Abstract
There is a need to understand the physio-morphological responses of northern tree species to climate change. The hypothesis of the current study was that provenance and light intensity were both influential in the control of intrinsic water-use efficiency (iWUE). Diameter at breast height (DBH)-increment was hypothesized as being more affected by provenance. Intrinsic water-use efficiency (iWUE), the ratio of photosynthesis (A) to stomatal conductance (gs), was assessed in foliage under two levels of photosynthetically active radiation (PAR; i.e., 300 and 1200 μmol m−2 s−1) in 63-year-old balsam fir [Abies balsamea (L.) Mill.] provenances derived from seed sources from across the species’ natural range (namely, within 44–51° N latitudes and 53–102° W longitudes) and cultivated in a common garden in eastern Canada. Diameter at breast height (DBH) of provenances from the common garden were measured when they were 42 and 58 years old (DBH1998, DBH2014). The results confirmed the hypotheses regarding the roles of provenance on iWUE and DBH (p < 0.05), but showed a diminished role of PAR on iWUE. The lowest and highest mean iWUE and DBH among the provenances ranged between 0.028 and 0.031 and 0.079–0.083 μmol mmol−1 and 11.82–12.78 and 16.38–18.44 cm, respectively. Stomatal conductance of balsam fir had a strong relationship with iWUE at both light settings, whereas A had a weaker relationship with iWUE. There were no significant relationships between iWUE at the two light settings and climatic variables at the provenance source (p > 0.05). Diameter at breast height in 2014 was significantly greater than DBH1998 (p < 0.05). The relationships between DBH2014 and climatic variables at the provenance source were statistically significant (p < 0.05). There was a significant positive relationship between iWUE and DBH measured in 2014. Survivorship of provenances was shown to vary with DBH-increment. The results show that for present-day and future forest management, (i) selection in balsam fir, in relation to iWUE should ideally be based on a criterion of intraspecific stomatal conductance; (ii) shade tolerance of balsam fir, population differentiation, and consistent pace of DBH-growth under variable climatic conditions are important factors in the species’ sustained growth under changes in forest dynamics projected to accompany changes in regional climate; (iii) temperature variables are strong indicators of DBH-increment in balsam fir; (iv) the effect of tree size on its survival is maintained under variable climatic conditions; and (v) there is a clear association between iWUE and the species’ radial growth.
1. Introduction
Increased temperatures and varied precipitation patterns resulting from the anthropogenic buildup of atmospheric carbon dioxide (CO2) concentrations and other greenhouse gases could have profound impacts on the temperate and boreal forests at mid to high latitudes in the Northern Hemisphere. This necessitates an understanding of the physio-morphological responses of northern tree species to climate change to facilitate the sustainable utilization of forests [1,2,3].
Plant assimilation of atmospheric CO2 during leaf photosynthesis is accompanied by water loss during transpiration, through leaf stomata [4,5]. Regulation of these leaf gas-exchange processes, which link the global carbon and hydrological cycles, is achieved by control of the stomata relative to changes in the environment [6,7,8,9]. The global carbon cycle is pivotally affected by terrestrial ecosystems that sequester a significant amount of anthropogenic CO2 emissions, which have caused a rise in global air temperatures since the mid-20th century [10]. Terrestrial ecosystems act as a key control on climate warming [11]. Forests cover ~4.1 billion ha of the Earth’s surface and hold 80% and 40% of terrestrial carbon above and belowground, respectively. Regional carbon stocks influence the dynamics of global carbon pools and fluxes [12,13,14]. The carbon cycle is characterized by gross primary production (GPP) from carbon assimilation resulting from photosynthesis. Net primary production (NPP) is the portion of GPP stored in plants after accounting for losses to respiration. Net ecosystem production (NEP) is NPP minus heterotrophic respiration [15]. The hydrological cycle of forest ecosystems commences with precipitation, which reaches and enters the soil through gaps in the forest-canopy, flows down tree trunks, and drips from tree crowns. A large portion of water that reaches the soil surface is absorbed by the roots of trees and understory plants, and then released into the atmosphere during transpiration or it recharges soil water. The rest, intercepted by the canopy, is evaporated back to the atmosphere [16]. Water-use efficiency (WUE), the rate of carbon uptake per unit water loss, is a key characteristic of ecosystem functioning. The variable enables the evaluation of tree performance in relation to water availability and consumption [4,17,18,19,20,21]. Intrinsic water-use efficiency (iWUE) is the ratio of the rate of photosynthesis (A) to stomatal conductance (gs), both of which can be obtained from leaf gas-exchange measurements [9,22]. Changes in iWUE influence ecosystem functioning, as higher iWUE, can result from reductions in stomatal conductance, increases in photosynthesis, or the combined effect of the two responses [22,23].
Light is one of the main environmental variables that affect plant development. It is an important energy source for plant photosynthesis and stomatal activity. To maximize their morphological growth and physiological processes, plants must be able to sense and respond to changes in light properties, which vary spatially and temporally in natural conditions, especially within forest canopies. There is a marked decrease in light intensity from the upper to the lower canopy, with upper-canopy foliage exposed to higher irradiance than lower-canopy foliage shaded by leaves and branches residing in the upper canopy [24,25,26,27,28]. There is a need to clarify how iWUE varies with light intensity. This is because the quantification of changes in plant WUE characteristics, their drivers as well as their relationships to climatic factors is important in modeling plant-growth response and regional forest carbon uptake [9,28,29,30].
Tree diameter-growth occurs through the formation of consecutive layers of structural tissue by the vascular cambium, inwardly toward the pith, and outwardly toward the bark, giving rise to the water and nutrient-conducting xylem, and photosynsynthate-conducting phloem, respectively. The presence of lignin in the xylem gives the tree trunk strength [31]. The anticipated effect of climate change on forest ecosystem processes has necessitated an increase in the knowledge of climate–growth relationships, with resultant studies at diurnal, seasonal, annual, and multi-annual timescales [32,33,34,35,36]. Studies of relationships between climatic factors and stem-increment dynamics will facilitate the understanding of the effects of changing climatic conditions on the growth of trees and enhance the modeling of tree–climate relationships [37,38]. Several studies have been conducted in the past to assess the response of conifers to climate change, with trials based on provenances of jack pine (Pinus banksiana Lamb.) [39], white spruce [Picea glauca (Moench.) Voss.] [40], black spruce [Picea mariana (Mill.) B.S.P.] [41], and Scots pine (Pinus sylvestris L.) [42]. Studies based on balsam fir [A. balsamea (L.) Mill.] are not common [43].
Our hypotheses are that (i) provenance and light intensity play a significant role in the control of iWUE, and (ii) provenance in the multi-year increment in diameter at breast height (DBH). Our study assesses these controls in provenances of balsam fir.
2. Materials and Methods
2.1. Study Site
The study was conducted at a research site of Natural Resources Canada, in northwestern New Brunswick (NB), Canada (47°7′ N, 67°32′ W). The study site is a balsam fir provenance trial established in 1961, with the planting of five-year-old balsam fir seedlings. The provenance trial was comprised of twelve provenances from locations within 44–51° N latitudes and 53–102° W longitudes across North America [44] (Table 1), along a climatic gradient with mean annual temperature and total annual precipitation from 1.5–6.7 °C and <500–1500 mm, respectively.

Table 1.
Provenance sources and provenance test site with their geographic coordinate position, key climatic variables for the period from 1981–2010, and Ecozone/Ecoregion classification [44,45,46,47,48]. Provenance sources and the test site (common garden) are ordered by mean annual temperature (MAT, °C). Other climatic variables in the table include mean winter temperature (MWT) and mean summer temperature (MST), also in °C, and total annual precipitation (TPPT) in mm. Abbreviations: SK = Saskatchewan, NL = Newfoundland, MB = Manitoba, NB = New Brunswick, QC = Quebec, and NY = New York State.
The common garden (test site) is located within the northern NB Uplands Ecoregion. All of NB falls in the Atlantic Maritime Ecozone [40]. Mean temperature and total precipitation during the study months of September and October were 12.2 °C and 150.6 mm, and 8 °C and 121.6 mm, respectively [49]. The soils are dominated by humo-ferric and ferro-humic podzols, with substantial amounts of gray luvisols. The soils are moderately well-drained, medium to fine loam up to a depth of 25 cm from the surface. Local parent material is of medium to fine loam to clay loam with coarse fragments. The depth to the compacted layer ranges from 30–65 cm [46,50].
2.2. Tree Sampling
Each provenance was grown in 0.04 ha plots, planted with 100 trees in 10 rows made up of 10 trees at a 1.8 m × 1.8 m spacing, one half of which was commercially thinned in 1998. Diameter at breast height of trees in the unthinned portion had been previously collected in 1998, when the provenances were 42 years old [51]. Diameter at breast height was again collected in 2014, when the provenances were 58 years old. The DBH data collected from the unthinned portion of each provenance-plot in 2014 were subsequently used to determine the mean DBH for all sampled plots. The mean DBH for each sampled plot (plot-DBH) was then used in the selection of representative sample trees for quantifying species evaluation of iWUE and DBH. Data from one of the provenances (i.e., MS-131) were only used in the study of DBH and not iWUE because of low tree-survival in 2014, and only one of the trees was alive in 2019.
For the study on iWUE, three representative sample trees were selected from each provenance plot (except MS-131) for foliar gas-exchange measurements. Measurements were acquired for the rate of photosynthesis (A; μmol m−2 s−1) and stomatal conductance (gs; mmol m−2 s−1), which were used in the calculation of iWUE (μmol mmol−1):
Measurements were made on a total of 33 foliage samples.
For the study on provenance DBH, five representative sample trees were selected from each provenance plot (the three trees from which branches were removed for foliar gas-exchange measurement and two additional trees) for the comparison of provenance DBH at the two points of measurement (i.e., DBH1988 and DBH2014, respectively). Measurements were made on a total of 60 foliage samples.
2.3. Gas-Exchange Measurements
The foliar gas-exchange measurements were conducted in 2019 when the provenances were 63 years old. Gas-exchange measurements were made over a 6-day period between September and October 2019 from 09:00–15:00 h (local daylight time) each day. In light of the heights of trees in the provenance plots (≥10 m), telescopic pole pruners were used to detach branches from each tree. Gas exchange in the detached foliage of trees can be affected by the time interval between branch cutting and gas-exchange measurement [52]. However, Clark [53], Koike and Sakagami [54], and Gauthier and Jacobs [55] showed the practicality of measuring gas exchange in detached foliage, as evidenced by unchanged gas-exchange rates associated with the foliage of species studied, ranging from seven to 20 min after detachment. Earlier measurements on attached and detached balsam fir foliage (unpublished data) showed no statistically significant differences in gas-exchange rates (p > 0.05).
Specifically, branches 50–80 cm long were detached from the south-facing, upper-canopy sections of each tree. Gas-exchange measurements were made immediately thereafter. Measurements were acquired for the rate of photosynthesis (A; μmol m−2 s−1) and stomatal conductance (gs; mmol m−2 s−1) in 1-year-old foliage, after current-year needles were removed with hand pruners. Measurements were taken under two photosynthetically active radiation (PAR) settings, with light perpendicularly incident on foliage, supplied by a tungsten halogen light unit attached to a CIRAS-2 Photosynthesis System (PP Systems, Amesbury, MD, USA). The two light settings were 1200 and 300 μmol m−2 s−1, with light varied downward from the former to the latter setting. Measurements were made on foliage of the detached tree branches, of which needles at the lower end were removed with tweezers. The remaining needles were completely enclosed in the conifer chamber (cuvette) with a CO2 concentration maintained at 400 μmol mol−1, together with a flow rate, chamber temperature, and relative humidity of 400 mL min−1, 18 °C, and 70%, respectively. Before starting each series of measurements, foliage was acclimated to cuvette conditions for 15 min at 1200 μmol m−2 s−1. Photosynthesis and gs were permitted to stabilize for two min at 1200 and 300 μmol m−2 s−1 (hereafter high and low light, respectively) before being measured.
On completion of each set of measurements, all needles (n = 20–90) on each foliage sample enclosed in the cuvette were removed and then scanned using a CanoScanLiDE 110 Flatbed Scanner (Canon Canada Inc, Mississauga, ON, Canada) for the determination of projected leaf area (i.e., ½ of total leaf area) with Image J software (version 1.49, National Institutes of Health, Bethesda, MD, USA). Projected leaf area was then used to estimate A and gs on a leaf area basis, following which iWUE was determined.
2.4. Data Analysis
2.4.1. Mixed Analysis of Variance (ANOVA)
Mixed analysis of variance (ANOVA) was performed on (i) iWUE at high and low light settings, and (ii) DBH at 42 and 58 years old. The analyses were performed with SPSS Statistical software (version 24, IBM Corp., New York, NY, USA) using the repeated measures option, with provenance, the between-subjects factor in (i) and (ii), and PAR and age, the within-subjects factors, and iWUE and DBH as the corresponding dependent variables. An assessment of the homogeneity of sample variances was carried out using Levene’s test. Mixed ANOVA was used to determine the significance level of the effects of provenance, PAR, and provenance × PAR interaction on iWUE, and the effects of provenance, growth period, and provenance × growth period interaction on DBH. The Bonferroni test was used subsequently to separate the effect means when significant differences were detected with respect to both between- and within-subjects factors.
2.4.2. Regression Analysis
Regression analysis was performed using the methods of Matyas and Yeatman [56] and Thomson and Parker [57] by relating the physio-morphological variables measured to climate at the provenance-source stands. This was done to identify climatic variables that exert selective pressures on the traits of the provenances in the development of climate models for balsam fir. Climate normals for the 1981–2010 period were generated from weather station data including (i) annual and seasonal temperature-variables (18, in total); (ii) precipitation variables (seven); indices of heat accumulation (i.e., growing degree-days > 5 and 10 °C) (two); annual and seasonal moisture indices (eight); and durations above specified temperature and precipitation-thresholds (ten, five for each grouping). The number of climatic variables used in the regression analysis was reduced using principal component analysis (PCA). The climatic variables extracted by PCA were then used in regressions of iWUE at low and high light settings, and DBH of provenances at 58 years old against the various provenance-source stand climatic variables. Note that regressions for DBH of provenances at 42 years old had already been determined [51]. Scatter plots of iWUE and climatic variables indicated nonlinear relationships, and as a result, quadratic and third-order polynomials were used in an expanded regression analysis. Equations used in the regressions involving iWUE and DBH2014 are as follows:
and
In Equations (2)–(4), Y, dependent variable is the provenance trait; X, the independent variable, is the explanatory climatic variable for the provenance; β0, β1, β2, and β3 are equation coefficients estimated by least squares regression; and ε is the provenance-source error. Climatic variables were assessed for suitability in balsam fir model development based on values of r2 > 0.40 and p < 0.05.
A 3-parameter Gaussian-equation was used, which related survivorship (lx) of trees in the provenance plots to DBH-increment over the 16-year period, 1998–2014. This was done using SigmaPlot Statistical software (version 11, Systat Software Inc., San Jose, CA, USA). Diameter-increment over the 16-year period for the sampled trees in each provenance plot was calculated from the difference between DBH1998 and DBH2014. Survivorship (i.e., lx) over the 16-year period was calculated for trees in the provenance plots [58,59] from which DBH was determined:
In Equation (5), n1 and n2 are the number of trees at the beginning and end of the 16-year period, respectively. The equation used in the regression analysis is as follows:
Here, Y is the survivorship; X is the DBH-increment; and a, b, and c are the equation coefficients. This equation was selected because the relationship between tree DBH-growth and survival is non-linear, and the representation of this relationship requires flexible equations that allow for variable curve forms [60,61].
Regression analysis was performed between iWUE at high and low light with DBH2014. This was done after an assessment of time series temperature and precipitation data for the study site over a ten-year period (i.e., 2010–2019), since both variables were measured in different years. The assessment was done to determine the similarity of climatic conditions at the study site when iWUE and DBH2014 were collected. An autocorrelation analysis was done with a regression of temperature and precipitation in a 5-year period (i.e., 2015–2019) on those for the preceding 5-year period (2010–2014) and Durbin–Watson values obtained (indicator of presence-absence of autocorrelation). Based on a strong similarity of the temperature data in the period assessed, the regression analysis relating iWUE at high and low light with DBH2014 was then performed excluding data from the MS-131 provenance.
3. Results
3.1. Intrinsic Water-Use Efficiency
The results showed that provenance and light setting had significant effects on iWUE in balsam fir (F(10) = 6.420, p < 0.05 and F(1) = 4.398, p = 0.048,respectively), but at a reduced level of significance in the latter relationship. The lowest and highest mean iWUE among the provenances ranged between 0.028 and 0.031 and 0.079 and 0.083 μmol mmol−1, respectively. There was no significant provenance × PAR interaction (F(10) = 1.084, p > 0.05).
Provenance, PAR, and provenance × PAR interaction accounted for 74.50, 16.70, and 33.0%, respectively, of the variation in iWUE (Table 2 and Table 3). Relative to the location of the common garden, the best-performing provenances were from Hawke’s Bay, NL (MS-126; 0.6 °C cooler and 74.8 mm drier), the Acadia Research Forest, NB (MS-118; 1.4 °C warmer and 90.8 mm wetter), Duck Mountain, SK (MS-130; 1.9 °C cooler and 635.7 mm drier), and the Adirondack Mountains, NY (MS-303; 2.9 °C warmer and 119.7 mm drier). Intrinsic WUE in most provenances increased with an increased light setting (Table 2).The components of iWUE for the provenances (i.e., A and gs) had mean ranges of 7.037(±1.956)–8.783(±2.402) µmol m−2 s−1 and 90.733(±25.353)–252.835(±76.863) mmol m−2 s−1, respectively (see Table 4 for provenance-specific results).

Table 2.
Mean intrinsic water-use efficiency (i.e., iWUE; μmol mmol−1) at photosynthetically active radiation (PAR) of 300 and 1200 μmol m−2 s−1 in provenances of balsam fir (n = 11, three samples per provenance) growing in a common garden established in northern New Brunswick, with standard deviations in parenthesis.

Table 3.
Output of mixed analysis of variance on iWUE (μmol mmol−1) at 300 and 1200 μmol m−2 s−1 in provenances of balsam fir growing in a common garden established in northern New Brunswick; “df” stands for degrees of freedom.

Table 4.
Mean rates of photosynthesis (i.e., A; μmol m−2 s−1) and stomatal conductance (i.e., gs; mmol m−2 s−1) in provenances of balsam fir growing in a common garden established in northern New Brunswick, with standard deviations and value ranges in parenthesis.
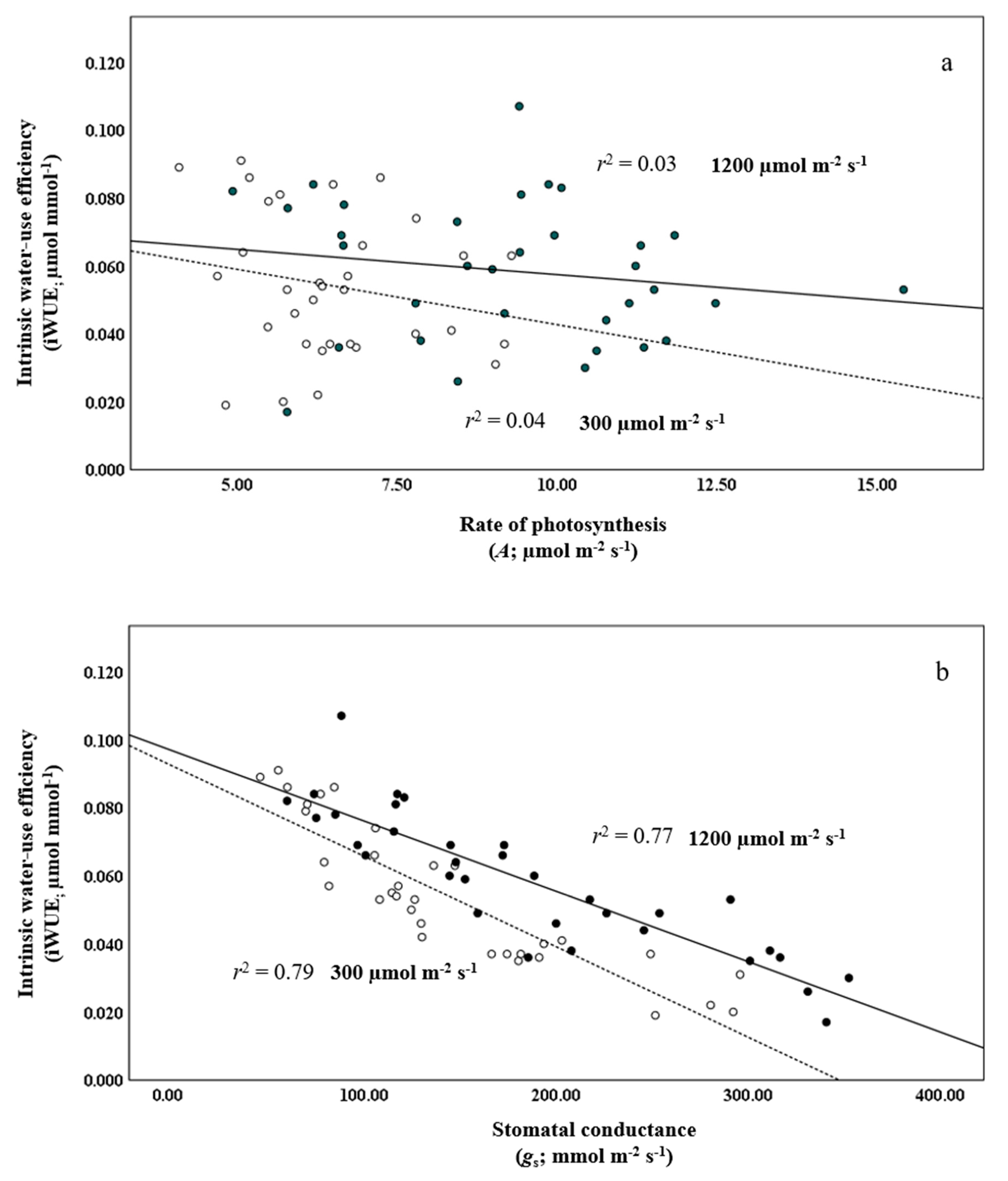
Three of the four best-performing provenances, those from Hawke’s Bay, NL, the Acadia Research Forest, NB, and Duck Mountain, SK, had the lowest gs, whereas the provenance from Bonne Bay, NL, with the lowest iWUE, had the highest gs. This indicates that stomatal conductance played an influential role in the water-use characteristics of the provenances. This is buttressed by regressions, which were performed against A and gs with iWUE. Rates of photosynthesis and stomatal conductance at low and high light produced weak and strong inverse linear relationships with iWUE, yielding r2’s of 0.04 and 0.03 and 0.79 and 0.77 (Figure 1a,b), respectively. The provenances from Hawke’s Bay, NL, and Duck Mountain, SK, had the second and third lowest mean A.
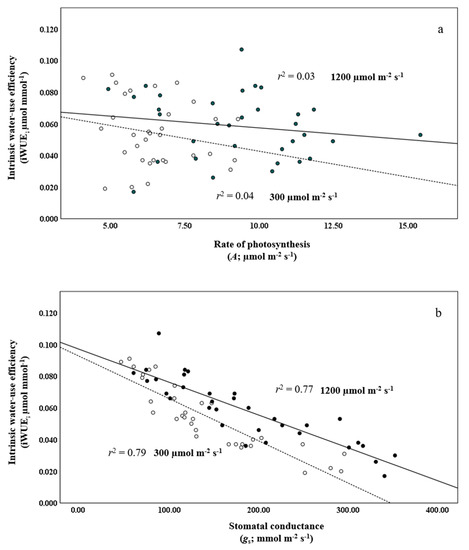
Figure 1.
Regression of intrinsic water-use efficiency (i.e., iWUE, µmol mmol−1) against (a) the rate of photosynthesis (A), and (b) stomatal conductance (gs) at 300 and 1200 µmol m−2 s−1 (open and closed circles, dashed and solid lines, respectively) in provenances of balsam fir growing in a common garden in northern New Brunswick.
3.2. Diameter at Breast Height Growth
The results showed that provenance had a significant effect on DBH in balsam fir (F(11) = 2.888, p < 0.05). Diameter at breast height in 2014 was significantly larger than DBH in 1998 (F(1) = 195.664, p < 0.05). The lowest and highest mean DBH across provenances ranged between 11.82 and 12.78 and 16.38 and 18.44 cm, respectively.
There was no significant provenance × growth period interaction (F(11) = 1.936, p > 0.05). Provenance, growth period, and provenance × growth period interaction accounted for 39.80, 80.30, and 30.70%, respectively, of the variation in DBH (Table 5 and Table 6). Over the course of the 40-year period between 1971 and 2010, there were 0.3, 0.7, and 0.1 ℃ increases in MAT, MWT, and MST, and a 49.4 mm decline in precipitation at the common garden (see Table 1 in Akalusi and Bourque [51] and Table 1 above).

Table 5.
Mean diameter at breast height (i.e., DBH; cm) in 1998 and 2014 in provenances of balsam fir (n = 12, five samples per provenance) growing in a common garden established in northern New Brunswick, with standard deviations in parenthesis.

Table 6.
Output of mixed analysis of variance on 16-year DBH-increment between 1998 and 2014 for provenances of balsam fir growing in a common garden established in northern New Brunswick; “df” stands for degrees of freedom.
3.3. Provenance–Climate Relationship
The climatic variables extracted using PCA showed that the principal components for each climatic-variable grouping; temperature, precipitation, temperature index, temperature/precipitation index, temperature and precipitation duration variables accounted for 93–99%of the variability in all variables in each grouping.
The r2 and p-values of quadratic and third-order polynomial regressions between iWUE at low and high light against temperature, precipitation, heat-accumulation and moisture indices, and duration above temperature thresholds at the provenance source exhibited weak relationships. The r2’s of quadratic and third-order polynomial regressions between iWUE at low and high light, against temperature-related variables, and precipitation-threshold variables at the provenance source (i.e., mean maximum annual temperature (MMAX; see Table 7 for definition of variables), lowest temperature of the warmest month (LTWM), number of days with precipitation and rainfall above 25 mm (DPPT25, DRnF25) ranged from <0.40 up to 0.84, with not all p-values suggesting statistical significance (Table 8).

Table 7.
Climatic variables and their abbreviations.

Table 8.
Coefficient of determination r2 and p-values from regressions of mean iWUE (µmol mmol−1) at light settings of 300 and 1200 µmol m−2 s−1 in balsam fir growing in northwestern New Brunswick, relative to climate at the point of origin. See Table 7 for climatic variable definitions.
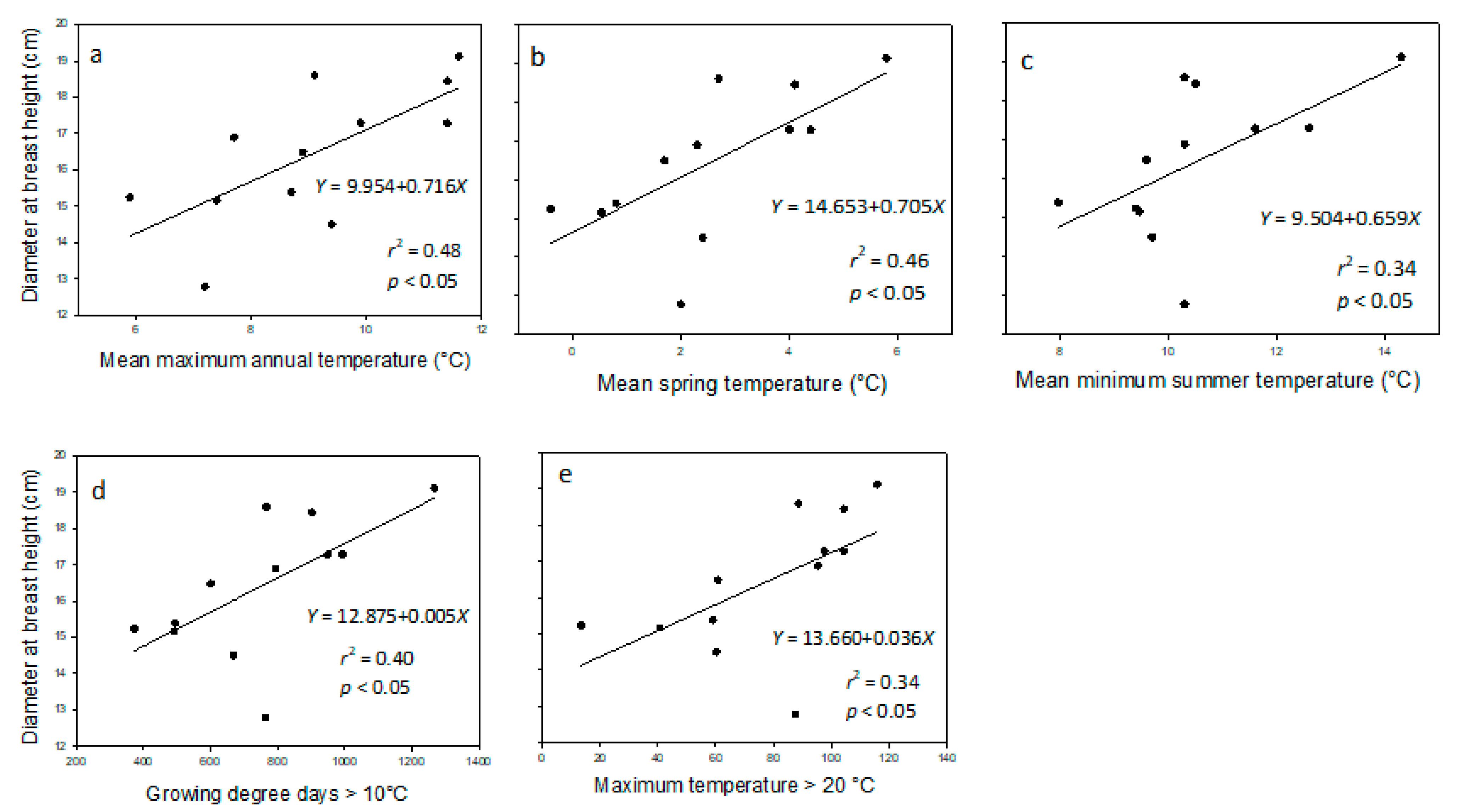
Regarding the DBH of provenances in 2014, three climatic variables (i.e., mean maximum annual temperature (MMAX), mean spring temperature (SpMN), and growing-degree days > 10 °C (GDD10)) had significant linear relationships (p < 0.05) with DBH (r2 = 0.48, 0.46, and 0.40, respectively). Two other climatic variables (i.e., mean minimum summer temperature (SMIN) and maximum temperature > 20 °C (DT > 20 °C), although with r2-values < 0.40 (r2 = 0.34 for both variables), also had statistically significant linear relationships with DBH (Figure 2).
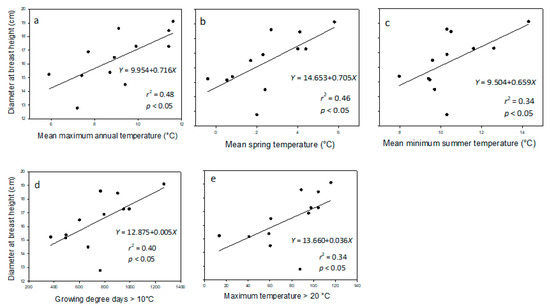
Figure 2.
Diameter at breast height (DBH, cm; n = 12, five samples per provenance) in provenances of balsam fir measured in 2014, in northwestern New Brunswick relative to (a) mean maximum annual temperature, (b) mean spring temperature, (c) mean minimum summer temperature, (d) growing degree-days with a base temperature > 10 °C, and (e) maximum temperature > 20 °C at the point of origin.
3.4. Survivorship-Diameter at Breast Height Relationship
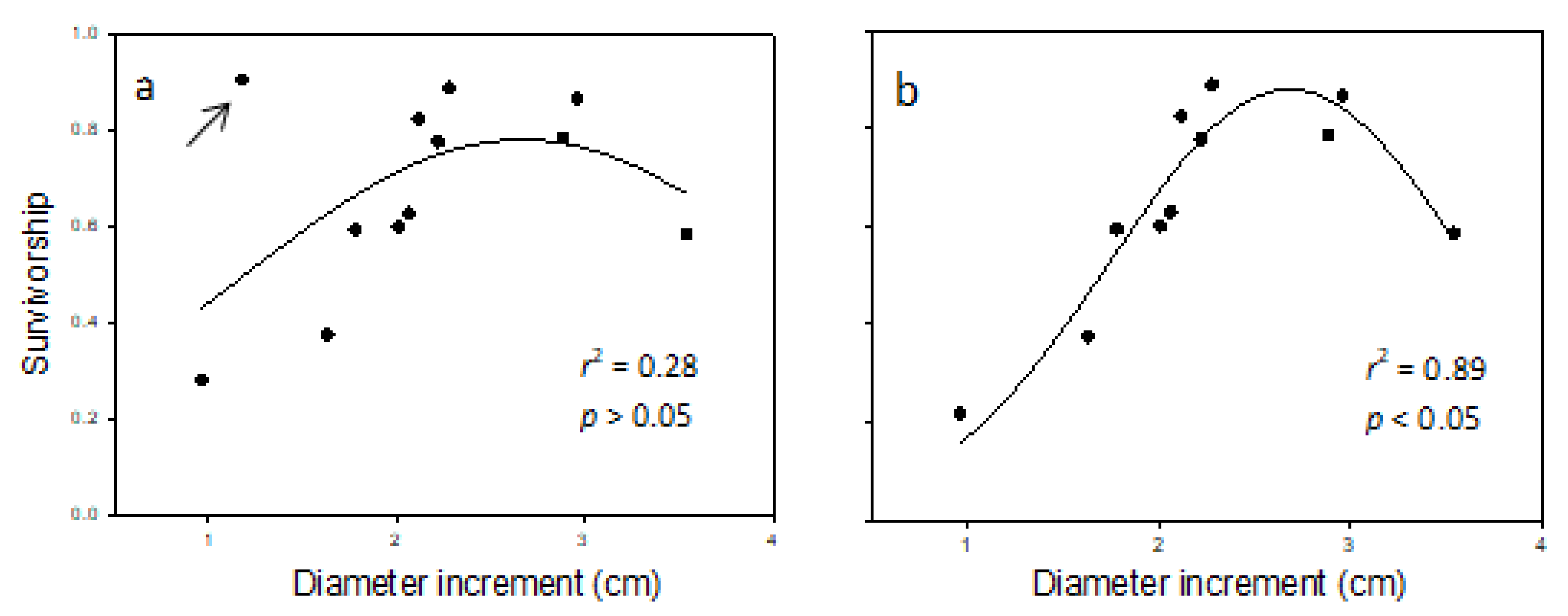
The regression analysis of survivorship (lx) against DBH-increment (Table 9) revealed a non-linear relationship (Figure 3a,b). The relationship showed low survival at low DBH-increment, increased survival at intermediate DBH-increment, followed by another decline at high DBH-increment. A data-point for one of the provenances (i.e., MS-133) appeared to be an outlier (indicated in Figure 3a). When all provenances were used in the regression, r2 was 0.28, and the unimodal relationship formed was not statistically significant (p > 0.05). When the provenance was excluded, r2 increased to 0.89 and the relationship formed was statistically significant (p < 0.5). Data from the provenance suspected of being an outlier departed from the expected trend. The reason for the uncharacteristic level of survival for the DBH-increment (Table 9) is unclear, but may have been less obvious with a larger sample size.

Table 9.
Mean DBH-increment (cm; n = 12, five samples per provenance) and survival for the period from 1998 to 2014 in provenances of balsam fir growing in a common garden established in northern New Brunswick.
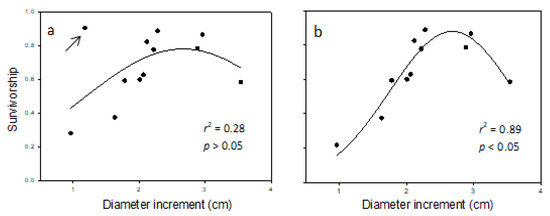
Figure 3.
Regression of survivorship against DBH-increment (cm; n = 12, five samples per provenance) in balsam fir growing in a common garden in northern New Brunswick over the 1998–2014 study period. Panel (a) gives the fit of the 3-parameter Gaussian equation (i.e., Equation (6)) with the suspected outlier (MS-133, identified by the arrow) included, whereas panel (b) gives the same regression excluding the suspected outlier.
3.5. Intrinsic Water-Use Efficiency-Diameter at Breast Height Relationship
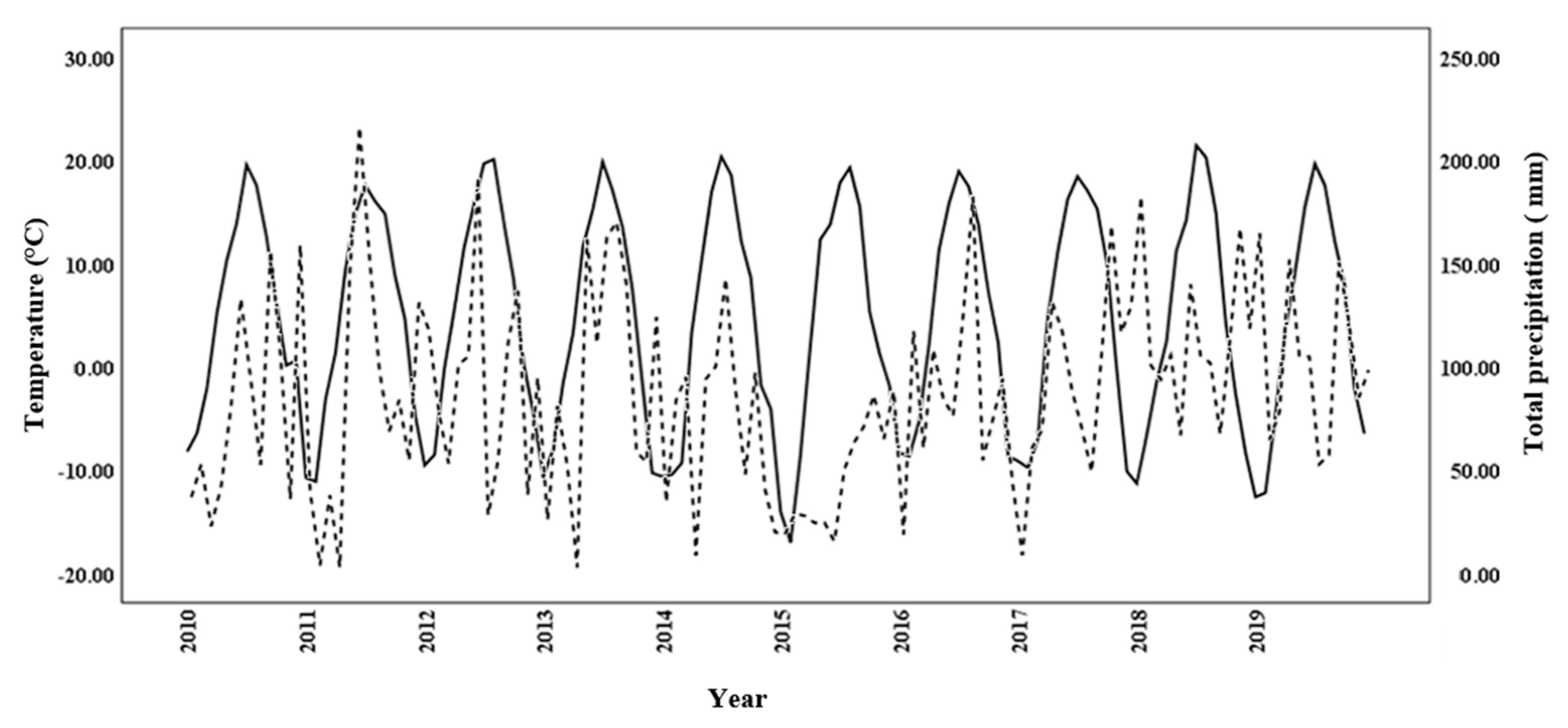
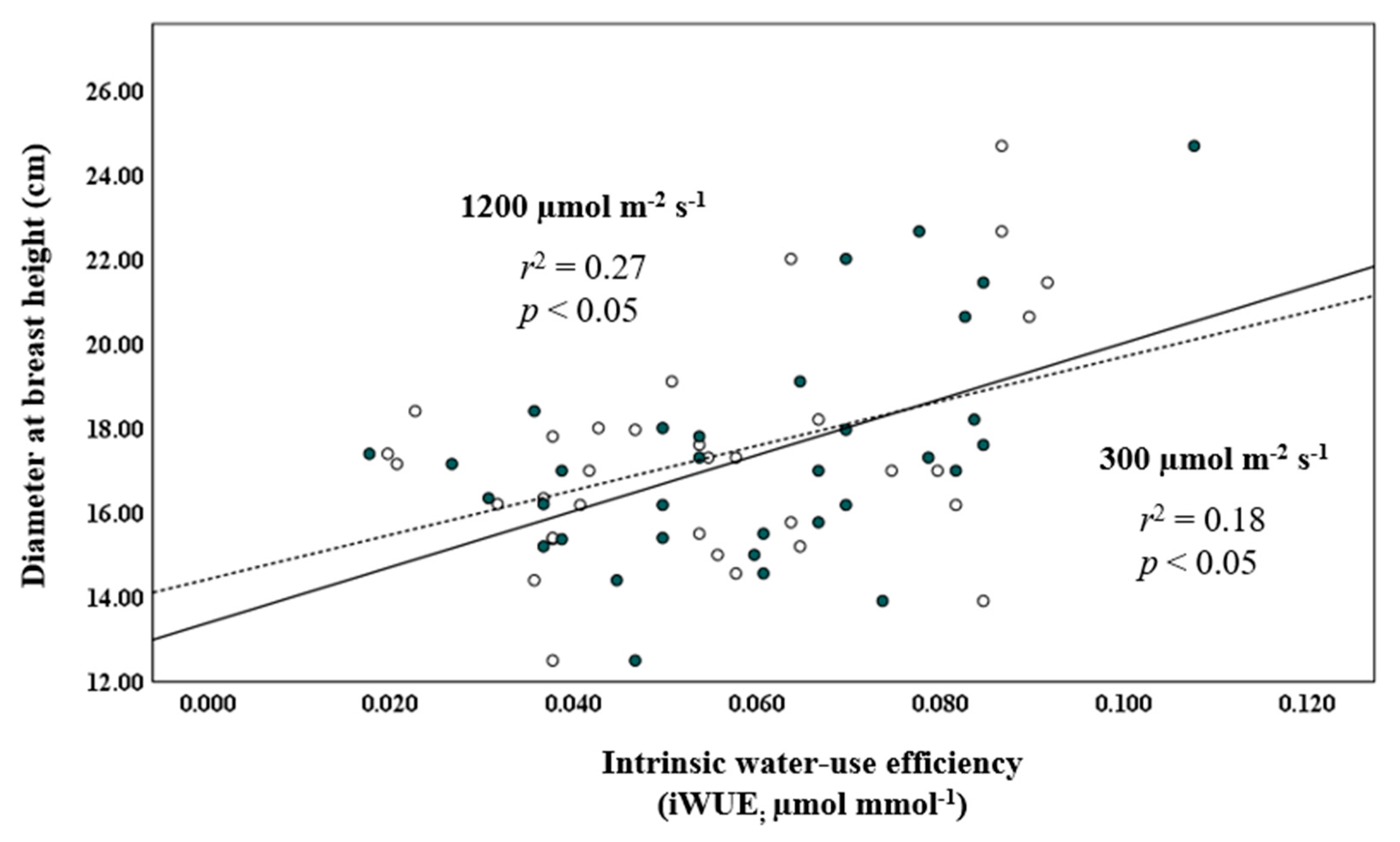
The regression of temperature and precipitation in the 2015–2019 period on those for the 2010–2014 period showed that temperature had a strong r2-value of 0.95, whereas precipitation was substantially weaker, at r2 = 0.02. Autocorrelation analysis of the regression done on the monthly temperature and precipitation data for the 10-year period, 2010–2019 (Figure 4), generated Durbin–Watson values of 1.337 and 1.143, respectively, indicating autocorrelation. The regression analysis relating iWUE at 300 and 1200 µmol m−2 s−1 with DBH2014 showed a statistically significant positive linear relationship (r2 = 0.18 and p < 0.05, and r2 = 0.27 and p < 0.05, respectively; Figure 5). Quadratic regression also showed a statistical significant relationship, but equation coefficients for linear regression revealed greater statistical significance.
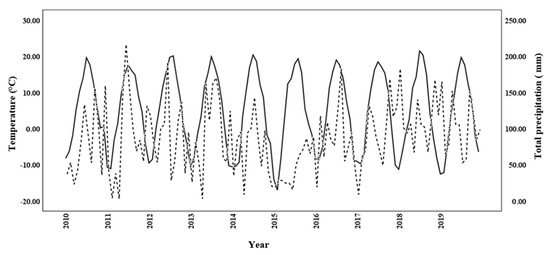
Figure 4.
Monthly temperature (solid line) and precipitation (dashed line) at the common garden in northern New Brunswick over the 2010–2019 study period.
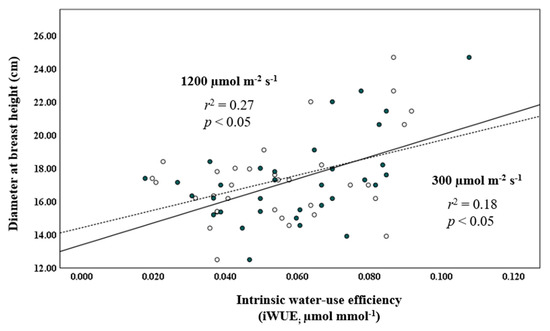
Figure 5.
Regression of DBH (cm) measured in 2014 against intrinsic water-use efficiency (i.e., iWUE, µmol mmol−1) at 300 and 1200 µmol m−2 s−1 (open and closed circles, dashed and solid lines, respectively) in provenances of balsam fir growing in a common garden in northern New Brunswick.
4. Discussion
The results showed a significant provenance effect on balsam fir iWUE (p < 0.05). Intraspecific variation in iWUE has been reported to have a genetic basis [62,63,64]. Intraspecific variation in iWUE is mainly the result of variations in gs, and not in A [65,66,67,68]. The values of iWUE obtained in this study were like those obtained by Medrano et al. [69], Hu et al. [21], and Roussel et al. [66], when compared at similar units of measurement.
Variation in iWUE in this study was a result of the marked differences in stomatal conductance among the provenances, as three of the four best-performing provenances had the lowest gs, whereas the provenance with the lowest iWUE had the highest gs. Furthermore, regressions between A and iWUE and gs and iWUE at low and high light settings showed a weak negative linear relationship regarding the former, and a stronger negative linear relationship with the latter. Stomatal conductance influences iWUE, with lower gs associated with higher iWUE. Increases in iWUE can be the result of reduced water loss via stomatal control, a higher level of photosynthesis, or a combination of changes in the two variables. A meta-analysis by Gago et al. [70] on iWUE in 237 species of trees, shrubs, and herbaceous species showed variations in iWUE correlated well with gs. Correlations with A, in contrast, were much lower, suggesting a stronger control of iWUE by gs. They observed this trend across the species studied, treatments applied, and in well-watered plants. A similar trend in the relationship between gs and iWUE was observed by Medrano et al. [69], Roussel et al. [66], and Galmes et al. [71].
The provenance with the highest iWUE had the lowest mean gs and lowest mean A. As gs and A are not independent [65], along with a reduction in gs is an increase in iWUE and, thereby, a reduction in water losses is expressed as a reduction in A as less substrate CO2 enters the leaves. Therefore, an increase in iWUE, due to a reduction in gs, results in reduced A, with the inverse being true as gs increases [68].
There was a reduced level of significance in the difference in provenance-based iWUE between light settings (p = 0.046). Catoni et al. [30] found that A and gs (the two components of iWUE) of sun leaves were significantly higher than those of shade leaves in a study of Quercus robur L., Corylus avellana L., Populus alba L., Acer campestre L., and Robinia pseudoacacia L. Murphy et al. [72] reported similar findings when A and gs in sun and shade leaves of Toona ciliate M. Roem. were assessed. The marginally significant difference in iWUE in provenances between light settings is likely an indication of the fact that balsam fir is a shade-tolerant species.
Shade tolerance, an important ecological driver behind forest succession, is an indication of the extent to which a plant can survive and grow in low light conditions. In relation to shade tolerance, tree species are classified into three broad categories: (i) shade-tolerant species that can reproduce and survive in low light conditions associated with heavy shading; (ii) intermediate shade-tolerant species with an ability to tolerate shade during some phases of the species’ life cycle; and (iii) shade-intolerant species requiring open, high-growth conditions [73,74,75,76,77]. Balsam fir, a shade-tolerant species, rates 9.8 on a ten-point tolerance scale developed by Graham [78]; also refer to Hett and Loucks [79]. The high shade tolerance of balsam fir is a result of its high light-capture efficiency [80,81,82]. This is because shade-tolerant species, to optimize light capture, typically assign a higher priority to foliage growth [83]. Furthermore, species in the genus Abies, to which balsam fir belongs, allocate resources in comparable proportions to branches and needles, presumably to hold branches horizontally to favor light interception [82,84]. It is likely that the shade tolerance of balsam fir may be an important factor in the survival of populations of the species under changes in forest dynamics projected to accompany climate change [85].
There were no significant relationships between iWUE and climatic variables at the provenance source despite the strong provenance effect on the parameter. This finding is consistent with that of Santini et al. [86], who found no relationship between C-isotope composition and climate at provenance source in a study of Pinus sylvestris populations. This finding differs from that of Ferrio and Voltas [87], who found relationships between the parameters in populations of Pinus halepensis Mill. and climatic variables at their source sites. C-isotope composition (i.e., δ13C) is reported to have a close relationship with iWUE, and as a result, can be used as an alternative measure of plant water consumption [20,86,88]. The lack of significant relationship between iWUE and climatic variables at the provenance source, despite the strong provenance effect on the parameter, may be an indication that climatic conditions at the provenance source do not play a significant role in intraspecific variation in iWUE in the species [86].
Diameter at breast height differed significantly among provenances. This is like the finding from the earlier study based on the 1998 provenance data at the same site [51]. Diameter at breast height in 2014 was significantly larger than DBH1998, which may be an indication that the rate of radial growth in the provenances during the 16-year period under review was not negatively impacted by the increases in temperature (via, e.g., MAT, MWT, and MST), and decline in precipitation (TPPT) at the common garden. Wang et al. [38] stated that tree DBH-increments are indicators of forest productivity, with the dynamics of such increments influenced by the times of initiation, cessation, and growth rate. The significant increase in DBH during the study period may be a result of the provenances maintaining good levels of productivity, even after several decades of growth.
Diameter at breast height in 2014 had significant linear relationships with temperature variables at provenance source. This reaffirms the earlier finding of the larger study at the same site, of the predominant effect of temperature on DBH [51]. The finding is also consistent with other studies of this kind (e.g., [41,56,89]). The pairwise relationships between DBH and MMAX, SpMN, GDD10, and SMIN are indicative of the importance of the growing season, which spans the period between the last and first frost, and periods of low temperature [31,90,91] to growth and development in balsam fir.
Survival declined at small and large DBH-increments and increased at intermediate DBH increments. This is an indication of the influence of size on tree survival [92,93] and is consistent with other studies that have reported increasing survival with increasing DBH up to a maximum, after which it begins to decrease [61,94]. High competition brought about by limited resource availability is responsible for the low survival at small DBH increases, resulting in improved spacing for trees at intermediate DBH-increments and higher levels of survival [95]. The decline in survival with large DBH-increments is the result of high maintenance costs relative to the photosynthate produced, associated with large trees, and size-limitations, which constrain water and nutrient transport to their canopies [95,96].
Intrinsic water-use efficiency at low and high light showed positive linear relationships with DBH2014. On a global scale, it has been found that increase in iWUE has not been accompanied by an increase in tree DBH-growth. Penuelas et al. [97] and Silva and Anand [98], however, state that the relationship between iWUE and tree DBH-growth varies latitudinally, with positive relationships between the two variables in alpine and boreal regions, and a trend toward a negative relationship with decreasing latitude.
5. Conclusions
A study was conducted on balsam fir provenances growing in a common garden in eastern Canada. Intrinsic water-use efficiency (iWUE) of the foliage of provenances, 63 years old, was assessed under low and high light. Diameter at breast height (DBH) of the provenances were independently measured when they were 42 and 58 years old. It was concluded from the findings of this study that stomatal conductance, not climatic factors, influences intraspecific variation in iWUE in balsam fir, and should therefore be used as a criterion in selection within the species under conditions of climate change. Shade tolerance, population differentiation, and the consistent pace of DBH-growth in balsam fir will facilitate sustained growth of the species under changes in forest dynamics projected to occur with climate change. The strong relationships of balsam fir DBH measured in 2014 with climate factors, particularly temperature-based, demonstrates that temperature variables are reliable indicators of variation in the trait. Under variable climatic conditions, the effect of size on tree survival is persistent. The observed positive relationship between iWUE and DBH confirms the association of these traits in the species.
Author Contributions
M.E.A. and C.P.-A.B. developed the ideas; M.E.A. collected the data, designed the methods, analyzed the data, and led the writing of the manuscript for the study; C.P.-A.B. reviewed the drafts, amended the manuscript, and gave final approval for its publication. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Data Availability Statement
The data presented in this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.13661207.v1.
Acknowledgments
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Oechel, W.C.; Hastings, S.J.; Vourlrtis, G.; Jenkins, M.; Riechers, G.H.; Grulke, N. Recent change of Arctic tundra ecosystems from a net carbon dioxide sink to a source. Nat. Cell Biol. 1993, 361, 520–523. [Google Scholar] [CrossRef]
- Huang, J.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Chang. Biol. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Adams, H.D.; Collins, A.D.; Dickman, L.T.; McBranch, N.; Michaletz, S.T.; Stockton, E.A.; Vigil, M.; McDowell, N.G. Precipitation, not air temperature, drives functional responses of trees in semi-arid ecosystems. J. Ecol. 2016, 105, 163–175. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nat. Cell Biol. 2013, 499, 324–327. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Aphalo, J.; Jarvis, P.G. Do stomata respond to relative humidity? Plant Cell Environ. 1991, 14, 127–132. [Google Scholar]
- Papanatsiou, M.; Amtmann, A.; Blatt, M.R. Stomatal clustering in Begonia associates with the kinetics of leaf gaseous exchange and influences water use efficiency. J. Exp. Bot. 2017, 68, 2309–2315. [Google Scholar] [CrossRef]
- Chen, B.; Chen, J.M.; Huang, L.; Tans, P.P. Modeling dynamics of stable carbon isotopic exchange between a boreal forest ecosystem and the atmosphere. Glob. Chang. Biol. 2006, 12, 1842–1867. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Belmecheri, S.; Chen, T.; Wu, G.; Wang, B.; Zeng, X.; Wang, W. Disentangling Contributions of CO2 Concentration and Climate to Changes in Intrinsic Water-Use Efficiency in the Arid Boreal Forest in China’s Altay Mountains. Forests 2018, 9, 642. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Summary for policymakers. In Climate Change 2007: The Physical Science Basis; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press: Cambridge, UK, 2007; pp. 1–18. [Google Scholar]
- Hayes, D.J.; McGuire, A.D.; Kicklighter, D.W.; Gurney, K.R.; Burnside, T.J.; Melillo, J.M. Is the northern high-latitude land-based CO2sink weakening? Glob. Biogeochem. Cycles 2011, 25. [Google Scholar] [CrossRef]
- Schroeder, P. Can intensive management increase carbon storage in forests? Environ. Manag. 1991, 15, 475–481. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon Pools and Flux of Global Forest Ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef]
- Vogt, K.A.; Vogt, D.J.; Palmiotto, P.A.; Boon, P.; O’Hara, J.; Asbjornsen, H. Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant Soil 1996, 187, 159–219. [Google Scholar] [CrossRef]
- Schulze, E.; Wirth, C.; Heimann, M. Managing forests after Kyoto. Science 2000, 289, 2058–2059. [Google Scholar] [CrossRef]
- Roberts, J.M. The role of forests in the hydrological cycle. For. For. Plants 2009, 3, 42–76. [Google Scholar]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; DeLay, D.; Petit, J.-M.; Barbaroux, C.; Le Thiec, D.; Brechet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides x Populus nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Aranda, J.; Marshall, J.D.; Winter, K. Large variation in whole-plant water-use efficiency among tropical tree species. New Phytol. 2006, 173, 294–305. [Google Scholar] [CrossRef]
- Barbour, M.M.; Tcherkez, G.; Bickford, C.P.; Mauve, C.; Lamothe, M.; Sinton, S.; Brown, H. δ13C of leaf-respired CO2 reflects intrinsic water-use efficiency in barley. Plant Cell Environ. 2011, 34, 792–799. [Google Scholar] [CrossRef]
- Battie-Laclau, P.; Delgado-Rojas, J.S.; Christina, M.; Nouvellon, Y.; Bouillet, J.-P.; Piccolo, M.D.C.; Moreira, M.Z.; Gonçalves, J.L.D.M.; Roupsard, O.; Laclau, J.-P. Potassium fertilization increases water-use efficiency for stem biomass production without affecting intrinsic water-use efficiency in Eucalyptus grandis plantations. For. Ecol. Manag. 2016, 364, 77–89. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, P.; Zhu, L.; Zhao, X.; Ni, G.; Ouyang, L.; Schäfer, K.V.; Shen, W. Responses of sap flux and intrinsic water use efficiency to canopy and understory nitrogen addition in a temperate broadleaved deciduous forest. Sci. Total. Environ. 2019, 648, 325–336. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; An, W.; Xu, G.; Zeng, X. Increased intrinsic water-use efficiency during a period with persistent decreased tree radial growth in northwestern China: Causes and implications. For. Ecol. Manag. 2012, 275, 14–22. [Google Scholar] [CrossRef]
- Liu, X.; Shao, X.; Wang, L.; Liang, E.; Qin, D.; Ren, J. Response and dendroclimatic implications of δ13C in tree rings to increasing drought on the northeastern Tibetan Plateau. J. Geophys. Res. Biogeosciences 2008, 113. [Google Scholar] [CrossRef]
- Walters, R.G.; Horton, P. Acclimation of Arabidopsis thaliana to the light environment: Changes in composition of the photosynthetic apparatus. Planta 1994, 195, 248–256. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, K.; Chen, L. Response of photosynthetic plasticity of Paeonia suffruticosa to changed light envi-ronments. Environ. Exp. Bot. 2003, 49, 121–133. [Google Scholar] [CrossRef]
- Legner, N.; Fleck, S.; Leuschner, C. Within-canopy variation in photosynthetic capacity, SLA and foliar N in tem-perate broad-leaved trees with contrasting shade tolerance. Trees 2014, 28, 263–280. [Google Scholar] [CrossRef]
- Niglas, A.; Papp, K.; Sękiewicz, M.; Sellin, A.; Goldstein, G. Short-term effects of light quality on leaf gas exchange and hydraulic properties of silver birch (Betula pendula). Tree Physiol. 2017, 37, 1218–1228. [Google Scholar] [CrossRef]
- Passos, L.C.; Da Silva, J.R.; Rodrigues, W.P.; Reis, F.D.O.; Vasconcellos, M.A.D.S.; Filho, J.A.M.; Campostrini, E. Leaf photosynthetic responses of passion fruit genotypes to varying sunlight exposure within the canopies. Theor. Exp. Plant Physiol. 2018, 30, 103–112. [Google Scholar] [CrossRef]
- Yu, M.; Xie, Y.; Zhang, X. Quantification of Intrinsic Water Use Efficiency along a Moisture Gradient in Northeastern China. J. Environ. Qual. 2005, 34, 1311–1318. [Google Scholar] [CrossRef]
- Catoni, R.; Gratani, L.; Sartori, F.; Varone, L.; Granata, M.U. Carbon gain optimization in five broadleaf deciduous trees in response to light variation within the crown: Correlations among morphological, anatomical and physiological leaf traits. Acta Bot. Croat. 2015, 74, 71–94. [Google Scholar] [CrossRef][Green Version]
- Barnes, B.V.; Zak, D.R.; Denton, S.R.; Spurr, S.H. Forest Ecology, 4th ed.; John Wiley and Sons Inc.: New York, NY, USA, 1998. [Google Scholar]
- Duchesne, L.; Houle, D. Modelling day-to-day stem diameter variation and annual growth of balsam fir (Abies balsamea (L.) Mill.) from daily climate. For. Ecol. Manag. 2011, 262, 863–872. [Google Scholar] [CrossRef]
- Subedi, N.; Sharma, M. Climate-diameter growth relationships of black spruce and jack pine trees in boreal Ontario, Canada. Glob. Chang. Biol. 2013, 19, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.A. Ecological Studies of Forest Trees at Chalk River, Ontario, Canada. I. Tree Species in Relation to Soil Moisture Sites. Ecology 1954, 35, 406. [Google Scholar] [CrossRef]
- Fritts, H.C. An Analysis of Radial Growth of Beech in a Central Ohio Forest during 1954–1955. Ecol. 1958, 39, 705–720. [Google Scholar] [CrossRef]
- Hofgaard, A.; Tardif, J.; Bergeron, Y. Dendroclimatic response of Picea mariana and Pinus banksiana along a longi-tudinal gradient in the eastern Canadian boreal forest. Can. J. For. Res. 1999, 29, 1333–1346. [Google Scholar] [CrossRef]
- Kulmala, L.; Read, J.; Nöjd, P.; Rathgeber, C.B.; Cuny, H.E.; Hollmén, J.; Mäkinen, H. Identifying the main drivers for the production and maturation of Scots pine tracheids along a temperature gradient. Agric. For. Meteorol. 2017, 232, 210–224. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, Y.; Dong, M.; Zhang, W.; Wang, B.; Zhang, Y.; Ding, X.; Kang, M.; Xu, H. The contributions of rate and duration of stem radial increment to annual increments of Picea meyeri in a sub-alpine habitat, North-Central China. Trees 2018, 32, 1029–1041. [Google Scholar] [CrossRef]
- Savva, Y.; Bergeron, Y.; Denneler, B.; Koubaa, A.; Tremblay, F. Effect of interannual climate variations on radial growth of jack pine provenances in Petawawa, Ontario. Can. J. For. Res. 2008, 38, 619–630. [Google Scholar] [CrossRef]
- Andalo, C.; Beaulieu, J.; Bousquet, J. The impact of climate change on growth of local white spruce populations in Québec, Canada. For. Ecol. Manag. 2005, 205, 169–182. [Google Scholar] [CrossRef]
- Thomson, A.M.; Riddell, C.L.; Parker, W.H. Boreal forest provenance tests used to predict optimal growth and response to climate change: 2. Black spruce. Can. J. For. Res. 2009, 39, 143–153. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Tchebakova, N.M.; Parfenova, Y.I.; Wykoff, W.R.; Kuzmina, N.A.; Milyutin, L.I. Intraspecific responses to climate change in Pinus sylvestris. Glob. Chang. Biol. 2002, 8, 912–929. [Google Scholar] [CrossRef]
- Carter, K. Provenance tests as Indicators of growth response to climate change in 10 north temperate tree species. Can. J. For. Res. 1996, 26, 1089–1095. [Google Scholar] [CrossRef]
- MacGillivray, H.G. Progress Report, Balsam Fir Provenance Planting 1956 sowing, Project M. 95; Related Studies: Petawawa Experiments Number 63, Number 175 and Number 176; Department of Forestry, Forest Research Branch, Canada: Kamloops, BC, Canada, 1963. [Google Scholar]
- Bailey, R.G. Descriptions of the Ecoregions of the United States; US Department of Agriculture Forest Service: Washington, DC, USA, 1995.
- Ecological Stratification Working Group. A National Ecological Framework for Canada; Environment Canada: Gatineau, QC, Canada, 1996; p. 76.
- Environment and Climate Change Canada. 2018. Available online: http://climate.weather.gc.ca/climate_normals/index_e.html (accessed on 24 April 2018).
- National Oceanic and Atmospheric Administration, United States of America. Comparative Climatic Data. 2018. Available online: https://www.ncdc.noaa.gov/ghcn/comparative-climatic-data (accessed on 24 April 2018).
- Environment and Climate Change Canada. 2019. Available online: https://climate.weather.gc.ca/prods_servs/cdn_climate_summary_e.html (accessed on 16 December 2019).
- Canadian Soil Information Service. 2019. Available online: http://sis.agr.gc.ca/cansis/publications/index.html (accessed on 12 December 2019).
- Akalusi, M.E.; Bourque, C.P.-A. Effect of climatic variation on the morphological characteristics of 37-year-oldbalsam fir provenances planted in a common garden in New Brunswick, Canada. Ecol. Evol. 2018, 8, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Berlyn, G.P. Changes in foliar spectral reflectance and chlorophyll fluorescence of four temperate species following branch cutting. Tree Physiol. 2002, 22, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Clark, J. The Immediate Effect of Severing on the Photosynthetic Rate of Norway Spruce Branches. Plant Physiol. 1954, 29, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Sakagami, Y. Examination of methods of measuring photosynthesis with detached parts of three species of birch in Hokkaido. J. Jpn. For. Soc. 1984, 66, 337–340. [Google Scholar]
- Gauthier, M.-M.; Jacobs, D.F. Reductions in net photosynthesis and stomatal conductance vary with time since leaf detachment in three deciduous angiosperms. Trees 2018, 32, 1247–1252. [Google Scholar] [CrossRef]
- Matyas, C.S.; Yeatman, C.W. Effect of geographical transfer on growth and survival of jack pine (Pinus banksiana Lamb.) populations. Silvae Genet. 1992, 41, 370–376. [Google Scholar]
- Thomson, A.M.; Parker, W.H. Boreal forest provenance tests used to predict optimal growth and response to climate change. 1. Jack pine. Can. J. For. Res. 2008, 38, 157–170. [Google Scholar] [CrossRef]
- Sheil, D.; Burslem, D.F.R.P.; Alder, D. The Interpretation and Misinterpretation of Mortality Rate Measures. J. Ecol. 1995, 83, 331. [Google Scholar] [CrossRef]
- Roman, L.A.; Battles, J.J.; McBride, J.R. Determinants of establishment survival for residential trees in Sacramento County, CA. Landsc. Urban Plan. 2014, 129, 22–31. [Google Scholar] [CrossRef]
- Buchman, R.G.; Pederson, S.P.; Walters, N.R. A tree survival model with application to species of the Great Lakes region. Can. J. For. Res. 1983, 13, 601–608. [Google Scholar] [CrossRef]
- Monserud, R.A.; Sterba, H. Modeling individual tree mortality for Austrian forest species. For. Ecol. Manag. 1999, 113, 109–123. [Google Scholar] [CrossRef]
- Martin, B.; Nienhuis, J.; King, G.; Schaefer, A. Restriction Fragment Length Polymorphisms Associated with Water Use Efficiency in Tomato. Science 1989, 243, 1725–1728. [Google Scholar] [CrossRef]
- Masle, J.; Gilmore, S.R.; Farquhar, G.D. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nat. Cell Biol. 2005, 436, 866–870. [Google Scholar] [CrossRef]
- Flexas, J.; Niinemets, U.; Galle, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmes, J.; Ribas-Carbo, M.; Rodriguez, P.L.; et al. Diffusional conductances to CO2 as a target for increasing photosynthesis and pho-tosynthetic water-use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Roussel, M.; Le Thiec, D.; Montpied, P.; Ningre, N.; Guehl, J.-M.; Brendel, O. Diversity of water use efficiency among Quercus robur genotypes: Contribution of related leaf traits. Ann. For. Sci. 2009, 66, 408. [Google Scholar] [CrossRef]
- Tomás, M.; Medrano, H.; Escalona, J.M.; Martorell, S.; Pou, A.; Ribas-Carbó, M.; Flexas, J. Variability of water use efficiency in grapevines. Environ. Exp. Bot. 2014, 103, 148–157. [Google Scholar] [CrossRef]
- Flexas, J.; Díaz-Espejo, A.; Conesa, M.A.; Coopman, R.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Medrano, H.; Ribas-Carbo, M.; et al. Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ. 2015, 39, 965–982. [Google Scholar] [CrossRef]
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2008, 317, 17–29. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Flexas, J.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef]
- Galmés, J.; Conesa, M.À.; Ochogavía, J.M.; Perdomo, J.A.; Francis, D.M.; Ribas-Carbó, M.; Savé, R.; Flexas, J.; Medrano, H.; Cifre, J. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant Cell Environ. 2010, 34, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.R.C.; Jordan, G.J.; Brodribb, T.J. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ. 2012, 35, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Shirley, H.L. Is tolerance the capacity to endure shade? J. For. 1943, 41, 339–345. [Google Scholar]
- Anderson, R.C.; Loucks, O.L.; Swain, A.M. Herbaceous response to canopy cover, light intensity and through fall precipitation in coniferous forests. Ecology 1969, 50, 255–263. [Google Scholar] [CrossRef]
- Kobe, R.K.; Pacala, S.W.; Silander, J.A., Jr.; Canham, C.D. Juvenile tree survivorship as a component of shade tol-erance. Ecol. Appl. 1995, 5, 517–532. [Google Scholar] [CrossRef]
- Kneeshaw, D.D.; Kobe, R.K.; Coates, K.D.; Messier, C. Sapling size influences shade tolerance ranking among southern boreal tree species. J. Ecol. 2006, 94, 471–480. [Google Scholar] [CrossRef]
- Humbert, L.; Gagnon, D.; Kneeshaw, D.; Messier, C. A shade tolerance index for common understory species of northeastern North America. Ecol. Indic. 2007, 7, 195–207. [Google Scholar] [CrossRef]
- Graham, S.A. Scoring Tolerance of Forest Trees; Research Note 4; University of Michigan, Department of Forestry, School of Natural Resources: Ann Arbor, MI, USA,, 1954. [Google Scholar]
- Hett, J.M.; Loucks, O.L. Age Structure Models of Balsam Fir and Eastern Hemlock. J. Ecol. 1976, 64, 1029. [Google Scholar] [CrossRef]
- Klinka, K.; Feller, M.C.; Green, R.N.; Meidinger, D.V.; Pojar, J.; Worrall, J. Ecological principles: Application. In Regenerating British Columbia’s Forests; Lavender, D.P., Parish, R., Johnson, C.M., Montgomery, G., Vyse, A., Willis, R.A., Winston, D., Eds.; University of British Columbia Press: Vancouver, BC, Canada, 1990; pp. 55–72. [Google Scholar]
- Sims, R.A.; Kershaw, H.M.; Wickware, G.M. The Autecology of Major Forest Species in the North Central Region of Ontario; Forestry Canada–Ontario region: Sault Ste. Marie, ON, Canada, 1990; 126p. [Google Scholar]
- Claveau, Y.; Messier, C.; Comeau, P.G. Interacting influence of light and size on aboveground biomass distribution in sub-boreal conifer saplings with contrasting shade tolerance. Tree Physiol. 2005, 25, 373–384. [Google Scholar] [CrossRef]
- King, D.A. Correlations Between Biomass Allocation, Relative Growth Rate and Light Environment in Tropical Forest Saplings. Funct. Ecol. 1991, 5, 485. [Google Scholar] [CrossRef]
- King, D.A. Branch growth and biomass allocation in Abies amabilis saplings in contrasting light environments. Tree Physiol. 1997, 17, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Saxe, H.; Cannell, M.G.R.; Johnsen, Ø.; Ryan, M.G.; Vourlitis, G. Tree and forest functioning in response to global warming. New Phytol. 2001, 149, 369–399. [Google Scholar] [CrossRef]
- Santini, F.; Ferrio, J.P.; Hereş, A.-M.; Notivol, E.; Piqué, M.; Serrano, L.; Shestakova, T.; Sin, E.; Vericat, P.; Voltas, J. Scarce population genetic differentiation but substantial spatiotemporal phenotypic variation of water-use efficiency in Pinus sylvestris at its western distribution range. Eur. J. For. Res. 2018, 137, 863–878. [Google Scholar] [CrossRef]
- Ferrio, J.P.; Voltas, J. Carbon and oxygen isotope ratios in wood constituents of Pinus halepensis as indicators of precipitation, temperature and vapour pressure deficit. Tellus B Chem. Phys. Meteorol. 2005, 57, 164–173. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhang, Q.; Zeng, X.; Xu, G.; Wu, G.; Wang, W. Species-specific tree growth and intrinsic water-use efficiency of Dahurian larch (Larix gmelinii) and Mongolian pine (Pinus sylvestris var. mongolica) growing in a boreal permafrost region of the Greater Hinggan Mountains, Northeastern China. Agric. For. Meteorol. 2018, 248, 145–155. [Google Scholar] [CrossRef]
- Schmidtling, R.C. Use of provenance tests to predict response to climate change: Loblolly pine and Norway spruce. Tree Physiol. 1994, 14, 805–817. [Google Scholar] [CrossRef]
- Frank, R.M. Abies balsamea. Silvics of North America: 1. Conifers; 2. Hardwoods. Agriculture Handbook 654 (Tech. Cords. R. M. Burns & B.H. Honkala); U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 1, 877p.
- Rossi, S.; DesLauriers, A.; Griçar, J.; Seo, J.-W.; Rathgeber, C.B.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Hamilton, D.A. A logistic model of mortality in thinned and unthinned mixed conifer stands of Northern Idaho. For. Sci. 1986, 32, 989–1000. [Google Scholar]
- Sims, A.; Kiviste, A.; Hordo, M.; Laarmann, D.; Von Gadow, K. Estimating Tree Survival: A Study Based on the Estonian Forest Research Plots Network. Ann. Bot. Fenn. 2009, 46, 336–352. [Google Scholar] [CrossRef]
- Cortini, F.; Comeau, P.G.; Strimbu, V.C.; Hogg, E.T.; Bokalo, M.; Huang, S. Survival functions for boreal tree species in northwestern North America. For. Ecol. Manag. 2017, 402, 177–185. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, H.Y. Competition, species interaction and ageing control tree mortality in boreal forests. J. Ecol. 2011, 99, 1470–1480. [Google Scholar] [CrossRef]
- Domec, J.-C.; Lachenbruch, B.; Meinzer, F.C.; Woodruff, D.R.; Warren, J.M.; McCulloh, K.A. Maximum height in a conifer is associated with conflicting requirements for xylem design. Proc. Natl. Acad. Sci. USA 2008, 105, 12069–12074. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Canadell, J.G.; Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2010, 20, 597–608. [Google Scholar] [CrossRef]
- Silva, L.C.R.; Anand, M. Probing for the influence of atmospheric CO2 and climate change on forest ecosystems across biomes. Glob. Ecol. Biogeogr. 2012, 22, 83–92. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).