Accumulated Heating and Chilling Are Important Drivers of Forest Phenology and Productivity in the Algonquin-to-Adirondacks Conservation Corridor of Eastern North America
Abstract
:1. Introduction
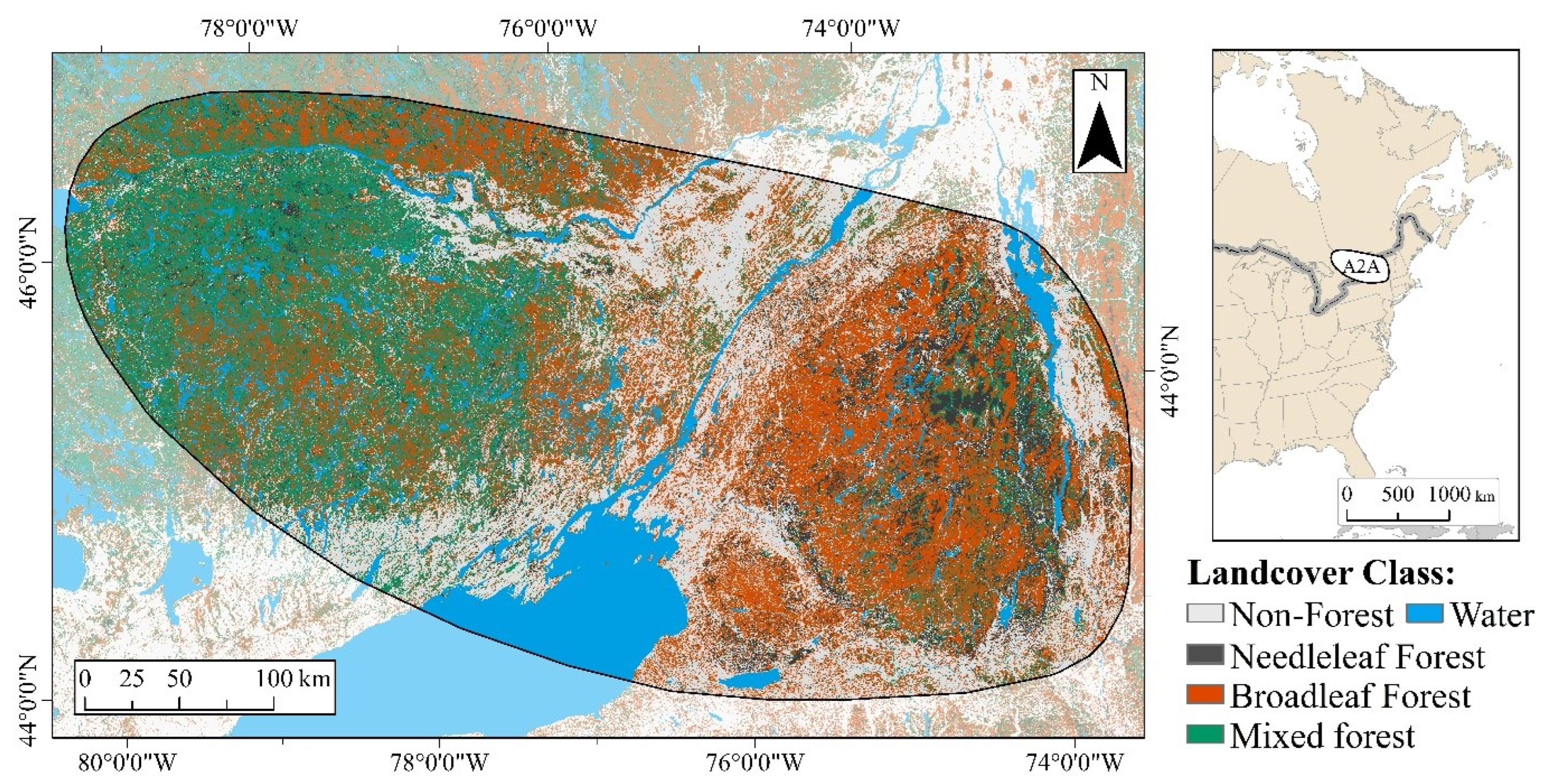
2. Study Area
3. Data
3.1. Forest Phenology and Productivity Indices
3.2. Climatological Variables
4. Methods
4.1. Modelling Schema
4.2. Training and Validation Data
4.3. Model Training
4.4. Model Validation
5. Results
5.1. Model Characteristics
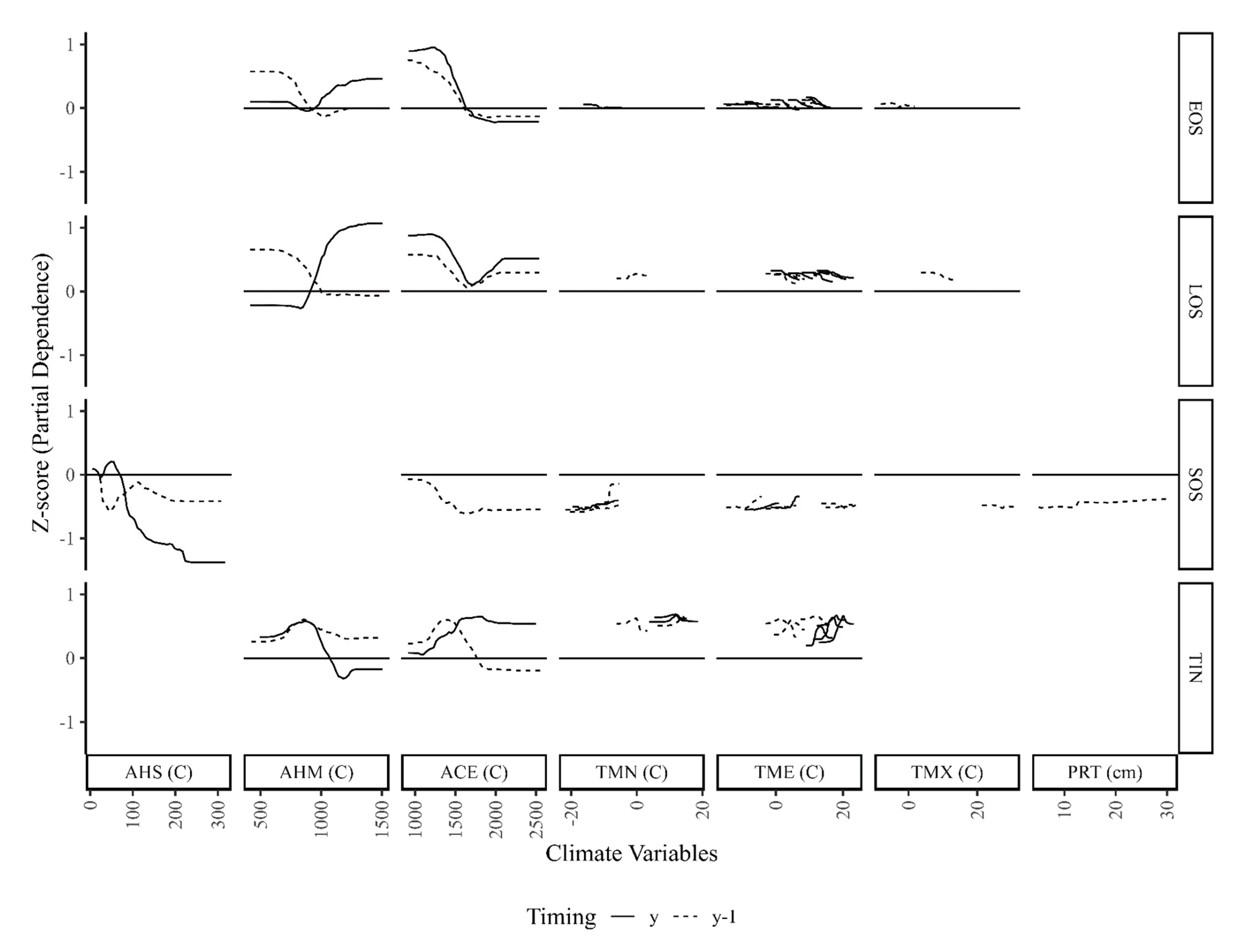
5.2. Forest-Climate Relationships
6. Discussion
7. Conclusions
- -
- A large proportion of year-to-year variability in forest phenology and productivity was explained by climatic variables, particularly heating and chilling accumulation;
- -
- Accumulated heating was most important for SOS, EOS, and TIN, but accumulated chilling was most important for EOS;
- -
- Phenology was most responsive to temperature accumulation at low or high extremes, while productivity was most responsive to more moderate accumulations;
- -
- Lagged responses of EOS and LOS to accumulated heating, and of SOS to accumulated chilling had important distinctions from current responses;
- -
- Precipitation, photoperiod, and other climate variables were not ranked as being among the most important drivers of phenology or productivity.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Precipitation | Minimum Temperature | Maximum Temperature | ||||
|---|---|---|---|---|---|---|
| 1988 | 2014 | 1988 | 2014 | 1988 | 2014 | |
| Number of Stations | 160 | 140 | 119 | 84 | 119 | 84 |
| R-squared of linear model | 0.581 | 0.632 | 0.977 | 0.973 | 0.969 | 0.965 |
| Pearson’s correlation coefficient | 0.762 | 0.795 | 0.988 | 0.986 | 0.984 | 0.982 |
| p-value of Pearson’s correlation | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| TIN | LOS | SOS | EOS | |||||
|---|---|---|---|---|---|---|---|---|
| p ≤ 0.05 | FDR | p ≤ 0.05 | FDR | p ≤ 0.05 | FDR | p ≤ 0.05 | FDR | |
| + | 3.2% | 0.0% | 8.6% | 0.0% | 0.4% | 0.0% | 7.0% | 0.0% |
| 0 | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| − | 1.1% | 0.0% | 0.0% | 0.0% | 0.7% | 0.0% | 0.1% | 0.0% |
References
- Zhu, Z.; Piao, S.; Myneni, R.B.; Huang, M.; Zeng, Z.; Canadell, J.G.; Ciais, P.; Sitch, S.; Friedlingstein, P.; Arneth, A.; et al. Greening of the Earth and its drivers. Nat. Clim. Chang. 2016, 6, 791–795. [Google Scholar] [CrossRef]
- Garonna, I.; de Jong, R.; Schaepman, M.E. Variability and evolution of global land surface phenology over the past three decades (1982–2012). Glob. Chang. Biol. 2016, 22, 1456–1468. [Google Scholar] [CrossRef]
- Reidmiller, D.R.; Avery, C.W.; Easterling, D.R.; Kunkel, K.E.; Lewis, K.L.M.; Maycock, T.K.; Stewart, B.C. (Eds.) USGCRP, 2018: Impacts, Risks, and Adaptation in the United States, Fourth National Climate Assessment, Volume II; U.S. Global Change Research Program: Washington, DC, USA, 2018.
- Bush, E.; Lemmen, D.S. (Eds.) Canada’s Changing Climate Report; Government of Canada: Ottawa, ON, Canada, 2019.
- Huntington, T.G.; Richardson, A.D.; McGuire, K.J.; Hayhoe, K. Climate and hydrological changes in the northeastern United States: Recent trends and implications for forested and aquatic ecosystems. Can. J. For. Res. 2009, 39, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Vincent, L.A.; Zhang, X.; Mekis, É.; Wan, H.; Bush, E.J. Changes in Canada’s Climate: Trends in Indices Based on Daily Temperature and Precipitation Data. Atmos. Ocean 2018, 56, 332–349. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.L.; Feng, Y.; Vincent, L.A. Observed changes in one-in-20 year extremes of Canadian surface air temperatures. Atmos. Ocean 2014, 52, 222–231. [Google Scholar] [CrossRef]
- Wan, H.; Zhang, X.; Zwiers, F. Human influence on Canadian temperatures. Clim. Dyn. 2019, 52, 479–494. [Google Scholar] [CrossRef]
- Hoerling, M.; Eischeid, J.; Perlwitz, J.; Quan, X.W.; Wolter, K.; Cheng, L. Characterizing recent trends in U.S. heavy precipitation. J. Clim. 2016, 29, 2313–2332. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Way, D.A.; Montgomery, R.A. Photoperiod constraints on tree phenology, performance and migration in a warming world. Plant Cell Environ. 2015, 38, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M.E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982-2008. Glob. Chang. Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Richardson, A.D.; Bailey, A.S.; Denny, E.G.; Martin, C.W.; O’Keefe, J. Phenology of a northern hardwood forest canopy. Glob. Chang. Biol. 2006, 12, 1174–1188. [Google Scholar] [CrossRef] [Green Version]
- Hufkens, K.; Friedl, M.A.; Keenan, T.F.; Sonnentag, O.; Mailey, A.; O’Keefe, J.; Richardson, A.D. Ecological impacts of a widespread frost event following early spring leaf-out. Glob. Chang. Biol. 2012, 18, 2365–2377. [Google Scholar] [CrossRef]
- Wang, X.; Piao, S.; Xu, X.; Ciais, P.; Macbean, N.; Myneni, R.B.; Li, L. Has the advancing onset of spring vegetation green-up slowed down or changed abruptly over the last three decades? Glob. Ecol. Biogeogr. 2015, 24, 621–631. [Google Scholar] [CrossRef]
- Dragoni, D.; Rahman, A.F. Trends in fall phenology across the deciduous forests of the Eastern USA. Agric. For. Meteorol. 2012, 157, 96–105. [Google Scholar] [CrossRef]
- Myneni, R.B.; Keeling, C.D.; Tucker, C.J.; Asrar, G.; Nemani, R.R. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 1997, 386, 698–702. [Google Scholar] [CrossRef]
- Richardson, A.D.; Black, T.A.; Ciais, P.; Delbart, N.; Friedl, M.A.; Gobron, N.; Hollinger, D.Y.; Kutsch, W.L.; Longdoz, B.; Luyssaert, S.; et al. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3227–3246. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fensholt, R. Temporal changes in coupled vegetation phenology and productivity are biome-specific in the Northern Hemisphere. Remote Sens. 2017, 9, 1277. [Google Scholar] [CrossRef] [Green Version]
- Mekonnen, Z.A.; Grant, R.F.; Schwalm, C. Contrasting changes in gross primary productivity of different regions of North America as affected by warming in recent decades. Agric. For. Meteorol. 2016, 218–219, 50–64. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Masek, J.G. The vegetation greenness trend in Canada and US Alaska from 1984-2012 Landsat data. Remote Sens. Environ. 2016, 176, 1–16. [Google Scholar] [CrossRef]
- Froelich, N.; Croft, H.; Chen, J.M.; Gonsamo, A.; Staebler, R.M. Trends of carbon fluxes and climate over a mixed temperate–boreal transition forest in southern Ontario, Canada. Agric. For. Meteorol. 2015, 211–212, 72–84. [Google Scholar] [CrossRef]
- Urbanski, S.; Barford, C.; Wofsy, S.; Kucharik, C.; Pyle, E.; Budney, J.; McKain, K.; Fitzjarrald, D.; Czikowsky, M.; Munger, J.W. Factors controlling CO2 exchange on timescales from hourly to decadal at Harvard Forest. J. Geophys. Res. Biogeosci. 2007, 112, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Seyednasrollah, B.; Young, A.M.; Li, X.; Milliman, T.; Ault, T.; Frolking, S.; Friedl, M.; Richardson, A.D. Sensitivity of Deciduous Forest Phenology to Environmental Drivers: Implications for Climate Change Impacts Across North America. Geophys. Res. Lett. 2020, 47. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Aubrecht, D.M.; Furze, M.E.; Nettles, R.; Heiderman, R.R.; Seyednasrollah, B.; Krassovski, M.B.; Latimer, J.M.; et al. Ecosystem warming extends vegetation activity but heightens vulnerability to cold temperatures. Nature 2018, 560, 368–371. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Wolkovich, E.M. Temperature and photoperiod drive spring phenology across all species in a temperate forest community. New Phytol. 2018, 219, 1353–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmett, K.D.; Renwick, K.M.; Poulter, B. Disentangling Climate and Disturbance Effects on Regional Vegetation Greening Trends. Ecosystems 2018, 22, 873–891. [Google Scholar] [CrossRef] [Green Version]
- Norman, S.P.; Hargrove, W.W.; Christie, W.M. Spring and Autumn Phenological Variability across Environmental Gradients of Great Smoky Mountains National Park, USA. Remote Sens. 2017, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Galiano, V.F.; Sanchez-Castillo, M.; Dash, J.; Atkinson, P.M.; Ojeda-Zujar, J. Modelling interannual variation in the spring and autumn land surface phenology of the European forest. Biogeosciences 2016, 13, 3305–3317. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Zheng, Y.; Piao, S.; Ciais, P.; Lombardozzi, D. Increased atmospheric vapor pressure deficit reduces global vegetation growth. Sci. Adv. 2019, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Forkel, M.; Carvalhais, N.; Verbesselt, J.; Mahecha, M.D.; Neigh, C.S.R.; Reichstein, M. Trend Change detection in NDVI time series: Effects of inter-annual variability and methodology. Remote Sens. 2013, 5, 2113–2144. [Google Scholar] [CrossRef] [Green Version]
- Rafferty, N.E.; Caradonna, P.J.; Burkle, L.A.; Iler, A.M.; Bronstein, J.L. Phenological overlap of interacting species in a changing climate: An assessment of available approaches. Ecol. Evol. 2013, 3, 3183–3193. [Google Scholar] [CrossRef]
- Stephenson, B. The Algonquin to Adirondack Conservation Initiative: A key macro-landscape linkage in eastern North America. In Crossing Boundaries in Park Management; The George Wright Society: Hancock, MI, USA, 2001; pp. 303–310. [Google Scholar]
- CEC. Land Cover, 2010 (Landsat, 30 m). 2010. Available online: http://www.cec.org/north-american-environmental-atlas/land-cover-2010-landsat-30m/ (accessed on 6 July 2017).
- Crins, W.J. Ecozones, Ecoregions and Ecodistricts of Ontario; Inventory, Monitoring and Assessment; Ontario Ministry of Natural Resources: Peterborough, ON, Canada, 2002.
- Widmann, R.H.; Crawford, S.; Kurtz, C.M.; Nelson, M.D.; Miles, P.D.; Morin, R.S.; Riemann, R. New York Forests, 2012; U.S Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2015.
- Eidenshink, J. A 16-year Time Series of 1 km AVHRR Satellite Data of the Conterminous United States and Alaska. Photogramm. Eng. Remote Sens. 2006, 72, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- de Beurs, K.M.; Henebry, G.M. Spatio-Temporal Statistical Methods for Modelling Land Surface Phenology. In Phenological Research: Methods for Environmental and Climate Change Analysis; Keatley, M.R., Hudson, I.L., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 147–158. ISBN 978-90-481-3334-5. [Google Scholar]
- Ji, L.; Brown, J.F. Effect of NOAA satellite orbital drift on AVHRR-derived phenological metrics. Int. J. Appl. Earth Obs. Geoinf. 2017, 62, 215–223. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. 2017. Available online: cran.r-project.org/package=raster (accessed on 4 July 2018).
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: r-project.org (accessed on 4 July 2018).
- Thornton, P.E.; Thornton, M.M.; Mayer, B.W.; Wei, Y.; Devarakonda, R.; Vose, R.S.; Cook, R.B. Daymet: Daily Surface Weather Data on a 1-km Grid for North America, 3rd ed.; 2017. Available online: daymet.ornl.gov (accessed on 4 July 2018).
- Menne, M.J.; Durre, I.; Vose, R.S.; Gleason, B.E.; Houston, T.G. An overview of the global historical climatology network-daily database. J. Atmos. Ocean. Technol. 2012, 29, 897–910. [Google Scholar] [CrossRef]
- Beguería, S.; Vicente-Serrano, S.M. Standardised Precipitation-Evapotranspiration Index. 2017. Available online: cran.r-project.org/package=SPEI (accessed on 4 July 2018).
- Beguería, S.; Vicente-Serrano, S.M.; Reig, F.; Latorre, B. Standardized precipitation evapotranspiration index (SPEI) revisited: Parameter fitting, evapotranspiration models, tools, datasets and drought monitoring. Int. J. Climatol. 2014, 34, 3001–3023. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- Thornton, M.M.; Thornton, P.E.; Wei, Y.; Vose, R.S.; Boyer, A.G. Daymet: Station-Level Inputs and Model Predicted Values for North America, 3rd ed.; 2017. Available online: https://daac.ornl.gov/DAYMET/guides/Daymet_V3_Stn_Level_CrossVal.html (accessed on 2 May 2019).
- Henn, B.; Newman, A.J.; Livneh, B.; Daly, C.; Lundquist, J.D. An assessment of differences in gridded precipitation datasets in complex terrain. J. Hydrol. 2018, 556, 1205–1219. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Genuer, R.; Poggi, J.M.; Tuleau-Malot, C. Variable selection using random forests. Pattern Recognit. Lett. 2010, 31, 2225–2236. [Google Scholar] [CrossRef] [Green Version]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R. News 2002, 2, 18–22. Available online: cran.r-project.org/package=randomForest (accessed on 2 May 2019).
- Microsoft; Weston, S. doSNOW: Foreach Parallel Adaptor for the “Snow” Package. 2017. Available online: cran.r-project.org/package=doSNOW (accessed on 2 May 2019).
- Microsoft; Weston, S. foreach: Provides Foreach Looping Construct for R. 2017. Available online: cran.r-project.org/package=foreach (accessed on 2 May 2019).
- Kuhn, M. caret: Classification and Regression Training. 2018. Available online: cran.r-project.org/package=caret (accessed on 2 May 2019).
- Genuer, R.; Poggi, J.M.; Tuleau-Malot, C.; Villa-Vialaneix, N. Random Forests for Big Data. Big Data Res. 2017, 9, 28–46. [Google Scholar] [CrossRef]
- Wei, P.; Lu, Z.; Song, J. Variable importance analysis: A comprehensive review. Reliab. Eng. Syst. Saf. 2015, 142, 399–432. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinf. 2007, 8. [Google Scholar] [CrossRef] [Green Version]
- Greenwell, B.M. pdp: An R Package for Constructing Partial Dependence Plots. R. J. 2017, 9, 421–436. Available online: cran.r-project.org/package=pdp (accessed on 2 May 2019). [CrossRef] [Green Version]
- Milborrow, S. Plotmo: Plot a Model’s Residuals, Response, and Partial Dependence Plots. 2018. Available online: cran.r-project.org/package=plotmo (accessed on 2 May 2019).
- Tang, J.; Körner, C.; Muraoka, H.; Piao, S.; Shen, M.; Thackeray, S.J.; Yang, X. Emerging opportunities and challenges in phenology: A review. Ecosphere 2016, 7, e01436. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Schwartz, M.D.; Donnelly, A.; Liang, L. An observation-based progression modeling approach to spring and autumn deciduous tree phenology. Int. J. Biometeorol. 2016, 60, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Piao, S.; Ciais, P.; Li, J.; Friedlingstein, P.; Koven, C.; Chen, A. Spring temperature change and its implication in the change of vegetation growth in North America from 1982 to 2006. Proc. Natl. Acad. Sci. USA 2011, 108, 1240–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers-Smith, I.H.; Kerby, J.T.; Phoenix, G.K.; Bjerke, J.W.; Epstein, H.E.; Assmann, J.J.; John, C.; Andreu-Hayles, L.; Angers-Blondin, S.; Beck, P.S.A.; et al. Complexity revealed in the greening of the Arctic. Nat. Clim. Chang. 2020, 10, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Körner, C.; Basler, D. Phenology Under Global Warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Hanson, P.J.; Post, W.M.A.C.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 Eastern US Spring Freeze: Increased Cold Damage in a Warming World? Bioscience 2008, 58, 253–262. [Google Scholar] [CrossRef]
- Schratz, P.; Muenchow, J.; Iturritxa, E.; Richter, J.; Brenning, A. Hyperparameter tuning and performance assessment of statistical and machine-learning algorithms using spatial data. Ecol. Modell. 2019, 406, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.; Reudenbach, C.; Hengl, T.; Katurji, M.; Nauss, T. Improving performance of spatio-temporal machine learning models using forward feature selection and target-oriented validation. Environ. Model. Softw. 2018, 101, 1–9. [Google Scholar] [CrossRef]
- Meyer, H.; Reudenbach, C.; Wöllauer, S.; Nauss, T. Importance of spatial predictor variable selection in machine learning applications—Moving from data reproduction to spatial prediction. Ecol. Modell. 2019, 411, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Hayhoe, K.; Wake, C.P.; Huntington, T.G.; Luo, L.; Schwartz, M.D.; Sheffield, J.; Wood, E.; Anderson, B.; Bradbury, J.; DeGaetano, A.; et al. Past and future changes in climate and hydrological indicators in the US Northeast. Clim. Dyn. 2007, 28, 381–407. [Google Scholar] [CrossRef]
- Xie, Y.; Ahmed, K.F.; Allen, J.M.; Wilson, A.M.; Silander, J.A. Green-up of deciduous forest communities of northeastern North America in response to climate variation and climate change. Landsc. Ecol. 2015, 30, 109–123. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Silander, J.A. Deciduous forest responses to temperature, precipitation, and drought imply complex climate change impacts. Proc. Natl. Acad. Sci. USA 2015, 112, 13585–13590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, S.; Pilon, P.; Phinney, B.; Cavadias, G. The influence of autocorrelation on the ability to detect trend in hydrological series. Hydrol. Process. 2002, 16, 1807–1829. [Google Scholar] [CrossRef]
- Bronaugh, D.; Werner, A. Zyp: Zhang + Yue-Pilon Trends Package. 2013. Available online: cran.r-project.org/package=zyp (accessed on 2 May 2019).
- Sen, P.K. Estimates of the Regression Coefficient Based on Kendall’s Tau. J. Am. Stat. Assoc. 1968, 63, 1379–1389. [Google Scholar] [CrossRef]
- McLeod, A.I. Kendall: Kendall Rank Correlation and Mann-Kendall Trend Test. 2011. Available online: cran.r-project.org/package=Kendall (accessed on 2 May 2019).
- Cortés, J.; Mahecha, M.; Reichstein, M.; Brenning, A. Accounting for multiple testing in the analysis of spatio-temporal environmental data. Environ. Ecol. Stat. 2020, 27, 293–318. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]

| Name | Abbreviation | Calculation | Description |
|---|---|---|---|
| Time- integrated NDVI | TIN | TIN = accumulated daily NDVI between SOS and EOS above NDVI values at SOS and EOS (values between 0 and 100) | Accumulated photosynthetic activity during the growing season, indicating foliage productivity |
| Length of growing season | LOS | LOS = EOS − SOS | Amount of time between spring green-up and autumn senescence, or the amount of time in a year in which trees are photosynthetically productive |
| Start of season date | SOS | SOS = date that NDVI exceeds the backward-looking delayed-moving-average trend from the previous 18 observations, interpolated within 14-day composites | Green-up of vegetation. This does not represent a distinct phenophase, but general ‘greening’ of vegetation that occurs in the spring |
| End of season date | EOS | EOS = date that NDVI becomes less than the forward-looking delayed-moving-average trend from the next 12 observations, interpolated within 14-day composites | Brown-down of vegetation. This does not represent a distinct phenophase, but a general ‘browning’ of vegetation that occurs in the fall |
| Name | Definition | Aggregate |
|---|---|---|
| Maximum temperature | Maximum air temperature at 2 m | Monthly Mean |
| Minimum temperature | Minimum air temperature at 2 m | Monthly Mean |
| Mean temperature | Mean air temperature at 2 m (mean of maximum and minimum air temperature) | Monthly Mean |
| Precipitation | Depth (mm) of precipitation in water equivalent | Monthly Sum |
| Snowpack | Weight (kg/m2) of on-ground snow, converted to water for measurement standardization | Monthly Sum |
| Spring heating | The sum of daily temperatures above 4 °C for the winter and spring (1 January to 31 May) | Annual Sum |
| Summer heating | The sum of daily temperatures above 4 °C during the winter, spring and summer (1 January to 31 July) | Annual Sum |
| Fall chilling | The sum of daily temperatures below 20 °C during the summer and fall (1 August to 31 October) | Annual Sum |
| Root-freeze risk | The number of days with minimum air temperatures below 0 °C and snow-water equivalent depth of 0 | Monthly Sum |
| SPEI (standardized precipitation—evapotranspiration index) | Index (mean = 0, SD = 1) of moisture stress, calculated using a 12-month rear-looking window [45,46] | Monthly (within ‘SPEI’ R package) |
| Day length | The amount of time between dawn and disk (s) | Monthly Mean |
| Solar radiation | Mean incident radiation flux density (W/m2) | Monthly Mean |
| Forest Growth | Model Inputs | Preliminary Model | Final Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Month | N | m | mtry | RMSE | pR2 | m | mtry | RMSE | pR2 | |
| TIN | Jany−1–Nov | 415,881 | 197 | 25 | 0.76 | 69.17 | 15 | 9 | 0.44 | 86.69 |
| LOS | Jany−1–Nov | 411,987 | 197 | 25 | 0.90 | 64.72 | 15 | 10 | 0.57 | 84.06 |
| SOS | Jany−1–Jun | 393,369 | 151 | 100 | 0.86 | 63.34 | 15 | 13 | 0.62 | 80.26 |
| EOS | Jany−1–Nov | 401,890 | 197 | 85 | 0.89 | 63.60 | 15 | 10 | 0.64 | 80.34 |
| Rank | TIN | LOS | SOS | EOS | ||||
|---|---|---|---|---|---|---|---|---|
| Variable | Imp. | Variable | Imp. | Variable | Imp. | Variable | Imp. | |
| 1 | Acc. Heat. | 21.03 | Acc. Heat. | 29.49 | Acc. Heat. | 29.71 | Acc. Chill | 35.10 |
| 2 | Acc. Chill. | 20.19 | Acc. Chill | 24.36 | Acc. Heat.y−1 | 17.86 | Acc. Heat.y−1 | 13.27 |
| 3 | Acc. Chill.y−1 | 16.76 | Acc. Heat.y−1 | 12.55 | Acc. Chill.y−1 | 17.64 | Acc. Chill.y−1 | 13.11 |
| 4 | Acc. Heat.y−1 | 14.77 | Acc. Chill.y−1 | 8.22 | TME.APR | 4.08 | Acc. Heat. | 11.87 |
| 5 | TME.SEP | 6.23 | TME.SEP | 4.31 | TMN.DECy−1 | 3.53 | TMN.MAR | 3.67 |
| 6 | TME.OCTy−1 | 3.71 | TME.MAY | 3.48 | TME.FEBy−1 | 3.33 | TME.MAR | 3.20 |
| 7 | TME.NOVy−1 | 2.84 | TME.AUG | 3.45 | TMX.JULy−1 | 3.32 | TME.SEP | 3.02 |
| 8 | TME.JUL | 2.41 | TME.JUL | 2.90 | TME.MAR | 3.30 | TME.SEPy−1 | 2.83 |
| 9 | TMN.APRy−1 | 2.37 | TME.APR | 2.58 | TMN.JAN | 3.10 | TME.NOVy−1 | 2.65 |
| 10 | TME.JUN | 2.28 | TMX.OCTy−1 | 1.84 | TME.NOVy−1 | 3.06 | TMX.FEBy−1 | 2.61 |
| 11 | TME.SEPy−1 | 1.74 | TME.NOVy−1 | 1.73 | TME.AUGy−1 | 2.49 | TME.MAY | 2.37 |
| 12 | TMN.AUGy−1 | 1.73 | TME.OCT | 1.50 | TME.JULy−1 | 2.46 | TME.OCT | 1.88 |
| 13 | TME.AUG | 1.61 | TMN.APRy−1 | 1.42 | PRT.NOVy−1 | 2.35 | TME.FEBy−1 | 1.73 |
| 14 | TMN.AUG | 1.18 | TME.APRy−1 | 1.17 | TMN.MARy−1 | 2.24 | TME.APR | 1.53 |
| 15 | TMN.JUL | 1.15 | TME.OCTy−1 | 1.00 | TMN.FEBy−1 | 1.73 | TME.FEB | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanuk, M.A.; Danby, R.K. Accumulated Heating and Chilling Are Important Drivers of Forest Phenology and Productivity in the Algonquin-to-Adirondacks Conservation Corridor of Eastern North America. Forests 2021, 12, 282. https://doi.org/10.3390/f12030282
Stefanuk MA, Danby RK. Accumulated Heating and Chilling Are Important Drivers of Forest Phenology and Productivity in the Algonquin-to-Adirondacks Conservation Corridor of Eastern North America. Forests. 2021; 12(3):282. https://doi.org/10.3390/f12030282
Chicago/Turabian StyleStefanuk, Michael A., and Ryan K. Danby. 2021. "Accumulated Heating and Chilling Are Important Drivers of Forest Phenology and Productivity in the Algonquin-to-Adirondacks Conservation Corridor of Eastern North America" Forests 12, no. 3: 282. https://doi.org/10.3390/f12030282
APA StyleStefanuk, M. A., & Danby, R. K. (2021). Accumulated Heating and Chilling Are Important Drivers of Forest Phenology and Productivity in the Algonquin-to-Adirondacks Conservation Corridor of Eastern North America. Forests, 12(3), 282. https://doi.org/10.3390/f12030282







