Radial Growth Response of European Larch Provenances to Interannual Climate Variation in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Climate
2.2. Sampling and Development of Chronologies
2.3. Grouping of Provenances and Climate–Growth Relationships
3. Results
3.1. Statistics of the Chronologies
3.2. Grouping of Provenances
3.3. Growth–Climate Relationships in Provenance Trials
3.4. Interpopulational Variability of Radial Growth Responses of Provenances to Climatic Conditions at the Provenance Trials
4. Discussion
4.1. Chronology Statistics
4.2. Common Radial Growth Responses of the Larch Provenances Growing at Three Provenance Trials
4.3. Differences in Radial Growth Responses between the Larch Provenances at the Individual Provenance Trials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kulej, M. Zmienność oraz wartość hodowlana modrzewi różnych pochodzeń z terenu Polski w warunkach Beskidu Sądeckiego. Zesz. Nauk. AR Krakowie Rozpr. 2001, 273, 1–159. [Google Scholar]
- Kulej, M. Resistance of larches of Polish provenances to larch crank Lachnellula willkommii (Hartig.) Dennis under mountain conditions of the Sącz Beskid. Electron. J. Pol. Agric. Univ. 2006, 9, 29. [Google Scholar]
- Socha, J.; Kulej, M. Variation of the tree from factor and taper in European larch of Polish provenances tested under conditions of the Beskid Sądecki mountain range (southern Poland). J. For. Sci. 2007, 53, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, J.; Matras, J.; Kulej, M.; Banach, J.; Barzdajn, W.; Kowalkowsk, W.; Szeligowski, H.; Buraczyk, W.; Chałupka, W.; Chmura, D.; et al. Genetyczne Uwarunkowania Procesów Adaptacyjnych u Wybranych Gatunków w Kontekście Przewidywanych Zmian Klimatycznych. Sprawozdanie Końcowe; IBL—ZHLiGDL: Sękocin Stary, Poland, 2015; pp. 1–146. Available online: https://tbr.lasy.gov.pl/apex/f?p=102,3,,,NO,,P3_TEMAT,3482 (accessed on 8 February 2021).
- Rubner, K. Die Ergebnisse zweier Lärchenherkunftsversuche im Tharandter Wald. Thar. Forst. Jb. 1938, 89, 465–491. [Google Scholar]
- Hunter-Blair, J. The polish larch (Larix decidua var. polonica). Scot. For. 1948, 3/4, 21–25. [Google Scholar]
- Göhrn, V. Proveniensforsög med Laerk. Forstl. Forsøgsv. Danmark 1956, 23, 1–124. [Google Scholar]
- Kocięcki, S. Porównanie dwóch upraw doświadczalnych modrzewia polskiego założonych w Czechosłowacji i Polsce. Sylwan 1959, 103, 39–43. [Google Scholar]
- Šťastný, T. Modifikovanie prejavu genetickej podstaty rastu Larix decidua Mill. vplyvom rozdielnych podmienok prostredia. Lesn. Štúd. VÚLH Zvolen 1971, 10, 1–101. [Google Scholar]
- Szeligowski, H. Proweniencyjne różnice w odporności modrzewia europejskiego (Larix decidua Mill.) na suszę. Sylwan 2001, 14, 65–78. [Google Scholar]
- Wilczyński, S.B.; Kulej, M. The influence of climate on the radial increment of larches of different provenances on the basis of the experiment in the Carpathian Mountains in Southern Poland. Eur. J. Forest. Res. 2013, 132, 919–929. [Google Scholar] [CrossRef]
- Szymański, N.; Wilczyński, S. Wrażliwość wybranych pochodzeń modrzewia europejskiego (Larix decidua Mill.) na warunki klimatyczne Wyżyny Kieleckiej (centralna Polska). EPISTEME Czasop. Nauk. Kult. 2016, 30, 149–160. [Google Scholar]
- Wilczyński, S.; Szymański, N.; Olejnik, M. Adaptacja wybranych pochodzeń modrzewia europejskiego do klimatu nizin centralnej Polski. Sylwan 2016, 160, 656–665. [Google Scholar]
- Serre, F. The dendroclimatological value of the European larch (Larix decidua Mill.) in the French Maritime Alps. Tree Ring Bull. 1978, 38, 25–34. [Google Scholar]
- Eckstein, D.; Aniol, R.W. Dendroclimatological reconstruction of the summer temperatures for an alpine region. Mitteil. Forst. Bundes Versuch Wien 1981, 142, 391–398. [Google Scholar]
- Rolland, C.; Petitcolas, V.; Michalet, R. Changes in radial tree growth for Picea abies; Larix decidua; Pinus cembra and Pinus uncinata near the alpine timberline since 1750. Trees 1998, 13, 40–53. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Age-dependent tree-ring growth response to climate in Larix decidua and Pinus cembra. Ecology 2004, 85, 730–740. [Google Scholar] [CrossRef]
- Carrer, M.; Urbinati, C. Long-term change in the sensitivity of tree-ring growth to climate forcing in Larix decidua. New Phytol. 2006, 170, 861–872. [Google Scholar] [CrossRef]
- Frank, D.; Esper, J. Characterization and climate response patterns of a high-elevation; multi-species tree-ring network in the European Alps. Dendrochronologia 2005, 22, 107–121. [Google Scholar] [CrossRef]
- Büntgen, U.; Frank, D.C.; Kaczka, R.J.; Verstege, A.; Zwijacz-Kozica, T.; Esper, J. Growth responses to climate in a multi-species tree-ring network in the Western Carpathian Tatra Mountains; Poland and Slovakia. Tree Physiol. 2007, 27, 689–702. [Google Scholar] [CrossRef] [Green Version]
- Danek, M. Wpływ warunków klimatycznych na szerokość przyrostów rocznych modrzewia (Larix decidua Mill.) rosnącego w północnej części województwa małopolskiego. Sylwan 2009, 153, 768–776. [Google Scholar]
- Wilczyński, S. Uwarunkowania przyrostu radialnego wybranych gatunków drzew z Wyżyny Kieleckiej w świetle analiz dendroklimatologicznych. Zesz. Nauk. UR Krakowie Rozpr. 2010, 1–464. [Google Scholar]
- Coppola, A.; Leonelli, G.; Salvatore, M.C.; Pelfini, M.; Baroni, C. Weakening climatic signal since mid-20th century in European larch tree-ring chronologies at different altitudes from the Adamello−Presanella Massif (Italian Alps). Quat. Res. 2012, 77, 344–354. [Google Scholar] [CrossRef]
- Koprowski, M. Long-term increase of March temperature has no negative impact on tree-rings of European larch (Larix decidua) in lowland Poland. Trees 2012, 26, 1895–1903. [Google Scholar] [CrossRef] [Green Version]
- Bijak, S. Climate signal in the radial growth of selected coniferous species from the Forest Experimental Station in Rogów. Leśn. Pr. Bad. 2013, 74, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef] [PubMed]
- Vitas, A. A Dendroclimatological Analysis of European Larch (Larix decidua Mill.) from Lithuania. Balt. For. 2015, 20, 65–72. [Google Scholar]
- Oleksyn, J.; Fritts, H. Influence of climatic factors upon tree-rings of Larix decidua and L. decidua x L. kaempferi from Puławy; Poland. Trees-Struct. Funct. 1991, 5, 75–82. [Google Scholar] [CrossRef]
- Oleksyn, J.; Fritts, H.C.; Hughes, M.K. Tree-ring analysis of different Pinus sylvestris provenances; Quercus robur; Larix decidua and L. decidua x L. kaempferi affected by air pollution. Arbor. Kórn. 1993, 38, 89–109. [Google Scholar]
- Klisz, M.; Koprowski, M.; Ukalska, J.; Nabais, C. Does the genotype have a significant effect on the formation of intra-annual density fluctuations? A case study using Larix decidua from Northern Poland. Front. Plant Sci. 2016, 7, 691. [Google Scholar] [CrossRef] [Green Version]
- Bolte, A.; Ammer, C.; Löf, M.; Nabuurs, G.-J.; Schall, P.; Spathelf, P. Adaptive Forest Management, A Prerequisite for Sustainable Forestry in the Face of Climate Change. In Sustainable Forest Management in a Changing World, a European Perspective; Spathelf, P., Ed.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK; New York, NY, USA, 2010; pp. 114–140. [Google Scholar]
- Andrzejczyk, T.; Bolibok, L.; Drozdowski, S.; Szeligowski, H. Sposób powstawania; struktura i produkcyjności drzewostanów bukowo-modrzewiowych w Polsce. Leśn. Pract. Bad. 2011, 72, 301–310. [Google Scholar]
- Tyszkiewicz, S. Las jako przedmiot gospodarki. Ważniejsze gatunki drzew leśnych jako przedmiot uprawy. In Hodowla i Uprawa Lasu; Tyszkiewicz, S., Obmiński, Z., Eds.; PWRiL: Warszawa, Poland, 1963; pp. 649–804. [Google Scholar]
- Kocięcki, S. Badania nad Wzrostem i Formą Modrzewia i Przydatnością Różnych Pochodzeń w Poszczególnych Krainach Przyrodniczo-leśnych; IBL: Warszawa, Poland, 1977; pp. 1–26. [Google Scholar]
- Distribution map of European Larch (Larix decidua). EUFORGEN. 2009. Available online: http://www.euforgen.org/species/larix-decidua (accessed on 1 August 2018).
- Poland—Hipsometric Map. Available online: https://commons.wikimedia.org/wiki/File:Poland-hipsometric_map.jpg (accessed on 10 February 2020).
- Institute of Meteorology and Water Management National Research Institute, Poland. Available online: https://www.imgw.pl/index.php/en (accessed on 10 February 2020).
- Hill, T.; Lewicki, P. Statistics, Methods and Applications; StatSoft: Tulsa, OK, USA, 2006; 832p. [Google Scholar]
- Cybis Elektronik & Data AB. Technical Writing; Software Development; Photography and Dendrochronology Software. Available online: https://www.cybis.se/ (accessed on 10 February 2021).
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 188–189. [Google Scholar]
- Douglass, A.E. Evidence of climate effects in the annual rings of trees. Ecology 1920, 1, 24–32. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic: London, UK, 1976; 582p. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series; with applications in dendroclimatology and hydrometeorology. J. Appl. Meteorol. Climatol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- De Witt, E.; Ames, M. Tree-Ring Chronologies of Eastern North America; Chronology Series IV; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1978; Volume 1, pp. 1–42. [Google Scholar]
- Richter, K.; Eckstein, D. The dendrochronological signal of pine trees (Pinus spp.) in Spain. Tree Ring Bull. 1991, 51, 1–13. [Google Scholar]
- Mazza, G.; Gallucci, V.; Manetti, M.C.; Urbinati, C. Climate-growth relationships of silver fir (Abies alba Mill.) in marginal populations of Central Italy. Dendrochronologia 2014, 32, 181–190. [Google Scholar] [CrossRef]
- Roibu, C.-C.; Popa, I.; Kirchhefer, A.J.; Palaghianu, C. Growth responses to climate in a tree-ring network of European beech (Fagus sylvatica L.) from the eastern limit of its natural distribution area. Dendrochronologia 2017, 42, 104–116. [Google Scholar] [CrossRef]
- StatSoft Polska. Available online: https//www.statsoft.pl/ (accessed on 8 February 2021).
- Holmes, R.L.; Lough, J.M. RESPO—Response and Correlation Function; Laboratory of Tree-Ring Research, University of Arizona: Tucson, AZ, USA, 1999. [Google Scholar]
- Danek, M.; Chuchro, M.; Walanus, A. Variability in larch (Larix decidua Mill.) tree-ring growth response to climate in the Polish Carpathian Mountains. Forests 2017, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Danek, M.; Chuchro, M.; Walanus, A. Tree-ring growth of larch (Larix decidua Mill.) in the Polish Sudetes—The influence of altitude and site-related factors on the climate–growth relationship. Forests 2018, 9, 663. [Google Scholar] [CrossRef] [Green Version]
- Sakulich, J.B. A Dendrochronological Approach for Analyzing the Geographic Range Structure of Tree Species. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2011. [Google Scholar]
- Rötzer, T.; Grote, R.; Pretzsch, H. The timing of bud burst and its effect on tree growth. Int. J. Biometeorol. 2004, 48, 109–118. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Gričar, J.; Seo, J.-W.; Rathgeber, C.B.K.; Anfodillo, T. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions, understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Wilczyński, S.; Wertz, B. Sygnał klimatyczny w seriach przyrostów radialnych drzew na przykładzie jodły pospolitej i modrzewia europejskiego. Stud. Mat. CEPL Rogowie 2012, 1, 66–74. [Google Scholar]
- Jansons, Ā.; Matisons, R.; Puriņa, L.; Neimane, U.; Jansons, J. Relationships between climatic variables and tree-ring width of European beech and European larch growing outside of their natural distribution area. Silva. Fenn. 2015, 49, 1255. [Google Scholar] [CrossRef] [Green Version]
- Moser, L.; Fonti, P.; Büntgen, U.; Esper, J.; Luterbacher, J.; Franzen, J.; Frank, D. Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol. 2009, 30, 225–233. [Google Scholar] [CrossRef]
- Holstener-Jørgensen, H. Influences of forest management and drainage on groundwater fluctuations. In Forest Hydrology; Sopper, W.E., Lull, H.W., Eds.; Pergamon Press: Oxford, UK, 1967; pp. 325–480. [Google Scholar]
- Jones, H. Stomatal control of photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 387–398. [Google Scholar] [CrossRef]
- Major, J.E.; Johnsen, K.H. Shoot water relations of mature black spruce families displaying a genotype × environment interaction in growth rate. III. Diurnal patterns as influenced by vapour pressure deficit and internal water status. Tree Physiol. 2001, 21, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Pichler, P.; Oberhuber, W. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. For. Ecol. Manag. 2007, 242, 688–699. [Google Scholar] [CrossRef]
- Danek, M.; Danek, T. Zastosowanie alternatywnych metod przetwarzania danych w analizie dendroklimatologicznej modrzewia Larix decidua Mill. z Polski Południowej. Sylwan 2011, 155, 147–158. [Google Scholar]
- Wilczyński, S.; Szymański, N.; Wertz, B.; Muter, E. Wpływ wieku na odpowiedź przyrostową drzew na czynnik klimatyczny na przykładzie modrzewia europejskiego. Stud. Mat. CEPL Rogowie 2014, 40, 256–264. [Google Scholar]
- Feliksik, E.; Wilczyński, S. Wpływ warunków termicznych i pluwialnych na przyrost drewna modrzewi (Larix decidua Mill.). Sylwan 1998, 142, 85–90. [Google Scholar]
- Hoffmann, G.; Lyr, H. Charakterisierung des Wachstumsverhaltens von Pflanzen durch Wachstumsschemata. Flora 1973, 162, 81–98. [Google Scholar] [CrossRef]
- Jaworski, A. Hodowla Lasu. Charakterystyka Hodowlana Drzew i Krzewów Leśnych; PWRiL: Warszawa, Poland, 2011; 556p. [Google Scholar]
- Holzer, K. The evolution of Alpine Norway spruce during immigration into high altitudes and its consequences. In Norway Spruce Provenances and Breeding, Proceedings of the IUFRO Symposium (WP S2.2-11), Riga, Latvia, 14–18 September 1993; Rone, V., Ed.; FAO: Rome, Italy, 1994; pp. 68–78. [Google Scholar]
- King, G.M.; Gugerli, F.; Fonti, P.; Frank, D.C. Tree growth response along an elevational gradient, climate or genetics? Oecologia 2013, 173, 1587–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simak, M. Photo- and thermoperiodic response of different Larch provenances (Larix decidua Mill.). Stud. Forest. Suec. 1970, 86, 1–31. [Google Scholar]
- Wilczyński, S.; Durło, G.; Feliksik, E. Przymrozki wczesne i późne na Kopciowej (Beskid Sadecki). Acta Agr. Silv. Ser. Silv. 2005, 43, 65–76. [Google Scholar]
- Owens, J.N.; Molder, M. Bud development in Larix occidentalis. II. Cone differentiation and early development. Can. J. Bot. 1979, 57, 1557–1572. [Google Scholar] [CrossRef]
- Fober, H. Relation between climatic factors and Scots pine (Pinus sylvestris) cone crops in Poland. Arbor. Kórn. 1976, 21, 367–374. [Google Scholar]
- Chałupka, W.; Giertych, M.; Królikowski, Z. The effect of cone crops on growth in Scots pine on tree diameter increment. Arbor. Kórn. 1976, 21, 361–366. [Google Scholar]
- Sabor, J. Elementy Genetyki i Hodowli Drzew Leśnych; CIPL: Warszawa, Poland, 2006; 672p. [Google Scholar]
- Wibig, J.; Brzóska, B.; Curyło, A.; Jaczewski, A.; Konca-Kędzierska, K.; Liszewska, M.; Pianko-Kluczyńska, M. Dynamiczne scenariusze zmian klimatu dla Polski na lata 2011–2030. In Warunki Klimatyczne i Oceanograficzne w Polsce i na Bałtyku Południowym—Spodziewane Zmiany i Wytyczne do Opracowania Strategii Adaptacyjnych w Gospodarce Krajowej; Wibig, J., Jakusik, E., Eds.; IMiGW-PIB: Warszawa, Poland, 2012; pp. 93–123. [Google Scholar]
- IPCC. Climate Change 2014, Impacts; Adaptation; and Vulnerability. Part, B., Regional Aspects; Cambridge University Press: Cambridge, NY, USA, 2014; 688p. [Google Scholar]
- Battipaglia, G.; Saurer, M.; Cherubini, P.; Siegwolf, R.T.W.; Cotrufo, M.F. Tree rings indicate different drought resistance of a native (Abies alba Mill.) and a nonnative (Picea abies (L.) Karst.) species co-occurring at a dry site in Southern Italy. For. Ecol. Manag. 2009, 257, 820–828. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Rathgeber, C.B.K.; Ulrich, E. Sensitivity of French temperate coniferous forests to climate variability and extreme events (Abies alba; Picea abies and Pinus sylvestris). J. Veg. Sci. 2010, 21, 364–376. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T. Assessment of cambial activity and xylogenesis by microsampling tree species, an example at the alpine timberline. IAWA J. 2006, 27, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wodzicki, T.J. Annual ring of wood formation and seasonal changes of natural growth-inhibitors in larch. Acta. Soc. Bot. Pol. 1965, 34, 117–151. [Google Scholar] [CrossRef] [Green Version]
- Maciejowski, K. Modrzew w lasach polskich. Sylwan 1956, 100, 6–50. [Google Scholar]
- Bałut, S. Udział modrzewia w lasach polskich w XVIII i XIX wieku. Sylwan 1967, 111, 75–87. [Google Scholar]
- Bałut, S. Zmienność szyszek modrzewia jako podstawa wyróżniania pochodzeń. Acta. Agr. Silv. Ser. Silv. 1969, 9, 3–109. [Google Scholar]
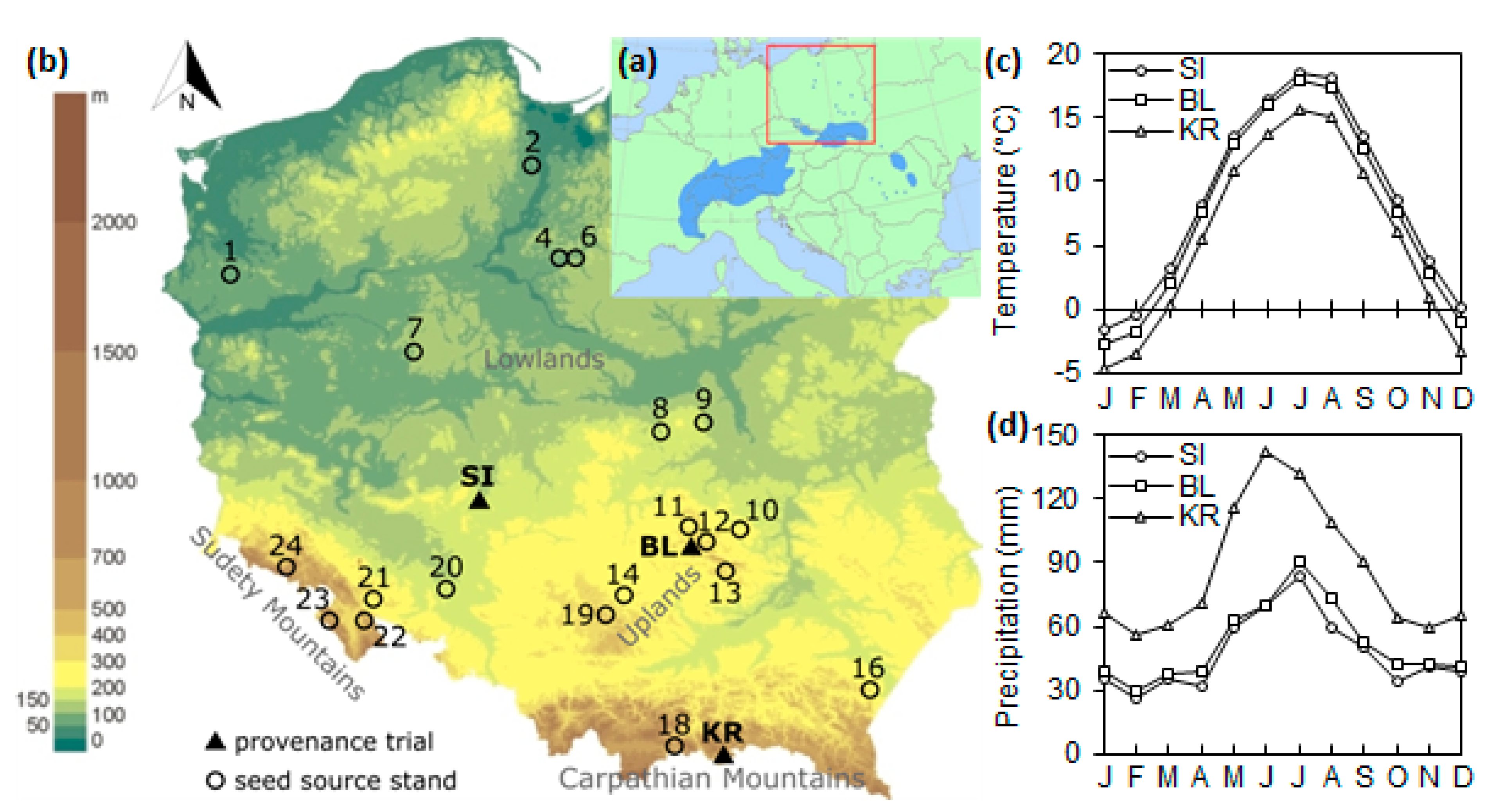


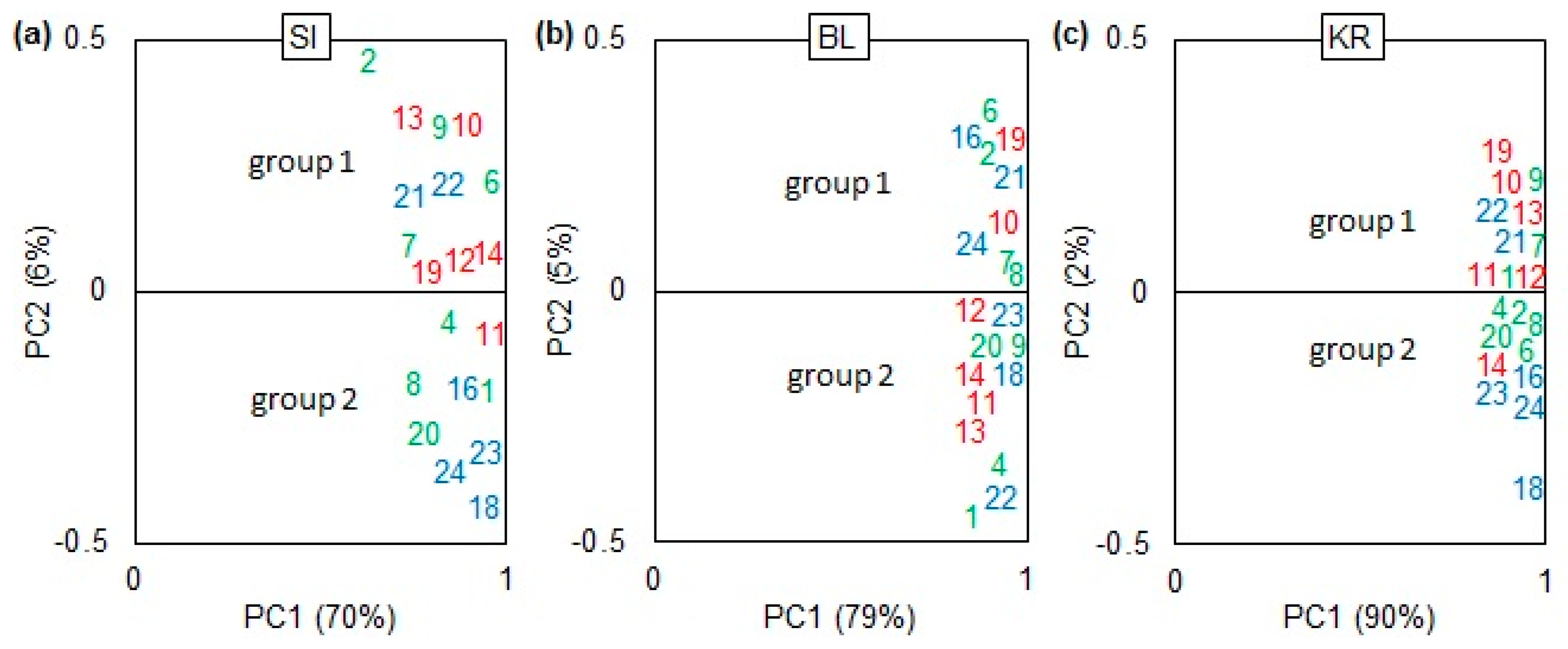
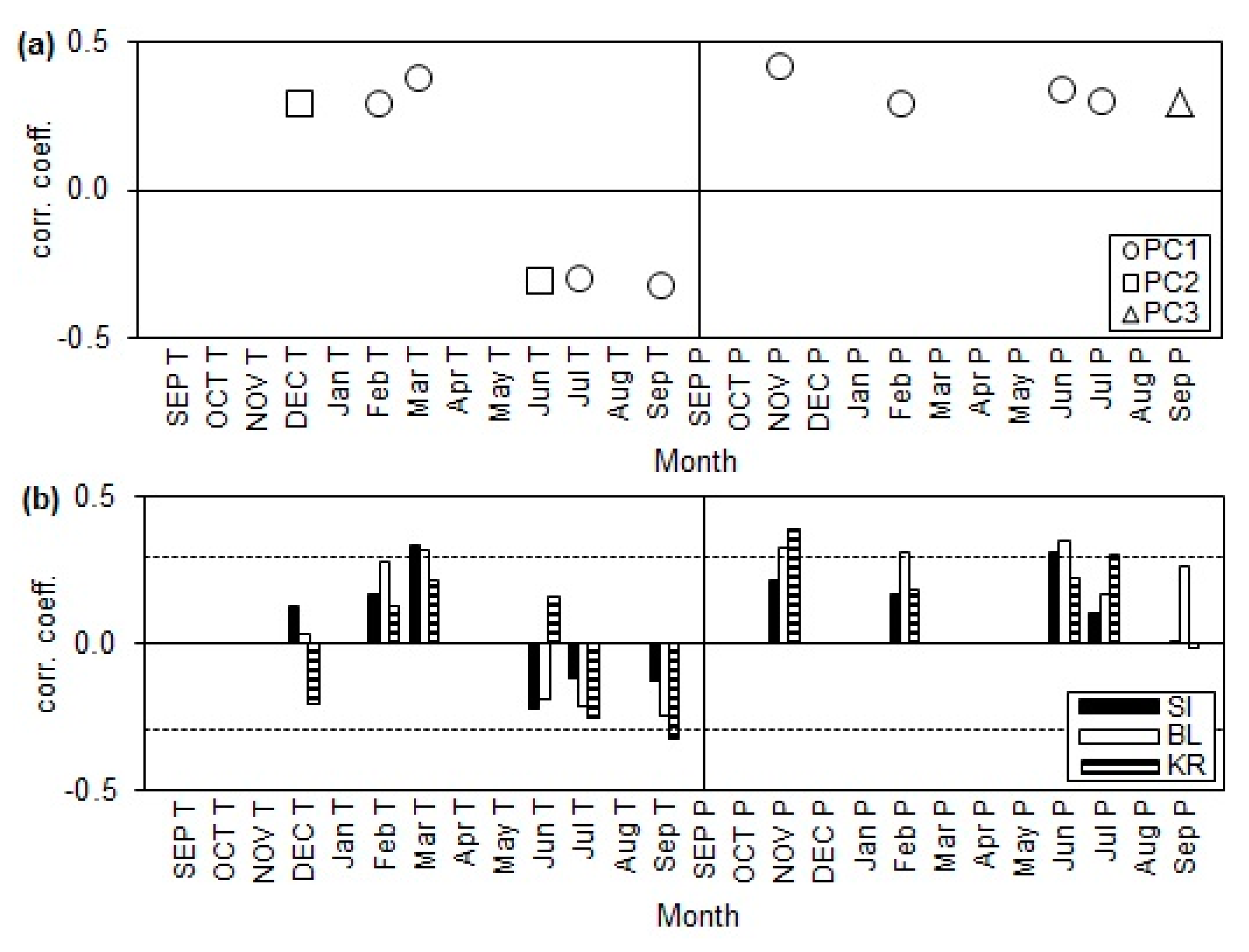

| Location | Siemianice (SI) | Bliżyn (BL) | Krynica (KR) |
|---|---|---|---|
| Latitude coordinate Longitude coordinate | 51°13′ N 18°03′ E | 51°02′ N 20°40′ E | 49°20′ N 20°59′ E |
| Altitude (m a.s.l.) | 170 | 305 | 790 |
| Slope aspect and inclination | flat | flat | SE, W, 5° (ridge) |
| Mean annual air temperature (°C) | 8.5 | 7.6 | 5.6 |
| Total annual precipitation (mm) | 565 | 618 | 1032 |
| Soil type | Haplic Podzol | Gleyic Podzol | Haplic Cambisol |
| Parent material | glacial sandstone | glacial sandstone | Carpathian flysch |
| Soil texture | sand | clay loam | silty clay loam |
| Humus type | moder/mor | mor | mull |
| Provenance trial size (m2) | 49,100 | 28,000 | 40,800 |
| Number of provenances at provenance trial | 21 | 23 | 21 |
| Plots per provenance (replications) | 5 | 3 | 5 |
| Plot size (m2) | 384 | 400 | 400 |
| Seedlings per plot | 96 | 100 | 121 |
| Planting spacing (m) | 2.0 × 2.0 | 2.0 × 2.0 | 1.8 × 1.8 |
| Planting year | 1967 | 1968 | 1967 |
| Current number of trees per provenance | 37–114 | 9–92 | 6–162 |
| No * | Name of Provenance | Region of Poland | Coordinate of Seed Stand | Elevation of Seed Stand (m a.s.l.) | Annual Air Temperature (°C) | Total Annual Precipitation (mm) | Mean DBH (mm) ** | Mean H (m) ** | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Latitude N | Longitude E | SI | BL | KR | SI | BL | KR | ||||||
| 1 | Myślibórz | northwestern lowland | 52°54′ | 14°52′ | 60 | 8.8 | 568 | 316.4 | 214.2 | 244.1 | 26.3 | 21.1 | 23.8 |
| 2 | Pelplin | north lowland | 53°56′ | 18°43′ | 60 | 8.2 | 510 | 309.1 | 180.9 | 260.9 | 24.6 | 19.2 | 23.7 |
| 4 | Płonne | north lowland | 53°07′ | 19°04′ | 60 | 8.4 | 540 | 325.8 | 199.3 | 258.2 | 25.9 | 20.4 | 24.8 |
| 6 | Tomkowo | north lowland | 53°07′ | 19°04′ | 60 | 8.4 | 540 | 297.8 | 230.7 | 268.3 | 24.7 | 21.5 | 23.9 |
| 7 | Czerniejewo | western lowland | 52°26′ | 17°30′ | 110 | 8.8 | 516 | 288.2 | 197.9 | 260.2 | 25.0 | 20.4 | 23.6 |
| 8 | Rawa | central lowland | 51°48′ | 20°15′ | 180 | 8.3 | 540 | 322.0 | 250.4 | 260.3 | 24.9 | 21.4 | 21.9 |
| 9 | Grójec | central lowland | 51°52′ | 20°52′ | 180 | 8.5 | 565 | 342.1 | 239.4 | 257.9 | 23.8 | 20.9 | 22.7 |
| 10 | Marcule | central upland | 51°08′ | 21°15′ | 210 | 7.8 | 662 | 327.8 | 235.7 | 258.7 | 25.0 | 20.7 | 21.3 |
| 11 | Skarżysko | Swietokrzyskie Mts | 51°10′ | 20°46′ | 375 | 7.6 | 687 | 308.9 | 253.8 | 245.6 | 24.8 | 22.9 | 22.4 |
| 12 | Bliżyn | Swietokrzyskie Mts | 51°05′ | 20°45′ | 300 | 7.6 | 618 | 319.8 | 214.0 | 247.9 | 26.5 | 21.1 | 23.0 |
| 13 | Chełmowa | Swietokrzyskie Mts | 50°55′ | 21°04′ | 350 | 6.8 | 769 | 400.6 | 234.1 | 259.1 | 28.2 | 20.9 | 23.3 |
| 14 | Moskorzew | central upland | 50°39′ | 19°56′ | 250 | 8.3 | 627 | 300.0 | 218.9 | 259.4 | 22.8 | 22.1 | 24.2 |
| 16 | Hołubla | Carpathian foothill | 49°48′ | 22°48′ | 325 | 8.2 | 670 | 330.5 | 202.0 | 266.5 | 26.4 | 19.4 | 24.0 |
| 18 | Krościenko | Carpathian Mts | 49°27′ | 20°26′ | 650 | 7.0 | 847 | 266.7 | 205.6 | 226.5 | 23.7 | 20.2 | 22.0 |
| 19 | Pilica | central upland | 50°28′ | 19°40′ | 450 | 8.0 | 655 | 285.3 | 231.7 | 254.9 | 23.6 | 21.8 | 23.2 |
| 20 | Prószków | western lowland | 50°35′ | 17°52′ | 180 | 9.0 | 604 | 319.1 | 182.0 | 249.9 | 24.7 | 19.5 | 24.5 |
| 21 | Henryków | Sudety foothill | 50°41′ | 17°01′ | 325 | 8.1 | 717 | 320.4 | 245.5 | 259.8 | 25.9 | 20.4 | 22.7 |
| 22 | Kłodzko | Sudety Mts | 50°22′ | 16°45′ | 375 | 7.6 | 592 | 311.8 | 191.8 | 277.5 | 25.5 | 20.9 | 24.0 |
| 23 | Szczytna | Sudety Mts | 50°25′ | 16°26′ | 525 | 6.5 | 921 | 289.0 | 214.8 | 242.0 | 24.4 | 23.8 | 24.3 |
| 24 | Kowary | Sudety Mts | 50°48′ | 15°50′ | 525 | 7.2 | 1028 | 323.4 | 208.0 | 245.9 | 26.2 | 21.3 | 24.4 |
| Mean for provenance trial | 315.5 | 218.1 | 255.2 | 25.2 | 20.9 | 23.4 | |||||||
| No | Name of Provenance | Mean TRW (mm) ** | MS | rbt | EPS | SNR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SI | BL | KR | SI | BL | KR | SI | BL | KR | SI | BL | KR | SI | BL | KR | ||
| 1 | Myślibórz | 3.40 | 3.22 | 3.19 | 0.25 | 0.23 | 0.27 | 0.70 | 0.67 | 0.57 | 0.98 | 0.98 | 0.96 | 45.7 | 39.8 | 26.5 |
| 2 | Pelplin | 3.35 | 2.43 | 3.12 | 0.27 | 0.26 | 0.35 | 0.56 | 0.63 | 0.75 | 0.96 | 0.95 | 0.98 | 25.7 | 19.0 | 58.7 |
| 4 | Płonne | 2.96 | 2.86 | 3.08 | 0.26 | 0.25 | 0.32 | 0.60 | 0.69 | 0.69 | 0.97 | 0.98 | 0.98 | 30.4 | 43.4 | 43.5 |
| 6 | Tomkowo | 2.81 | 2.97 | 3.03 | 0.28 | 0.20 | 0.34 | 0.58 | 0.56 | 0.74 | 0.97 | 0.96 | 0.98 | 28.1 | 24.9 | 56.7 |
| 7 | Czerniejewo | 2.80 | 2.72 | 2.84 | 0.27 | 0.22 | 0.34 | 0.54 | 0.61 | 0.69 | 0.96 | 0.97 | 0.98 | 23.7 | 31.2 | 45.1 |
| 8 | Rawa | 3.05 | 2.77 | 3.00 | 0.25 | 0.18 | 0.34 | 0.55 | 0.68 | 0.81 | 0.96 | 0.98 | 0.99 | 24.5 | 43.3 | 87.2 |
| 9 | Grójec | 3.69 | 2.70 | 2.70 | 0.22 | 0.19 | 0.31 | 0.47 | 0.71 | 0.69 | 0.95 | 0.98 | 0.98 | 17.9 | 48.0 | 43.6 |
| 10 | Marcule | 3.50 | 3.00 | 3.35 | 0.26 | 0.19 | 0.31 | 0.61 | 0.59 | 0.73 | 0.97 | 0.97 | 0.98 | 31.6 | 29.3 | 54.4 |
| 11 | Skarżysko | 3.18 | 3.16 | 2.96 | 0.24 | 0.20 | 0.31 | 0.60 | 0.65 | 0.63 | 0.97 | 0.97 | 0.97 | 30.5 | 37.5 | 34.0 |
| 12 | Bliżyn | 2.95 | 2.84 | 2.97 | 0.23 | 0.18 | 0.32 | 0.38 | 0.72 | 0.66 | 0.93 | 0.98 | 0.97 | 12.4 | 51.4 | 38.1 |
| 13 | Chełmowa | 3.44 | 3.43 | 2.84 | 0.23 | 0.19 | 0.31 | 0.67 | 0.53 | 0.78 | 0.98 | 0.96 | 0.99 | 41.2 | 22.8 | 72.0 |
| 14 | Moskorzew | 3.08 | 3.08 | 2.87 | 0.25 | 0.20 | 0.33 | 0.55 | 0.52 | 0.48 | 0.96 | 0.96 | 0.95 | 24.9 | 21.9 | 18.5 |
| 16 | Hołubla | 4.01 | 2.68 | 3.24 | 0.29 | 0.19 | 0.34 | 0.63 | 0.57 | 0.81 | 0.97 | 0.96 | 0.99 | 34.3 | 26.3 | 83.7 |
| 18 | Krościenko | 2.94 | 2.80 | 2.82 | 0.32 | 0.21 | 0.37 | 0.75 | 0.63 | 0.77 | 0.98 | 0.97 | 0.99 | 61.0 | 34.7 | 66.3 |
| 19 | Pilica | 3.14 | 2.80 | 2.92 | 0.31 | 0.21 | 0.35 | 0.72 | 0.63 | 0.69 | 0.98 | 0.97 | 0.98 | 51.2 | 34.7 | 46.3 |
| 20 | Prószków | 3.35 | 2.54 | 2.89 | 0.27 | 0.20 | 0.34 | 0.54 | 0.59 | 0.68 | 0.96 | 0.97 | 0.98 | 23.0 | 29.3 | 42.8 |
| 21 | Henryków | 3.38 | 2.67 | 3.21 | 0.26 | 0.19 | 0.30 | 0.67 | 0.56 | 0.70 | 0.98 | 0.96 | 0.98 | 39.9 | 25.1 | 47.4 |
| 22 | Kłodzko | 3.24 | 2.76 | 3.12 | 0.27 | 0.22 | 0.30 | 0.55 | 0.64 | 0.65 | 0.96 | 0.97 | 0.97 | 24.1 | 35.4 | 24.6 |
| 23 | Szczytna | 3.00 | 3.07 | 2.78 | 0.31 | 0.20 | 0.32 | 0.55 | 0.71 | 0.72 | 0.96 | 0.98 | 0.98 | 24.1 | 48.2 | 51.6 |
| 24 | Kowary | 3.74 | 2.94 | 3.03 | 0.27 | 0.21 | 0.30 | 0.49 | 0.60 | 0.66 | 0.95 | 0.97 | 0.98 | 18.9 | 30.2 | 38.4 |
| Mean for provenance trial | 3.25 * | 2.87 * | 3.00 * | 0.26 * | 0.21 * | 0.32 * | 0.59 | 0.62 | 0.69 * | 0.96 | 0.97 | 0.98 | 30.7 | 33.8 | 49.0 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymański, N.; Wilczyński, S. Radial Growth Response of European Larch Provenances to Interannual Climate Variation in Poland. Forests 2021, 12, 334. https://doi.org/10.3390/f12030334
Szymański N, Wilczyński S. Radial Growth Response of European Larch Provenances to Interannual Climate Variation in Poland. Forests. 2021; 12(3):334. https://doi.org/10.3390/f12030334
Chicago/Turabian StyleSzymański, Norbert, and Sławomir Wilczyński. 2021. "Radial Growth Response of European Larch Provenances to Interannual Climate Variation in Poland" Forests 12, no. 3: 334. https://doi.org/10.3390/f12030334






