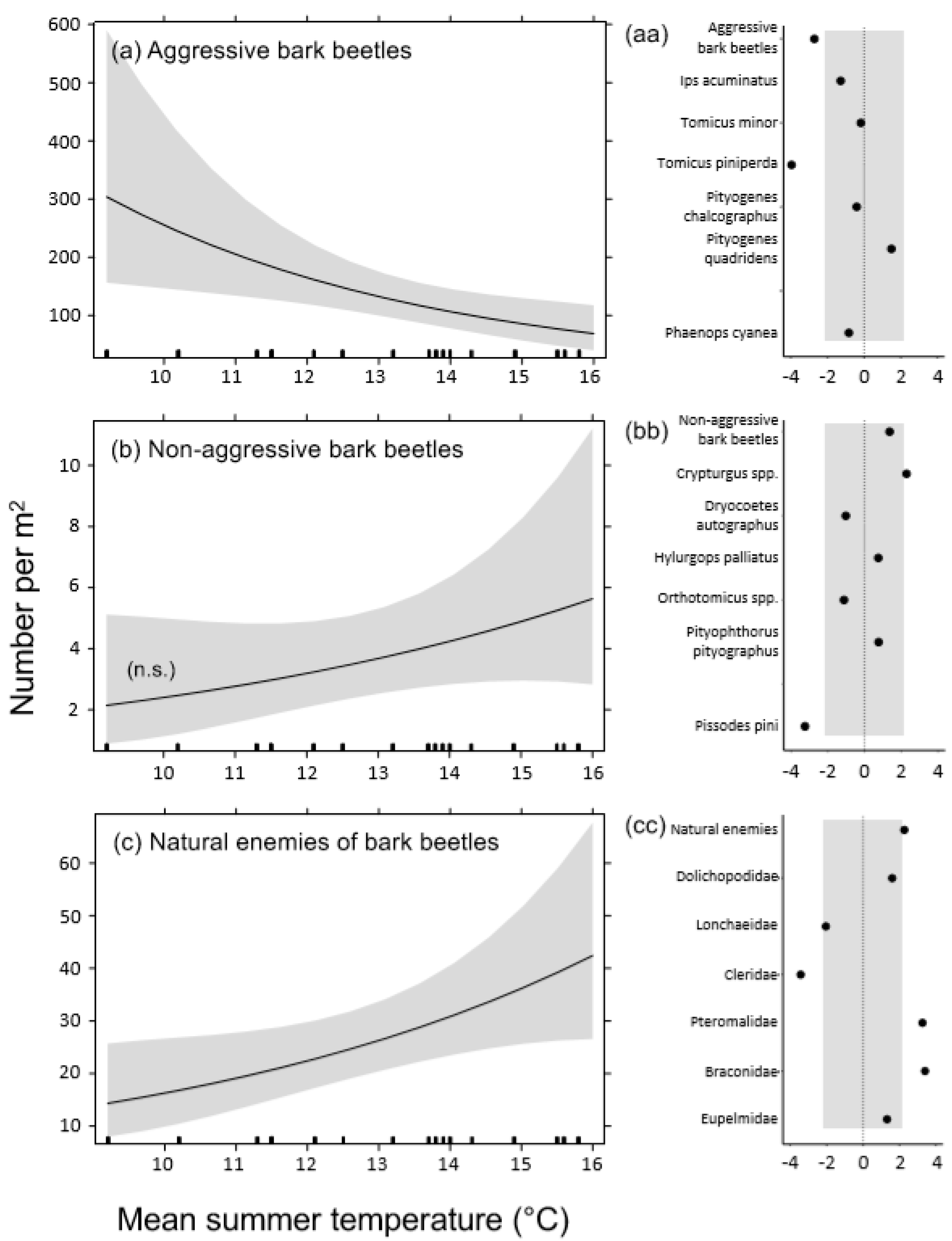
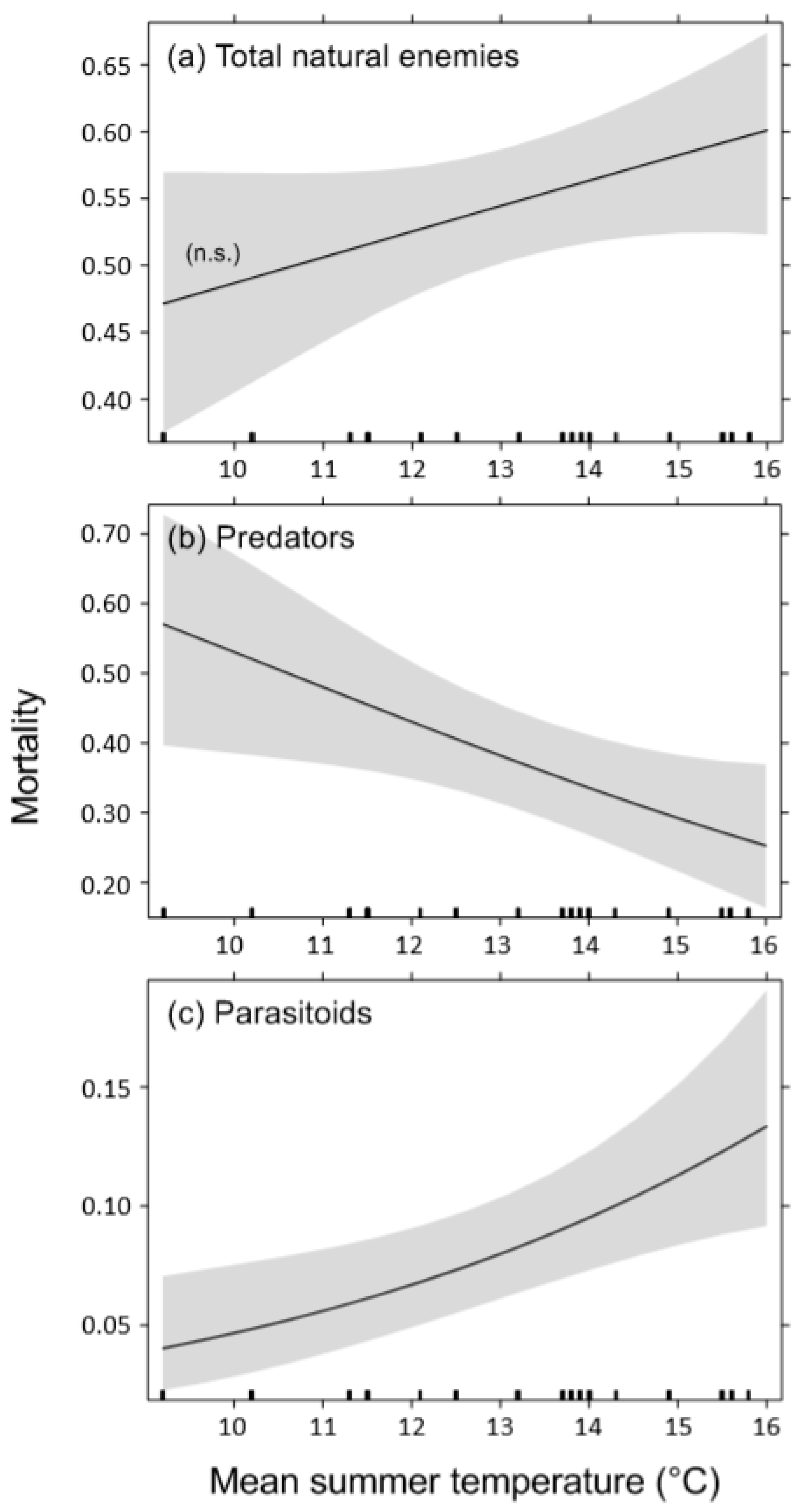
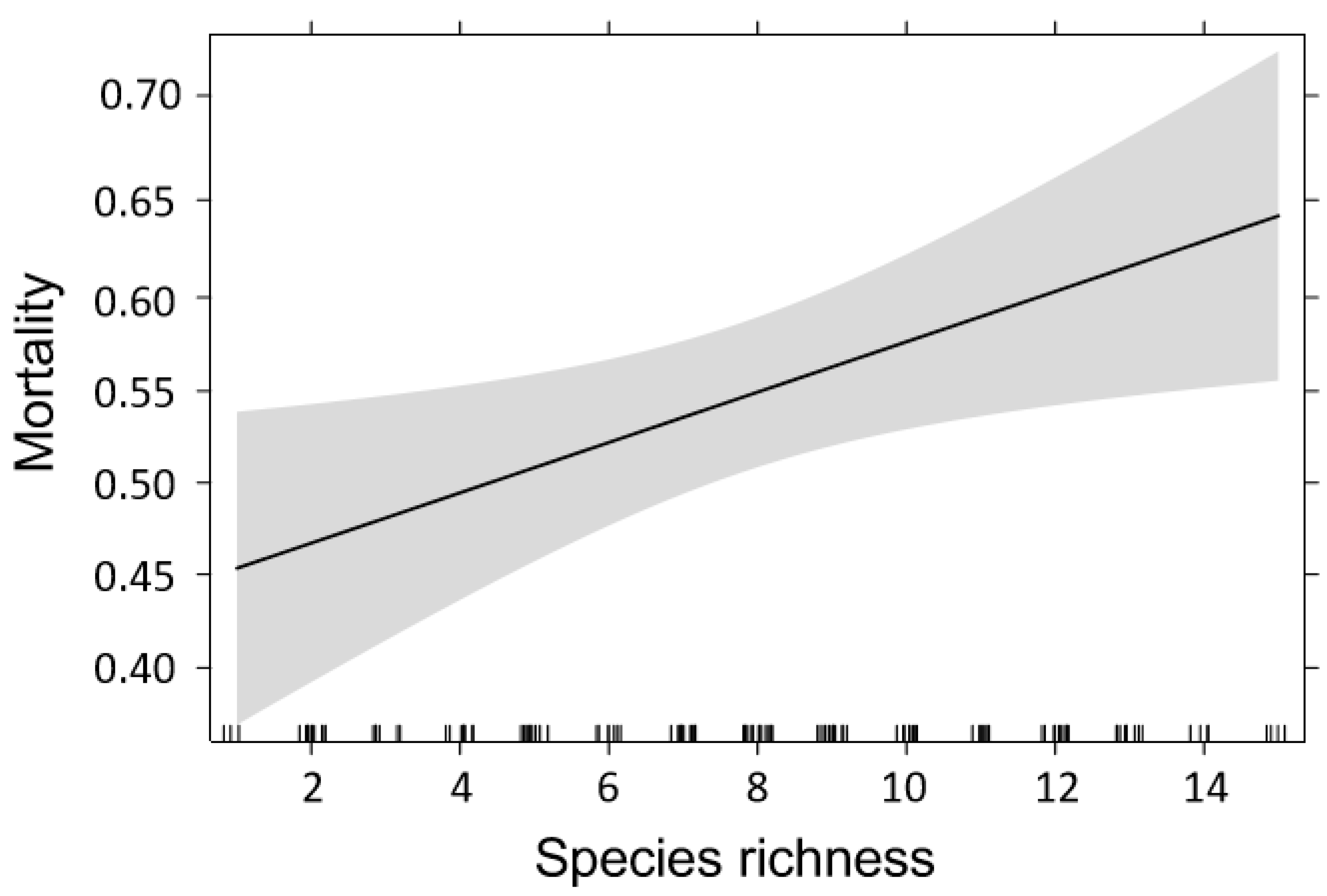
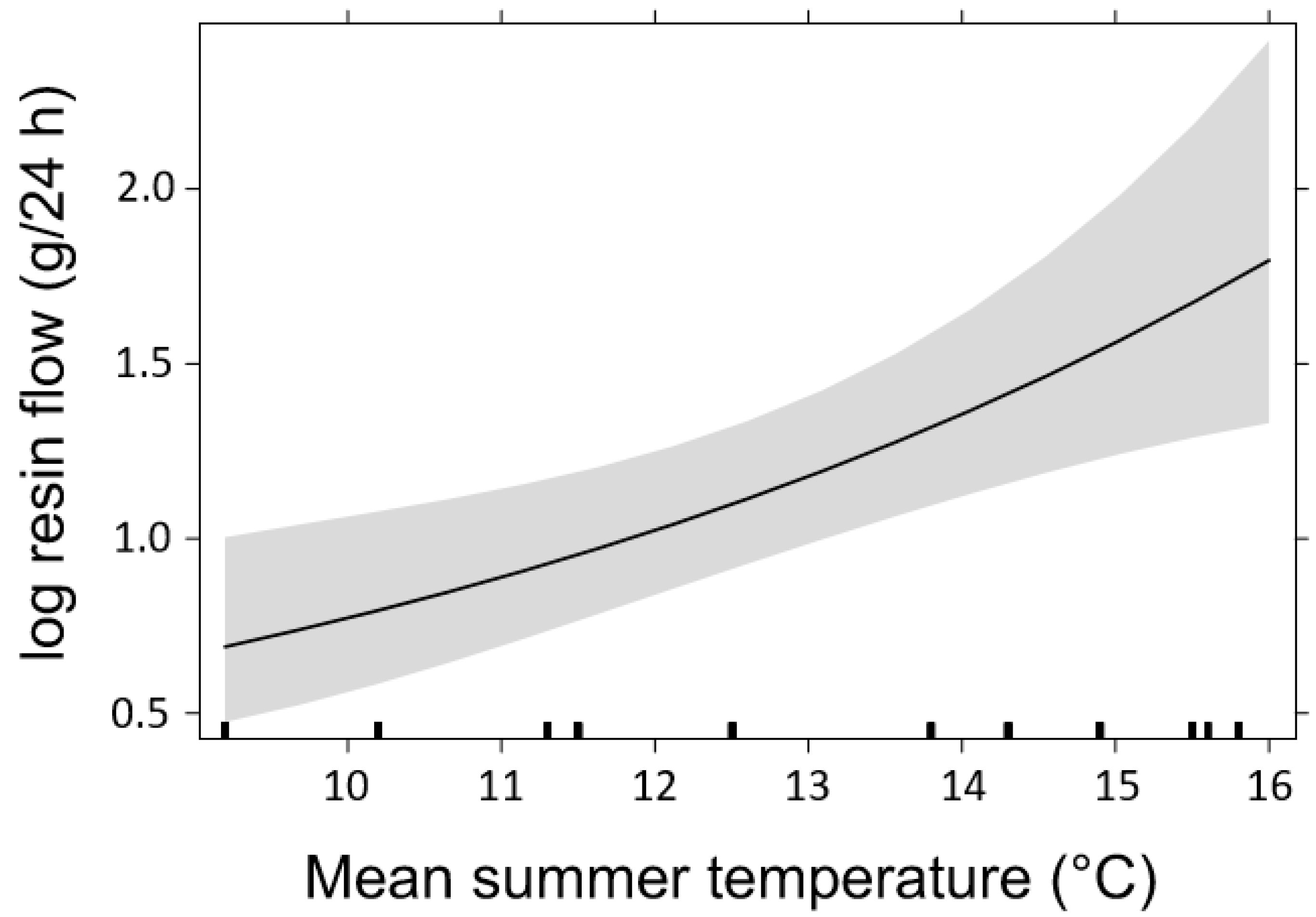
3. Results
3.1. Densities of Emerged Insects
3.2. Resin Flow
4. Discussion
4.1. Effects of Temperature on Bark Beetle Populations
4.2. Effects of Temperature on Bark Beetle Mortality
4.3. Effects of Temperature on Host Tree Defense
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 129. [Google Scholar] [CrossRef]
- Young, D.J.N.; Stevens, J.T.; Earles, J.M.; Moore, J.; Ellis, A.; Jirka, A.L.; Latimer, A.M. Long-term climate and competition explain forest mortality patterns under extreme drought. Ecol. Lett. 2017, 20, 78–86. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Chang. 2014, 4, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; McDowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef]
- Rosenberger, D.W.; Venette, R.C.; Aukema, B.H. Development of an aggressive bark beetle on novel hosts: Implications for outbreaks in an invaded range. J. Appl. Ecol. 2018, 55, 1526–1537. [Google Scholar] [CrossRef]
- Bentz, B.J.; Jönsson, A.M.; Schroeder, M.; Weed, A.; Wilcke, R.A.I.; Larsson, K. Ips typographus and Dendroctonus ponderosae models project thermal suitability for intra- and inter-continental establishment in a changing climate. Front. For. Glob. Change 2019, 2, 1. [Google Scholar] [CrossRef]
- Wermelinger, B. Ecology and management of the spruce bark beetle Ips typographus—A review of recent research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W. Climate change amplifies the interactions between wind and bark beetle disturbances in forest landscapes. Landsc. Ecol. 2017, 32, 1485–1498. [Google Scholar] [CrossRef]
- Paine, T.D.; Raffa, K.F.; Harrington, T.C. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef]
- Krokene, P. Conifer defense and resistance to bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: London, UK, 2015; pp. 177–207. [Google Scholar]
- Raffa, K.F.; Grégoire, J.C.; Lindgren, B.S. Natural history and ecology of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: London, UK, 2015; pp. 1–40. [Google Scholar]
- Damien, M.; Tougeron, K. Prey–predator phenological mismatch under climate change. Curr. Opin. Ins. Sci. 2019, 35, 60–68. [Google Scholar] [CrossRef]
- Huang, J.B.; Kautz, M.; Trowbridge, A.M.; Hammerbacher, A.; Raffa, K.F.; Adams, H.D.; Goodsman, D.W.; Xu, C.G.; Meddens, A.J.H.; Kandasamy, D.; et al. Tree defence and bark beetles in a drying world: Carbon partitioning, functioning and modelling. New Phytol. 2020, 225, 26–36. [Google Scholar] [CrossRef]
- Coley, P.D.; Bryant, J.P.; Chapin, F.S.I. Resource availability and plant antiherbivore defense. Science 1985, 230, 895–899. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.; He, S. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Boege, K.; Barton, K.E.; Dirzo, R. Influence of tree ontogeny on plant-herbivore interactions. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Amsterdam, The Netherlands, 2011; pp. 193–214. [Google Scholar]
- Ferrenberg, S.; Kane, J.M.; Langenhan, J.M. To grow or defend? Pine seedlings grow less but induce more defences when a key resource is limited. Tree Physiol. 2015, 35, 107–111. [Google Scholar] [CrossRef]
- Gaylord, M.L.; Kolb, T.E.; Pockman, W.T.; Plaut, J.A.; Yepez, E.A.; Macalady, A.K.; Pangle, R.E.; McDowell, N.G. Drought predisposes piñon-juniper woodlands to insect attacks and mortality. New Phytol. 2013, 198, 567–578. [Google Scholar] [CrossRef]
- Netherer, S.; Matthews, B.; Katzensteiner, K.; Blackwell, E.; Henschke, P.; Hietz, P.; Pennerstorfer, J.; Rosner, S.; Kikuta, S.; Schume, H. Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 2015, 205, 1128–1141. [Google Scholar] [CrossRef]
- Mulock, P.; Christiansen, E. The threshold of successful attack by Ips typographus on Picea abies: A field experiment. For. Ecol. Manag. 1986, 14, 125–132. [Google Scholar] [CrossRef]
- Nelson, W.A.; Lewis, M.A. Connecting host physiology to host resistance in the conifer-bark beetle system. Theor. Ecol. 2008, 1, 163–177. [Google Scholar] [CrossRef]
- Ayres, M.P.; Lombardero, M.J. Assessing the consequences of global change for forest disturbance from herbivores and pathogens. Sci. Total Environ. 2000, 262, 263–286. [Google Scholar] [CrossRef]
- Jakoby, O.; Lischke, H.; Wermelinger, B. Climate change alters elevational phenology patterns of the European spruce bark beetle (Ips typographus). Glob. Change Biol. 2019, 25, 4048–4063. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Harding, S.; Krokene, P.; Lange, H.; Lindelöw, A.; Økland, B.; Ravn, H.P.; Schroeder, L.M. Modelling the potential impact of global warming on Ips typographus voltinism and reproductive diapause. Clim. Chang. 2011, 109, 695–718. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Kolb, T.E. Responses of tree-killing bark beetles to a changing climate. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CAB International: Oxfordshire, UK, 2015; pp. 173–201. [Google Scholar]
- Wermelinger, B.; Seifert, M. Temperature-dependent reproduction of the spruce bark beetle Ips typographus, and analysis of the potential population growth. Ecol. Entomol. 1999, 24, 103–110. [Google Scholar] [CrossRef]
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of forest insect pests to climate change: Not so simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef]
- Weber, P.; Bugmann, H.; Rigling, A. Radial growth responses to drought of Pinus sylvestris and Quercus pubescens in an inner-Alpine dry valley. J. Veg. Sci. 2007, 18, 777–792. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Štefková, K.; Okrouhlík, J.; Doležal, P. Development and survival of the spruce bark beetle, Ips typographus (Coleoptera: Curculionidae: Scolytinae) at low temperatures in the laboratory and the field. Eur. J. Entomol. 2017, 114, 1–6. [Google Scholar] [CrossRef]
- Henn, M.W.; Schopf, R. Response of beech (Fagus sylvatica) to elevated CO2 and N: Influence on larval performance of the gypsy moth Lymantria dispar (Lep., Lymantriidae). J. Appl. Entomol. 2001, 125, 501–505. [Google Scholar] [CrossRef]
- Stiling, P.; Cornelissen, T. How does elevated carbon dioxide (CO2) affect plant–herbivore interactions? A field experiment and meta-analysis of CO2-mediated changes on plant chemistry and herbivore performance. Glob. Chang. Biol. 2007, 13, 1823–1842. [Google Scholar] [CrossRef]
- Körner, C.; Asshoff, R.; Bignucolo, O.; Hättenschwiler, S.; Keel, S.G.; Peláez-Riedl, S.; Pepin, S.; Siegwolf, R.T.W.; Zotz, G. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science 2005, 309, 1360–1362. [Google Scholar] [CrossRef]
- Novick, K.A.; Katul, G.G.; McCarthy, H.R.; Oren, R. Increased resin flow in mature pine trees growing under elevated CO2 and moderate soil fertility. Tree Physiol. 2012, 32, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Péré, C.; Jactel, H.; Kenis, M. Response of insect parasitism to elevation depends on host and parasitoid life-history strategies. Biol. Lett. 2013, 9. [Google Scholar] [CrossRef]
- Rasmann, S.; Pellissier, L.; Defossez, E.; Jactel, H.; Kunstler, G. Climate-driven change in plant–insect interactions along elevation gradients. Funct. Ecol. 2014, 28, 46–54. [Google Scholar] [CrossRef]
- Ferrenberg, S.; Langenhan, J.M.; Loskot, S.A.; Rozal, L.M.; Mitton, J.B. Resin monoterpene defenses decline within three widespread species of pine (Pinus) along a 1530-m elevational gradient. Ecosphere 2017, 8, e01975. [Google Scholar] [CrossRef]
- Moreira, X.; Petry, W.K.; Mooney, K.A.; Rasmann, S.; Abdala-Roberts, L. Elevational gradients in plant defences and insect herbivory: Recent advances in the field and prospects for future research. Ecography 2018, 41, 1485–1496. [Google Scholar] [CrossRef]
- Krause, A.M.; Townsend, P.A.; Lee, Y.; Raffa, K.F. Predators and competitors of the mountain pine beetle Dendroctonus ponderosae (Coleoptera: Curculionidae) in stands of changing forest composition associated with elevation. Agric. For. Entomol. 2017, 20, 402–413. [Google Scholar] [CrossRef]
- Kenis, M.; Wermelinger, B.; Grégoire, J.C. Research on parasitoids and predators of Scolytidae—A review. In Bark and Wood Boring Insects in Living Trees in Europe—A synthesis; Lieutier, F., Day, K.R., Battisti, A., Grégoire, J.C., Evans, H.F., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 237–290. [Google Scholar]
- Wermelinger, B.; Rigling, A.; Schneider Mathis, D.; Dobbertin, M. Assessing the role of bark- and wood-boring insects in the decline of Scots pine (Pinus sylvestris) in the Swiss Rhone valley. Ecol. Entomol. 2008, 33, 239–249. [Google Scholar] [CrossRef]
- Karsky, D.; Strom, B.; Thistle, H. An Improved Method for Collecting and Monitoring Pine Oleoresin; US Department of Agriculture, USDA Forest Service, Technology and Development: Missoula, MT, USA, 2004.
- Gärtner, H.; Heinrich, I. The formation of traumatic rows of resin ducts in Larix decidua and Picea abies (Pinaceae) as a result of wounding experiments in the dormant season. IAWA J. 2009, 30, 199–215. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 3.6.0. Available online: https://www.R-project.org (accessed on 10 August 2020).
- Pinheiro, J.C.; Bates, D.M.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-139. Available online: http://CRAN.R-project.org/package=nlme (accessed on 10 August 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Software 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; SAGE: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Fox, J. Effect displays in R for generalised linear models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef]
- Leingärtner, A.; Hoiss, B.; Krauss, J.; Steffan-Dewenter, I. Combined effects of extreme climatic events and elevation on nutritional quality and herbivory of alpine plants. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Sevruk, B. Regional dependency of precipitation–altitude relationship in the Swiss Alps. Clim. Chang. 1997, 36, 355–369. [Google Scholar] [CrossRef]
- Lahr, E.C.; Sala, A. Species, elevation, and diameter affect whitebark pine and lodgepole pine stored resources in the sapwood and phloem: Implications for bark beetle outbreaks. Can. J. For. Res. 2014, 44, 1312–1319. [Google Scholar] [CrossRef]
- Amman, G.D. Mountain pine beetle brood production in relation to thickness of lodgepole pine phloem. J. Econ. Entomol. 1972, 65, 138–140. [Google Scholar] [CrossRef]
- Boone, C.K.; Aukema, B.H.; Bohlmann, J.; Carroll, A.L.; Raffa, K.F. Efficacy of tree defense physiology varies with bark beetle population density: A basis for positive feedback in eruptive species. Can. J. For. Res. 2011, 41, 1174–1188. [Google Scholar] [CrossRef]
- Rubin-Aguirre, A.; Saenz-Romero, C.; Lindig-Cisneros, R.; del-Rio-Mora, A.A.; Tena-Morelos, C.A.; Campos-Bolaños, R.; del-Val, E. Bark beetle pests in an altitudinal gradient of a Mexican managed forest. For. Ecol. Manag. 2015, 343, 73–79. [Google Scholar] [CrossRef]
- Soto-Correa, J.C.; Avilés-Carrillo, I.; Giron-Gutiérrez, D.; Cambrón-Sandoval, V.H. Altitudinal abundance of Dendroctonus frontalis (Coleoptera: Curculionidae) in relation to climatic variables in Hidalgo, Mexico. Rev. Biol. Trop. 2019, 67, 370–379. [Google Scholar] [CrossRef]
- Williams, D.T.; Cull, T.; Forster, J. Investigating the abundance and flight period of bark beetles (Coleoptera: Curculionidae: Scolytinae) over elevational gradients in Sitka spruce forests. Agric. For. Entomol. 2020. [Google Scholar] [CrossRef]
- Chinellato, F.; Faccoli, M.; Marini, L.; Battisti, A. Distribution of Norway spruce bark and wood-boring beetles along Alpine elevational gradients. Agric. For. Entomol. 2014, 16, 111–118. [Google Scholar] [CrossRef]
- Kleinman, S.J.; DeGomez, T.E.; Snider, G.B.; Williams, K.E. Large-scale pinyon Ips (Ips confusus) outbreak in southwestern United States tied with elevation and land cover. J. For. 2012, 110, 194–200. [Google Scholar] [CrossRef]
- Seidl, R.; Baier, P.; Rammer, W.; Schopf, A.; Lexer, M.J. Modelling tree mortality by bark beetle infestation in Norway spruce forests. Ecol. Model. 2007, 206, 383–399. [Google Scholar] [CrossRef]
- Paz-Kagan, T.; Brodrick, P.G.; Vaughn, N.R.; Das, A.J.; Stephenson, N.L.; Nydick, K.R.; Asner, G.P. What mediates tree mortality during drought in the southern Sierra Nevada? Ecol. Appl. 2017, 27, 2443–2457. [Google Scholar] [CrossRef]
- Galmán, A.; Abdala-Roberts, L.; Zhang, S.; Berny-Mier y Teran, J.C.; Rasmann, S.; Moreira, X. A global analysis of elevational gradients in leaf herbivory and its underlying drivers: Effects of plant growth form, leaf habit and climatic correlates. J. Ecol. 2018, 106, 413–421. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, J.F.; Baz, A. The effects of elevation on the butterfly communities of a Mediterranean mountain, Sierra de Javalambre, Central Spain. J. Lepidopt. Soc. 1995, 49, 192–207. [Google Scholar]
- Röder, J.; Bässler, C.; Brandl, B.; Dvořak, L.; Floren, A.; Gossner, M.M.; Gruppe, A.; Jarzabek-Müller, A.; Vojtech, O.; Wagner, C.; et al. Arthropod species richness in the Norway Spruce (Picea abies (L.) Karst.) canopy along an elevation gradient. For. Ecol. Manag. 2010, 259, 1513–1521. [Google Scholar] [CrossRef]
- Reymond, A.; Purcell, J.; Cherix, D.; Guisan, A.; Pellissier, L. Functional diversity decreases with temperature in high elevation ant fauna. Ecol. Entomol. 2013, 38, 364–373. [Google Scholar] [CrossRef]
- Altmann, S.H.; Claros, S. Insect abundance and damage on the deciduous Nothofagus macrocarpa increase with altitude at a site in the Mediterranean climate zone of Chile. Austral. Entomol. 2015, 54, 402–410. [Google Scholar] [CrossRef]
- Azrag, A.G.A.; Pirk, C.W.W.; Yusuf, A.A.; Pinard, F.; Niassy, S.; Mosomtai, G.; Babin, R. Prediction of insect pest distribution as influenced by elevation: Combining field observations and temperature-dependent development models for the coffee stink bug, Antestiopsis thunbergii (Gmelin). PLoS ONE 2018, 13, e0199569. [Google Scholar] [CrossRef]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Reeve, J.D. Predation and bark beetle dynamics. Oecologia 1997, 112, 48–54. [Google Scholar] [CrossRef]
- Williams, K.K.; McMillin, J.D.; DeGomez, T.E. Relative and seasonal abundance of three bark beetle predators (Coleoptera: Trogositidae, Cleridae) across an elevation gradient in ponderosa pine forests of north central Arizona. West. N. Am. Nat. 2009, 69, 351–363. [Google Scholar] [CrossRef]
- Corcos, D.; Cerretti, P.; Mei, M.; Taglianti, A.V.; Paniccia, D.; Santoiemma, G.; De Biase, A.; Marini, L. Predator and parasitoid insects along elevational gradients: Role of temperature and habitat diversity. Oecologia 2018, 188, 193–202. [Google Scholar] [CrossRef]
- Camacho, L.F.; Aviles, L. Decreasing predator density and activity explains declining predation of insect prey along elevational gradients. Am. Nat. 2019, 194, 334–343. [Google Scholar] [CrossRef]
- Libra, M.; Tulai, S.; Novotny, V.; Hrcek, J. Elevational contrast in predation and parasitism risk to caterpillars in a tropical rainforest. Entomol. Exp. Appl. 2019, 167, 922–931. [Google Scholar] [CrossRef]
- Fink, U.; Völkl, W. The effect of abiotic factors on foraging and oviposition success of the aphid parasitoid, Aphidius rosae. Oecologia 1995, 103, 371–378. [Google Scholar] [CrossRef]
- Raffa, K.F. Terpenes tell different tales at different scales: Glimpses into the chemical ecology of conifer-bark beetle-microbial interactions. J. Chem. Ecol. 2014, 40, 1–20. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef]
- DeRose, R.J.; Bekker, M.F.; Long, J.N. Traumatic resin ducts as indicators of bark beetle outbreaks. Can. J. For. Res. 2017, 47, 1168–1174. [Google Scholar] [CrossRef]
- Denham, S.O.; Coyle, D.R.; Oishi, A.C.; Bullock, B.P.; Heliövaara, K.; Novick, K.A. Tree resin flow dynamics during an experimentally induced attack by Ips avulsus, I. calligraphus, and I. grandicollis. Can. J. For. Res. 2019, 49, 53–63. [Google Scholar] [CrossRef]
- Wimmer, R.; Grabner, M. Effects of climate on vertical resin duct density and radial growth of Norway spruce [Picea abies (L.) Karst.]. Trees 1997, 11, 271–276. [Google Scholar] [CrossRef]
- Rigling, A.; Brühlhart, H.; Bräker, O.U.; Forster, T.; Schweingruber, F.H. Effects of irrigation on diameter growth and vertical resin duct production in Pinus sylvestris L. on dry sites in the central Alps, Switzerland. For. Ecol. Manag. 2003, 175, 285–296. [Google Scholar] [CrossRef]
- Ruel, J.J.; Ayres, M.P.; Lorio, P.L.J. Loblolly pine responds to mechanical wounding with increased resin flow. Can. J. For. Res. 1998, 28, 596–602. [Google Scholar] [CrossRef]
- Gely, C.; Laurance, S.G.W.; Stork, N.E. How do herbivorous insects respond to drought stress in trees? Biol. Rev. 2019. [Google Scholar] [CrossRef]
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.; Carroll, A.; Forster, B.; Grégoire, J.C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce forests. Ecography 2017, 40, 1426–1435. [Google Scholar] [CrossRef]
- Mezei, P.; Jakuš, R.; Pennerstorfer, J.; Havašová, M.; Škvarenina, J.; Ferenčík, J.; Slivinský, J.; Bičárová, S.; Bilčík, D.; Blaženec, M.; et al. Storms, temperature maxima and the Eurasian spruce bark beetle Ips typographus—An infernal trio in Norway spruce forests of the Central European High Tatra Mountains. Agric. For. Meteorol. 2017, 242, 85–95. [Google Scholar] [CrossRef]
- Seybold, S.J.; Bentz, B.J.; Fettig, C.J.; Lundquist, J.E.; Progar, R.A.; Gillette, N.E. Management of western North American bark beetles with semiochemicals. Annu. Rev. Entomol. 2018, 63, 407–432. [Google Scholar] [CrossRef] [PubMed]
- Hlásny, T.; Krokene, P.; Liebhold, A.; Montagné-Huck, C.; Müller, J.; Qin, H.; Raffa, K.; Schelhaas, M.J.; Seidl, R.; Svoboda, M.; et al. From Science to Policy 8: Living with Bark Beetles: Impacts, Outlook and Management Options; European Forest Institute: Joensuu, Finland, 2019. [Google Scholar]
- Rouault, G.; Candau, J.N.; Lieutier, F.; Nageleisen, L.M.; Martin, J.C.; Warzee, N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann. For. Sci. 2006, 63, 613–624. [Google Scholar] [CrossRef]
- Wetherington, M.T.; Jennings, D.E.; Shrewsbury, P.M.; Duan, J.J. Climate variation alters the synchrony of host-parasitoid interactions. Ecol. Evol. 2017, 7, 8578–8587. [Google Scholar] [CrossRef] [PubMed]
| Region | Sampling Years | Location | Slope Aspect | Elevation [m a.s.l.] (=Site) | Stand | # Trees for Colonization | TREE Height [m] | # Trees for Resin Flow | # Trees for Resin Ducts | Mean Summer Temp. [°C] | Mean Winter Temp. [°C] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Valais (CH) | 2010, 2011 | Salgesch | South | 900 | Pin,Spr | 8 | 11.1 ± 2.57 | 16 | - | 15.6 | −0.4 |
| 46.3299, 7.5866 | 1200 | Pin,Spr | 8 | 14.6 ± 2.73 | - | - | 13.7 | −0.6 | |||
| 1600 | Pin,Spr | 8 | 16.6 ± 1.66 | 14 | - | 11.3 | −2.8 | ||||
| Visp | North | 900 | Pin,Lar,Spr | 8 | 14.3 ± 2.74 | 16 | 10 | 15.5 | −0.2 | ||
| 46.2930, 7.8101 | 1200 | Pin,Lar,Spr | 8 | 16.9 ± 2.83 | - | - | 14.0 | −1.2 | |||
| 1500 | Pin,Spr | 8 | 15.5 ± 1.61 | 15 | 10 | 11.5 | −3.1 | ||||
| Grisons (CH) | 2011, 2012 | Scharans | South | 900 | Pin,Spr | 4a | 9.1 ± 2.43 | 12 | - | 14.3 | −0.3 |
| 46.7204, 9.4715 | 1200 | Pin,Spr | 8 | 17.3 ± 2.93 | - | - | 13.2 | −1.1 | |||
| 1700 | Pin,Spr | 8 | 16.9 ± 2.74 | 11 | - | 9.2 | −3.3 | ||||
| Felsberg | South | 800 | Pin,Bee,Spr | 8 | 14.1 ± 1.27 | 12 | 10 | 14.9 | 1.5 | ||
| 46.8584, 9.4722 | 1300 | Pin,Spr,Lar | 8 | 16.7 ± 2.16 | - | - | 12.1 | −0.8 | |||
| 1600 | Pin,Spr,Lar | 8 | 20.7 ± 4.03 | 11 | 10 | 10.2 | −2.9 | ||||
| Aosta (IT) | 2010, 2011 | Sarre | South | 900 | NA | ||||||
| 45.7319, 7.2472 | 1200 | Pin | 8 | 16.7 ± 2.19 | 15 | - | 13.8 | −1.9 | |||
| 1600 | Pin,Lar,Spr | 8 | 16.4 ± 2.06 | 15 | - | 11.5 | −3.2 | ||||
| Aymavilles | North | 900 | Spr,Pin | 8 | 8.6 ± 1.25 | 17 | - | 15.8 | −0.2 | ||
| 45.6861, 7.2416 | 1200 | Spr,Pin | 8 | 11.8 ± 2.73 | - | - | 13.9 | −1.7 | |||
| 1500 | Spr,Pin,Lar | 8 | 12.8 ± 2.13 | 16 | - | 12.5 | −2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wermelinger, B.; Rigling, A.; Schneider Mathis, D.; Kenis, M.; Gossner, M.M. Climate Change Effects on Trophic Interactions of Bark Beetles in Inner Alpine Scots Pine Forests. Forests 2021, 12, 136. https://doi.org/10.3390/f12020136
Wermelinger B, Rigling A, Schneider Mathis D, Kenis M, Gossner MM. Climate Change Effects on Trophic Interactions of Bark Beetles in Inner Alpine Scots Pine Forests. Forests. 2021; 12(2):136. https://doi.org/10.3390/f12020136
Chicago/Turabian StyleWermelinger, Beat, Andreas Rigling, Doris Schneider Mathis, Marc Kenis, and Martin M. Gossner. 2021. "Climate Change Effects on Trophic Interactions of Bark Beetles in Inner Alpine Scots Pine Forests" Forests 12, no. 2: 136. https://doi.org/10.3390/f12020136
APA StyleWermelinger, B., Rigling, A., Schneider Mathis, D., Kenis, M., & Gossner, M. M. (2021). Climate Change Effects on Trophic Interactions of Bark Beetles in Inner Alpine Scots Pine Forests. Forests, 12(2), 136. https://doi.org/10.3390/f12020136





