Understory Vegetation Composition and Stand Are Mainly Limited by Soil Moisture in Black Locust Plantations of Loess Plateau
Abstract
:1. Introduction
2. Materials and Methods
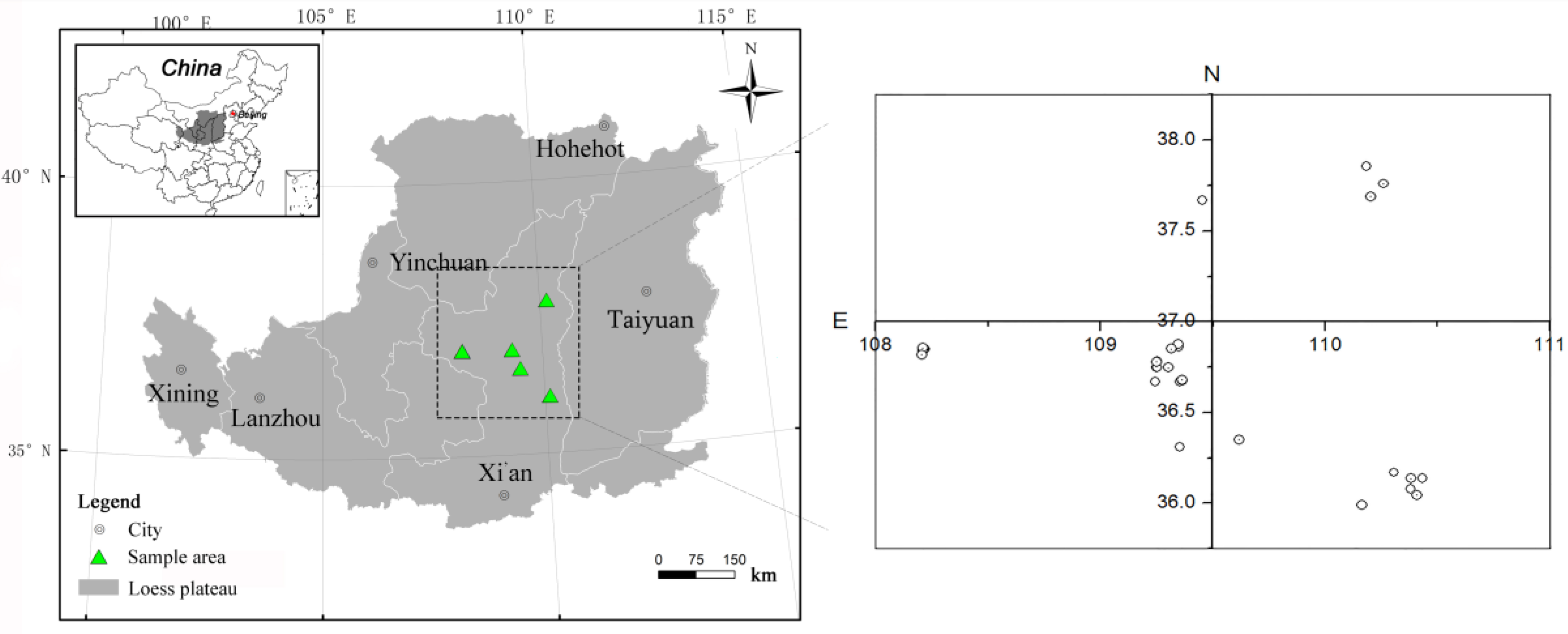
2.1. Study Sites and Experimental Design
2.2. Measurement of Soil Physicochemical Properties
2.3. Diversity Analysis
2.4. Data Analysis
3. Results
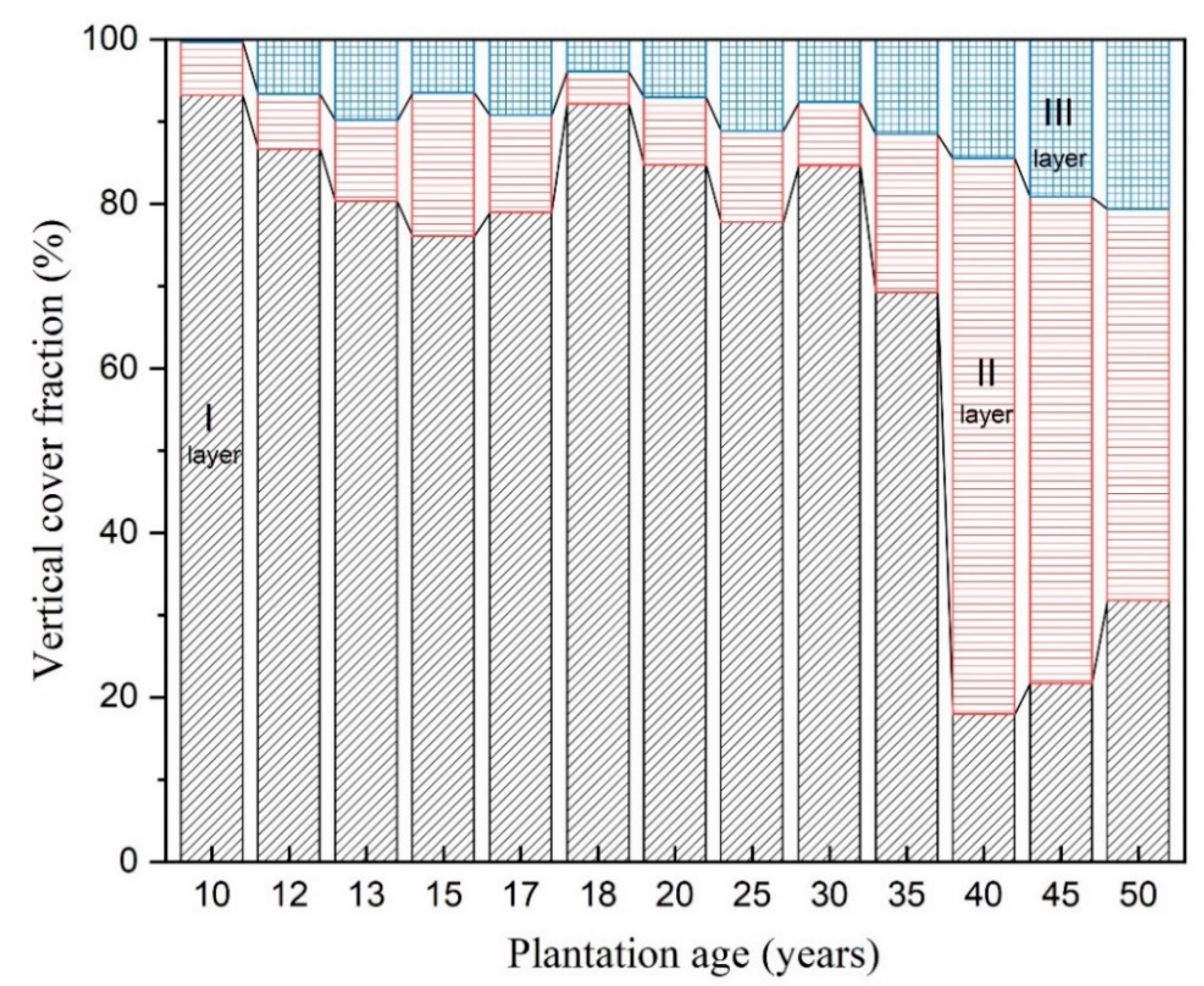
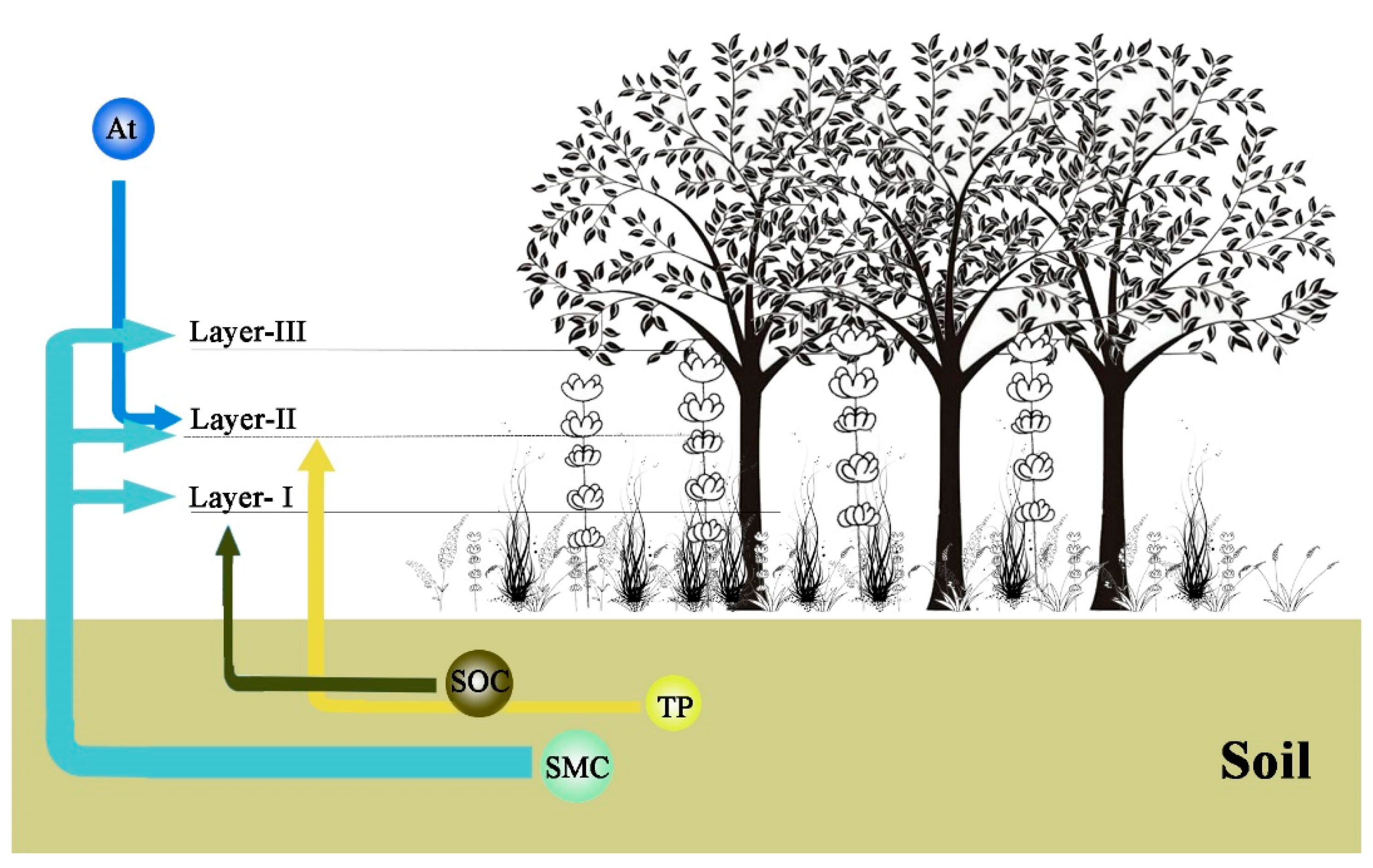
3.1. Vertical Structure
3.1.1. Stratification
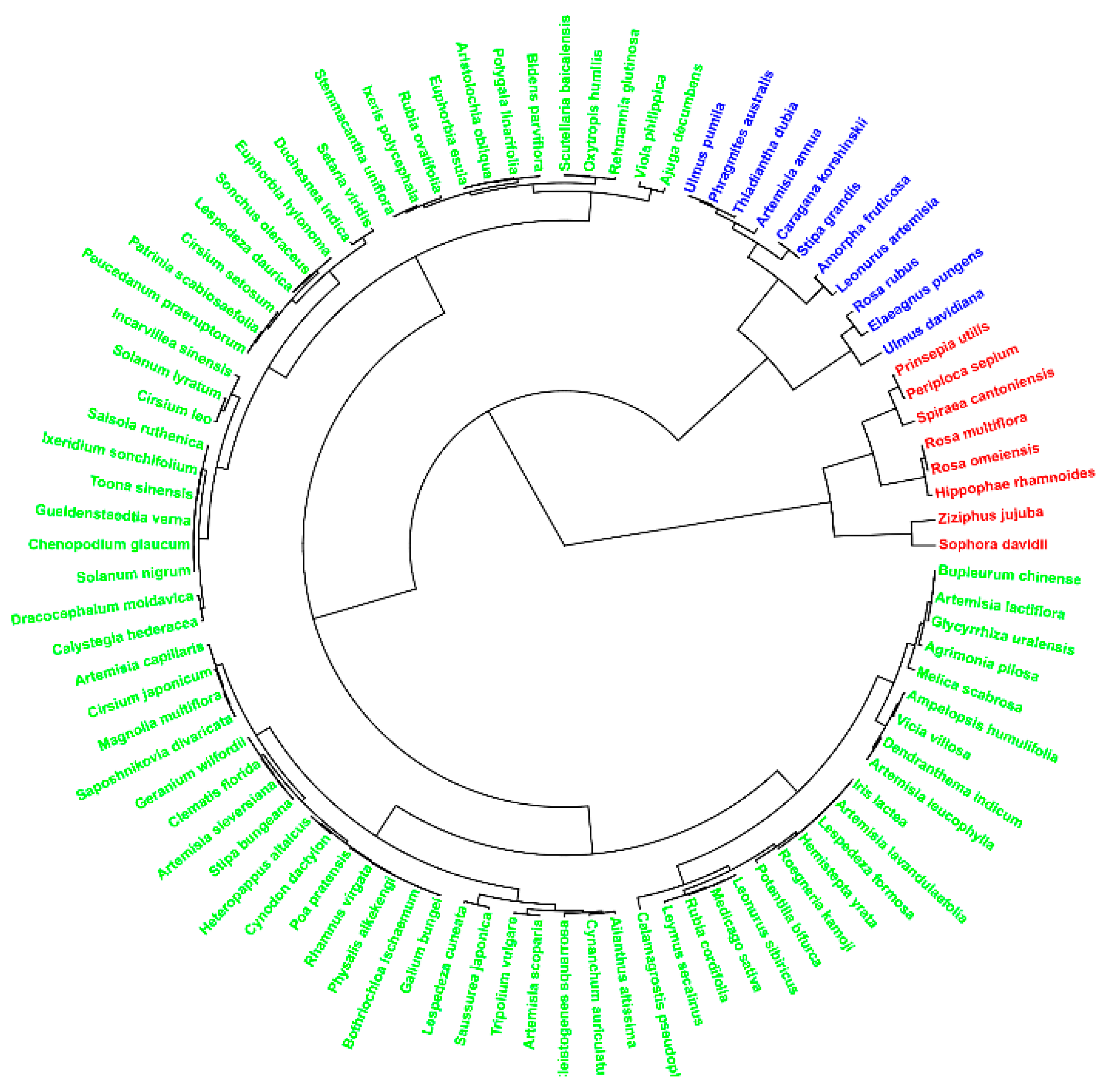
3.1.2. Understory Composition and Vertical Fractional Cover Dynamics
3.1.3. Diversity
3.2. Environmental Analysis
3.2.1. Soil Properties
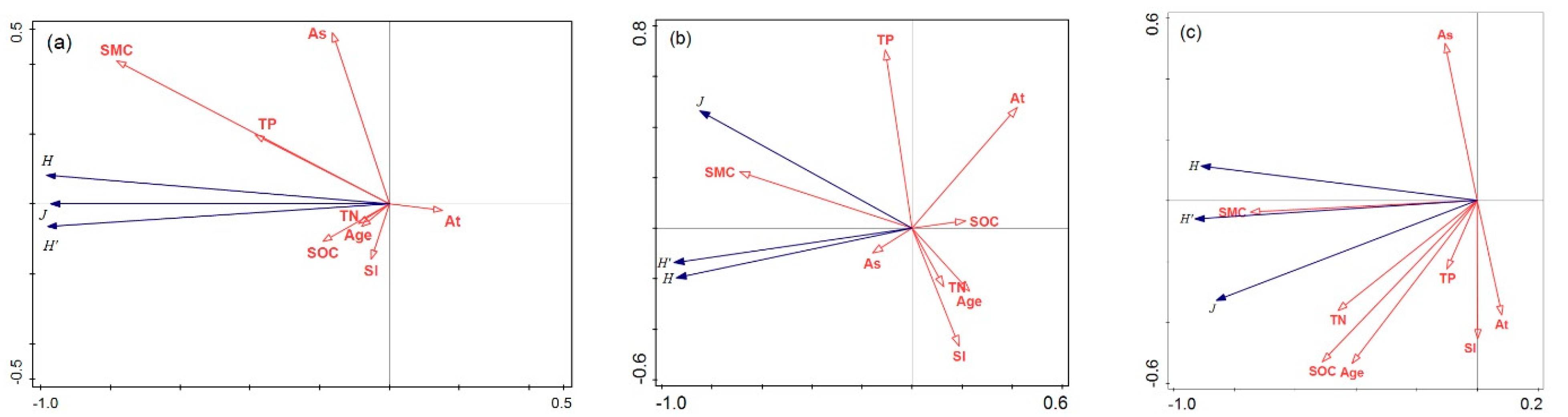
3.2.2. Redundancy Analysis
4. Discussion
4.1. Understory Stratification and Dynamic
4.2. Differential Responses of Understory Stand and Composition to Environmental Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Song, L. A review of ecological mechanisms for management practices of protective forests. J. For. Res. 2020, 1–14. [Google Scholar] [CrossRef]
- Franklin, J.F.; Donato, D.C. Variable retention harvesting in the Douglas-fir region. Ecol. Process. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Brandle, J.; Hintz, D. 34. Windbreaks for the future. Agric. Ecosyst. Environ. 1988, 22, 593–596. [Google Scholar] [CrossRef]
- Zhang, L.; Schwärzel, K. China’s Land Resources Dilemma: Problems, Outcomes, and Options for Sustainable Land Restoration. Sustainability 2017, 9, 2362. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.-J.; Zheng, X. The prospects of development of the Three-North Afforestation Program (TNAP): On the basis of the results of the 40-year construction general assessment of the TNAP. Chin. J. Ecol. 2019, 5, 1600–1610. [Google Scholar]
- Chen, C.; Park, T.; Wang, X.; Piao, S.; Xu, B.; Chaturvedi, R.K.; Fuchs, R.; Brovkin, V.; Ciais, P.; Fensholt, R.; et al. China and India lead in greening of the world through land-use management. Nat. Sustain. 2019, 2, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Luo, Y.; Shao, M.; Li, X.; Sun, L.; Jia, X. Environmental controls on sap flow in black locust forest in Loess Plateau, China. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. Annex I: Atlas of Global and Regional Climate Projections. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Cambridge, UK; New York, NY, USA, 2013; p. 1311. [Google Scholar]
- Majasalmi, T.; Rautiainen, M. The impact of tree canopy structure on understory variation in a boreal forest. For. Ecol. Manag. 2020, 466, 118100. [Google Scholar] [CrossRef]
- Majasalmi, T.; Rautiainen, M.; Stenberg, P.; Manninen, T. Validation of MODIS and GEOV1 fPAR Products in a Boreal Forest Site in Finland. Remote Sens. 2015, 7, 1359–1379. [Google Scholar] [CrossRef] [Green Version]
- Tonteri, T.; Salemaa, M.; Rautio, P.; Hallikainen, V.; Korpela, L.; Merilä, P. Forest management regulates temporal change in the cover of boreal plant species. For. Ecol. Manag. 2016, 381, 115–124. [Google Scholar] [CrossRef]
- Gilliam, F.S. The Ecological Significance of the Herbaceous Layer in Temperate Forest Ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Goulden, M.L.; Crill, P.M. Automated measurements of CO2 exchange at the moss surface of a black spruce forest. Tree Physiol. 1997, 17, 537–542. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, M.C.; Wardle, D.A. Understory Vegetation as a Forest Ecosystem Driver: Evidence from the Northern Swedish Boreal Forest. Front. Ecol. Environ. 2005, 3, 421–428. [Google Scholar] [CrossRef]
- Van Der Veken, S.; Bellemare, J.; Verheyen, K.; Hermy, M. Life-history traits are correlated with geographical distribution patterns of western European forest herb species. J. Biogeogr. 2007, 34, 1723–1735. [Google Scholar] [CrossRef]
- Collins, L.; Bradstock, R.A.; De Dios, V.R.; Duursma, R.A.; Velasco, S.; Boer, M.M. Understorey productivity in temperate grassy woodland responds to soil water availability but not to elevated [CO2]. Glob. Chang. Biol. 2018, 24, 2366–2376. [Google Scholar] [CrossRef]
- Zellweger, F.; De Frenne, P.; Van Calster, H.; Chudomelová, M.; Decocq, G.; Dirnböck, T.; Durak, T.; Heinken, T.; Jaroszewicz, B.; Kopecký, M.; et al. Forest microclimate dynamics drive plant responses to warming. Science 2020, 368, 772–775. [Google Scholar] [CrossRef]
- Feroz, S.M.; Yoshimura, K.; Hagihara, A. Stand stratification and woody species diversity of a subtropical forest in limestone habitat in the northern part of Okinawa Island. J. Plant Res. 2008, 121, 329–337. [Google Scholar] [CrossRef]
- Gao, T.; Qiu, L.; Hammer, M.; Gunnarsson, A. The Importance of Temporal and Spatial Vegetation Structure Information in Biotope Mapping Schemes: A Case Study in Helsingborg, Sweden. Environ. Manag. 2011, 49, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Laurans, M.; Herault, B.; Vieilledent, G.; Vincent, G. Vertical stratification reduces competition for light in dense tropical forests. For. Ecol. Manag. 2014, 329, 79–88. [Google Scholar] [CrossRef]
- Staab, M.; Blüthgen, N.; Klein, A.-M. Tree diversity alters the structure of a tri-trophic network in a biodiversity experiment. Oikos 2014, 124, 827–834. [Google Scholar] [CrossRef]
- Barros, C.; Thuiller, W.; Georges, D.; Boulangeat, I.; Münkemüller, T. N-dimensional hypervolumes to study stability of complex ecosystems. Ecol. Lett. 2016, 19, 729–742. [Google Scholar] [CrossRef] [Green Version]
- Zehm, A.; Nobis, M.; Schwabe, A. Multiparameter analysis of vertical vegetation structure based on digital image processing. Flora Morphol. Distrib. Funct. Ecol. Plants 2003, 198, 142–160. [Google Scholar] [CrossRef] [Green Version]
- Hamraz, H.; Contreras, M.A.; Zhang, J. Forest understory trees can be segmented accurately within sufficiently dense airborne laser scanning point clouds. Sci. Rep. 2017, 7, 6770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jules, M.J.; Sawyer, J.O.; Jules, E.S. Assessing the relationships between stand development and understory vegetation using a 420-year chronosequence. For. Ecol. Manag. 2008, 255, 2384–2393. [Google Scholar] [CrossRef]
- Nan, M.-X.; Sen, Z.-Y.; Lei, Z.; Ying, X. Population structure and understory species diversity of different aged Pinus sylvestris var. mongolica plantations in Nenjiang Sandy Land of Northeast China. Chin. J. Appl. Ecol. 2012, 23, 2332. [Google Scholar] [CrossRef]
- Niu, R.; Liu, J.; Zhao, X.; Qin, Y. Ecological benefit of different revegetated covers in the middle of Hexi corridor, northwestern China. Environ. Earth Sci. 2015, 74, 5699–5710. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Gray, A.; Vanbergen, A.J.; Bergès, L.; Bohner, A.; Brooker, R.W.; De Bruyn, L.; De Cinti, B.; Dirnböck, T.; Grandin, U.; et al. Functional traits and local environment predict vegetation responses to disturbance: A pan-European multisite experiment. J. Ecol. 2011, 99, 777–787. [Google Scholar] [CrossRef]
- Connolly, J.; Wayne, P. Asymmetric competition between plant species. Oecologia 1996, 108, 311–320. [Google Scholar] [CrossRef]
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Canopy gap size influences niche partitioning of the ground-layer plant community in a northern temperate forest. J. Plant Ecol. 2013, 6, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Fourrier, A.; Bouchard, M.; Pothier, D. Effects of canopy composition and disturbance type on understorey plant assembly in boreal forests. J. Veg. Sci. 2015, 26, 1225–1237. [Google Scholar] [CrossRef]
- Rüger, N.; Wirth, C.; Wright, S.J.; Condit, R. Functional traits explain light and size response of growth rates in tropical tree species. Ecology 2012, 93, 2626–2636. [Google Scholar] [CrossRef]
- Plewa, R.; Jaworski, T.; Hilszczański, J.; Horák, J. Investigating the biodiversity of the forest strata: The importance of vertical stratification to the activity and development of saproxylic beetles in managed temperate deciduous forests. For. Ecol. Manag. 2017, 402, 186–193. [Google Scholar] [CrossRef]
- Batterman, S.A.; Hedin, L.O.; Van Breugel, M.; Ransijn, J.; Craven, D.J.; Hall, J.S. Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 2013, 502, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Kargar-Chigani, H.; Javadi, S.A.; Zahedi-Amiri, G.; Khajeddin, S.J.; Jafari, M. Vegetation composition differentiation and species-environment relationships in the northern part of Isfahan Province, Iran. J. Arid. Land 2017, 9, 161–175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, W.; Yu, M.; Wang, G.G.; Wu, T. Latitudinal patterns of leaf N, P stoichiometry and nutrient resorption of Metasequoia glyptostroboides along the eastern coastline of China. Sci. Total Environ. 2018, 618, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.F.; Scheffers, B.R. Vertical stratification influences global patterns of biodiversity. Ecography 2018, 42, 249. [Google Scholar] [CrossRef] [Green Version]
- Ohsawa, M. Latitudinal comparison of altitudinal changes in forest structure, leaf-type, and species richness in humid monsoon Asia. Plant Ecol. 1995, 121, 3–10. [Google Scholar] [CrossRef]
- Schwärzel, K.; Zhang, L.; Montanarella, L.; Wang, Y.; Sun, G. How afforestation affects the water cycle in drylands: A process-based comparative analysis. Glob. Chang. Biol. 2020, 26, 944–959. [Google Scholar] [CrossRef] [Green Version]
- Neumann, M.; Starlinger, F. The significance of different indices for stand structure and diversity in forests. For. Ecol. Manag. 2001, 145, 91–106. [Google Scholar] [CrossRef]
- Wang, H.; Deng, N.; Wu, D.; Hu, S.; Kou, M. Long-term net transformation and quantitative molecular mechanisms of soil nitrogen during natural vegetation recovery of abandoned farmland on the Loess Plateau of China. Sci. Total Environ. 2017, 607, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis Part 2; Chemical and Microbial Properties: Madison, WI, USA, 1982; pp. 360–383. [Google Scholar]
- Su, Y.Z.; Zhao, W.Z.; Su, P.X.; Zhang, Z.H.; Wang, T.; Ram, R. Ecological effects of desertification control and desertified land reclamation in an oasis–desert ecotone in an arid region: A case study in Hexi Corridor, northwest China. Ecol. Eng. 2007, 29, 117–124. [Google Scholar] [CrossRef]
- Lindgren, P.M.; Sullivan, T.P. Influence of alternative vegetation management treatments on conifer plantation attributes: Abundance, species diversity, and structural diversity. For. Ecol. Manag. 2001, 142, 163–182. [Google Scholar] [CrossRef]
- Reyes, G.P.; Kneeshaw, D.D. Moderate-severity disturbance dynamics in Abies balsamea-Betula spp. forests: The relative importance of disturbance type and local stand and site characteristics on woody vegetation response. Écoscience 2008, 15, 241–249. [Google Scholar] [CrossRef]
- Perry, D.A. The scientific basis of forestry. Annu. Rev. Ecol. Syst. 1998, 29, 435–466. [Google Scholar] [CrossRef]
- Inoue, T.; Fukuzawa, K.; Watanabe, T.; Yoshida, T.; Shibata, H. Spatial pattern of soil nitrogen availability and its relationship to stand structure in a coniferous-broadleaved mixed forest with a dense dwarf bamboo understory in northern Japan. Ecol. Res. 2017, 32, 227–241. [Google Scholar] [CrossRef]
- Zhang, P.; Shao, M.; Zhang, X. Spatial pattern of plant species diversity and the influencing factors in a Gobi Desert within the Heihe River Basin, Northwest China. J. Arid. Land 2017, 9, 379–393. [Google Scholar] [CrossRef]
- Ricotta, C.; Avena, G. On the relationship between Pielou’s evenness and landscape dominance within the context of Hill’s diversity profiles. Ecol. Indic. 2003, 2, 361–365. [Google Scholar] [CrossRef]
- Bergholz, K.; May, F.; Giladi, I.; Ristow, M.; Ziv, Y.; Jeltsch, F. Environmental heterogeneity drives fine-scale species assembly and functional diversity of annual plants in a semi-arid environment. Perspect. Plant Ecol. Evol. Syst. 2017, 24, 138–146. [Google Scholar] [CrossRef]
- Correia, D.L.P.; Raulier, F.; Filotas, É.; Bouchard, M. Stand height and cover type complement forest age structure as a biodiversity indicator in boreal and northern temperate forest management. Ecol. Indic. 2017, 72, 288–296. [Google Scholar] [CrossRef]
- Zuo, X.; Zhao, X.; Zhao, H.; Zhang, T.; Li, Y.; Wang, S.; Li, W.; Powers, R. Scale dependent effects of environmental factors on vegetation pattern and composition in Horqin Sandy Land, Northern China. Geoderma 2012, 173, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Dong, Y.; Chang, X.; Nie, X.; Liu, L.; Xiao, H.; Lu, Y.; Zeng, G. Response of soil organic carbon and nitrogen stocks to soil erosion and land use types in the Loess hilly–gully region of China. Soil Tillage Res. 2017, 166, 1–9. [Google Scholar] [CrossRef]

| Age (y) | SOC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | SMC (%) |
|---|---|---|---|---|
| 10 | 2.86 ± 0.10 g | 0.44 ± 0.02 f | 0.55 ± 0.01 c | 5.92 ± 0.1 d |
| 12 | 3.87 ± 0.29 f | 0.48 ± 0.03 f | 0.62 ± 0.03 c | 5.58 ± 0.13 d |
| 13 | 4.57 ± 0.33 e | 0.53 ± 0.02 ef | 0.57 ± 0.01 c | 6.90 ± 0.45 c |
| 15 | 4.58 ± 0.20 e | 0.54 ± 0.05 ef | 0.78 ± 0.07 b | 7.45 ± 0.14 b |
| 17 | 7.22 ± 0.29 d | 0.65 ± 0.07 d | 1.11 ± 0.13 a | 7.66 ± 0.33 b |
| 18 | 7.33 ± 0.57 d | 0.63 ± 0.04 d | 1.08 ± 0.14 a | 7.64 ± 0.10 b |
| 20 | 7.48 ± 0.42 d | 0.66 ± 0.06 d | 1.06 ± 0.08 a | 7.13 ± 0.22 b |
| 25 | 7.41 ± 0.70 d | 0.65 ± 0.02 de | 1.13 ± 0.12 a | 8.17 ± 0.33 a |
| 30 | 10.48 ± 0.03 b | 1.10 ± 0.05 a | 0.60 ± 0.08 c | 8.00 ± 0.20 a |
| 35 | 8.65 ± 0.08 c | 0.96 ± 0.02 bc | 0.58 ± 0.07 c | 8.17 ± 0.28 a |
| 40 | 10.48 ± 0.18 b | 1.10 ± 0.15 a | 0.60 ± 0.06 c | 7.45 ± 0.50 b |
| 45 | 11.55 ± 0.03 a | 1.05 ± 0.01 ab | 0.61 ± 0.08 c | 6.20 ± 0.20 ab |
| 50 | 9.87 ± 0.21 b | 0.90 ± 0.08 c | 0.62 ± 0.06 c | 7.64 ± 0.40 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-F.; Gao, T.; Zhang, W.; Li, G.; Hao, W.-F. Understory Vegetation Composition and Stand Are Mainly Limited by Soil Moisture in Black Locust Plantations of Loess Plateau. Forests 2021, 12, 195. https://doi.org/10.3390/f12020195
Wu H-F, Gao T, Zhang W, Li G, Hao W-F. Understory Vegetation Composition and Stand Are Mainly Limited by Soil Moisture in Black Locust Plantations of Loess Plateau. Forests. 2021; 12(2):195. https://doi.org/10.3390/f12020195
Chicago/Turabian StyleWu, Hui-Feng, Tian Gao, Wei Zhang, Gang Li, and Wen-Fang Hao. 2021. "Understory Vegetation Composition and Stand Are Mainly Limited by Soil Moisture in Black Locust Plantations of Loess Plateau" Forests 12, no. 2: 195. https://doi.org/10.3390/f12020195





