Coexistent Heteroblastic Needles of Adult Pinus canariensis C.Sm. ex DC. in Buch Trees Differ Structurally and Physiologically
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Leaf Anatomy
2.3. Chlorophyll Fluorescence Analyses
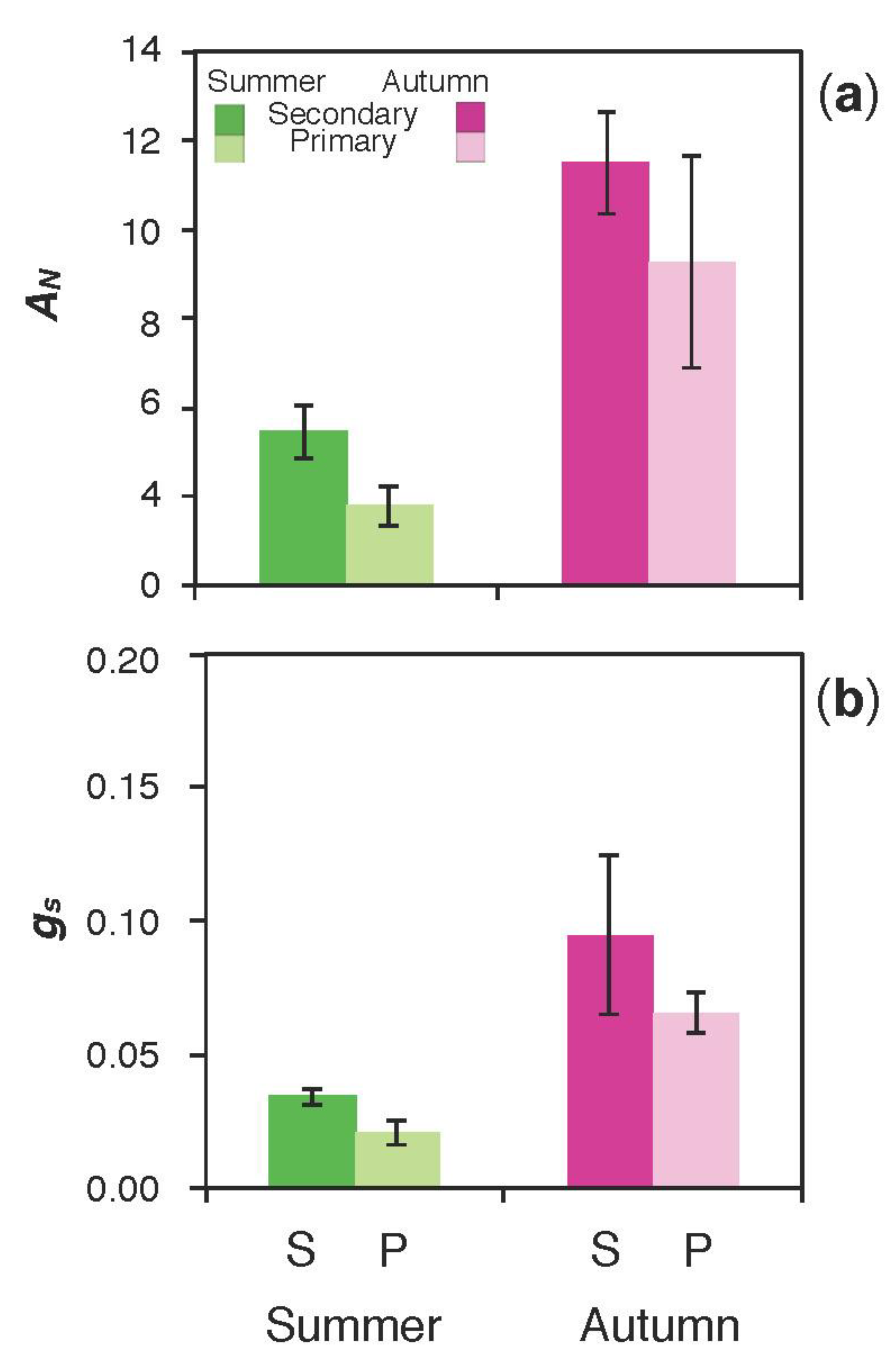
2.4. Gas Exchange Measurements
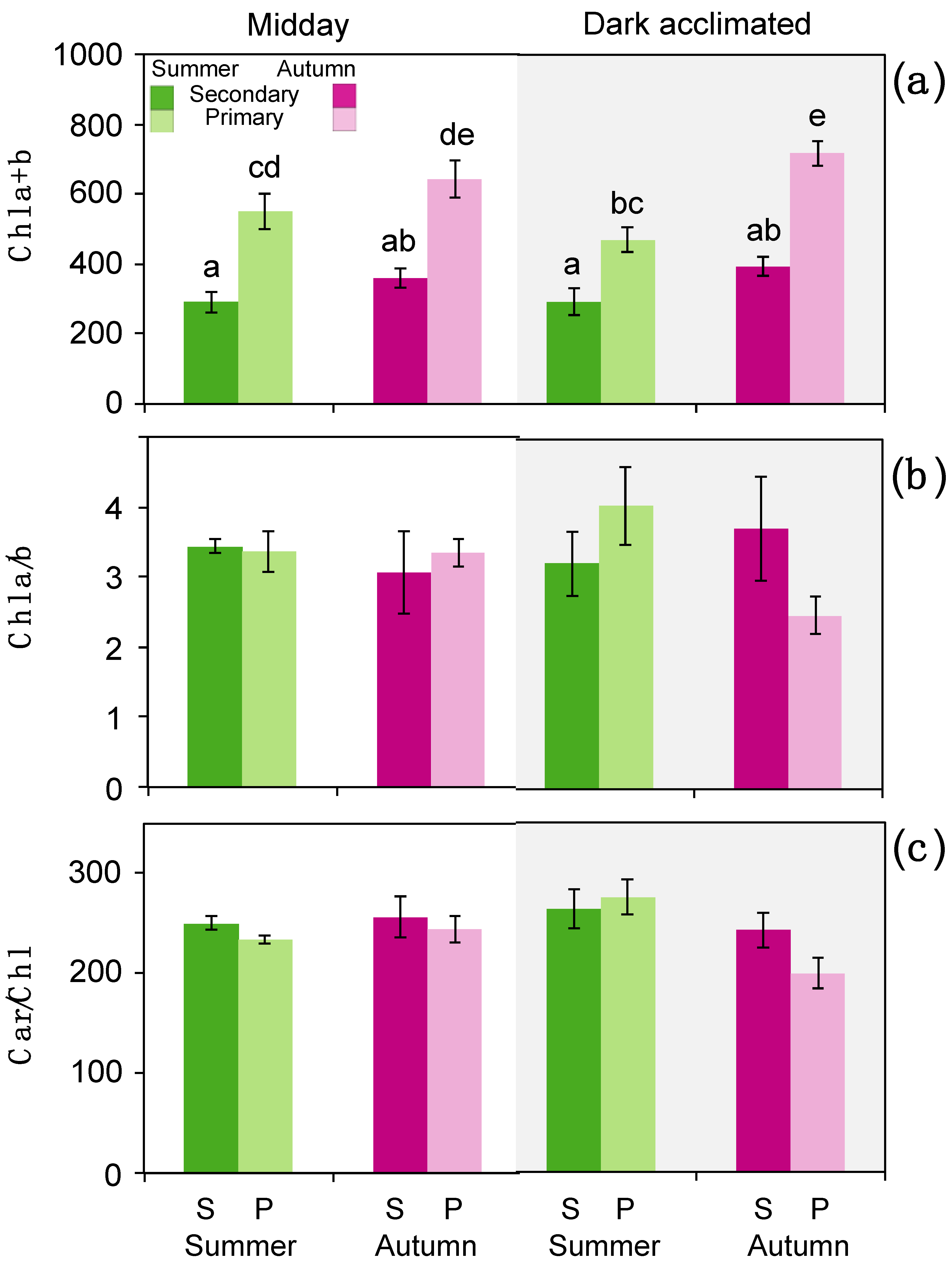
2.5. Analysis of Photosynthetic Pigments
2.6. Estimation of Needle Wettability through Contact Angle
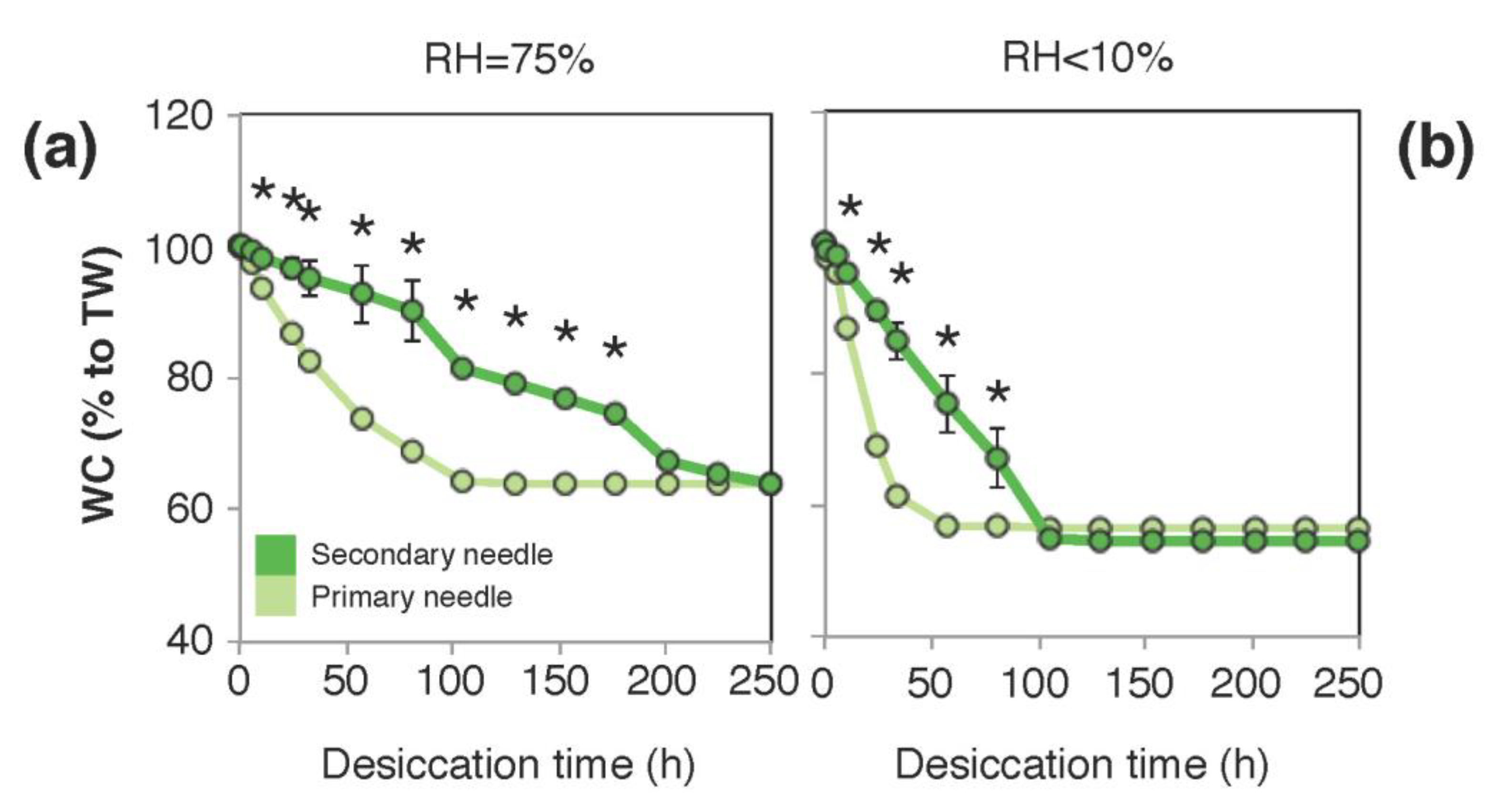
2.7. Desiccation Experiments
2.8. Thermo-Tolerance Assessment
2.9. Statistical Analyses
3. Results
3.1. Morpho-Anatomical Features of Needles
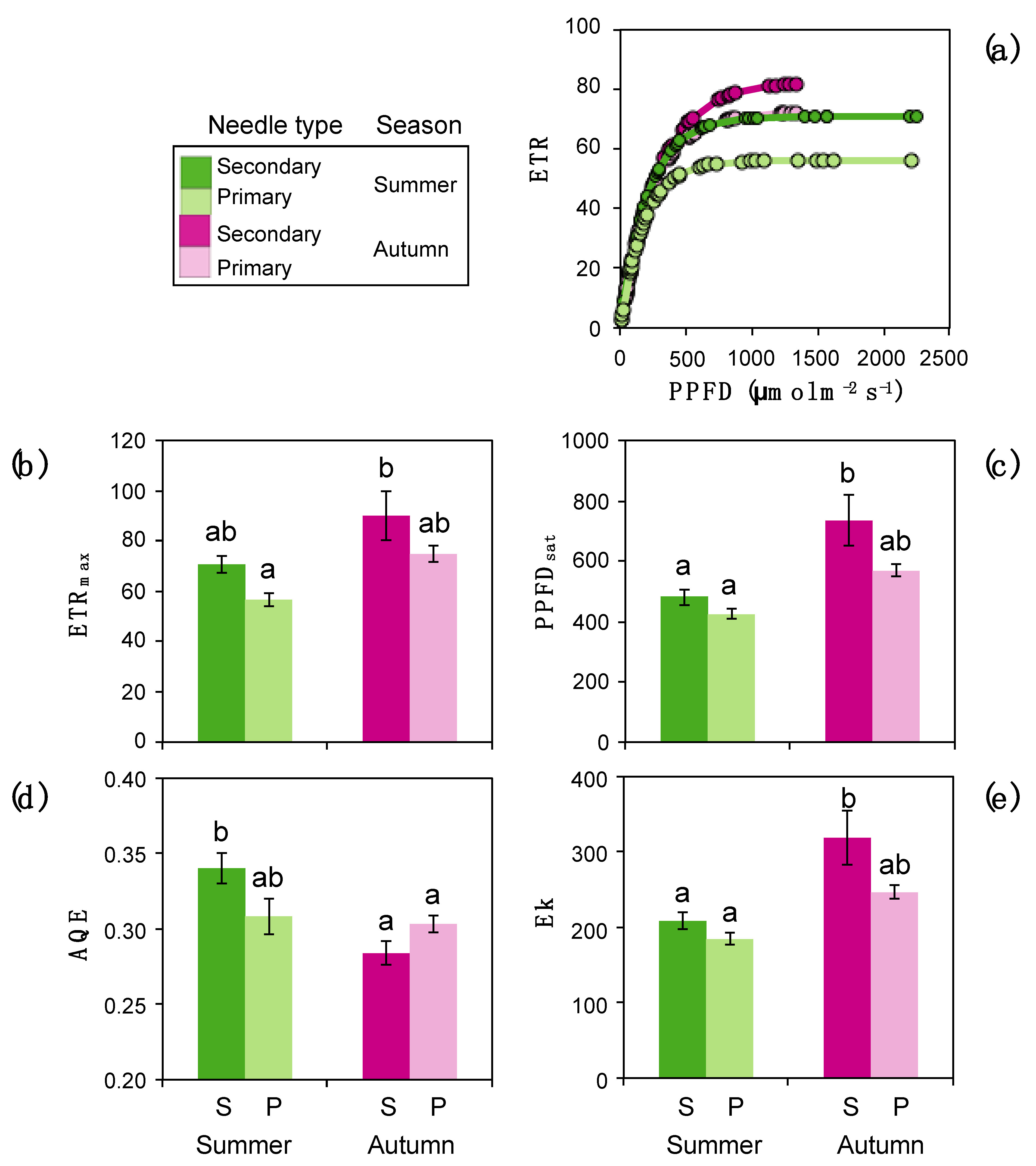
3.2. Photochemistry and Gas Exchange
3.3. Tolerance to Desiccation and Dehydration Kinetics
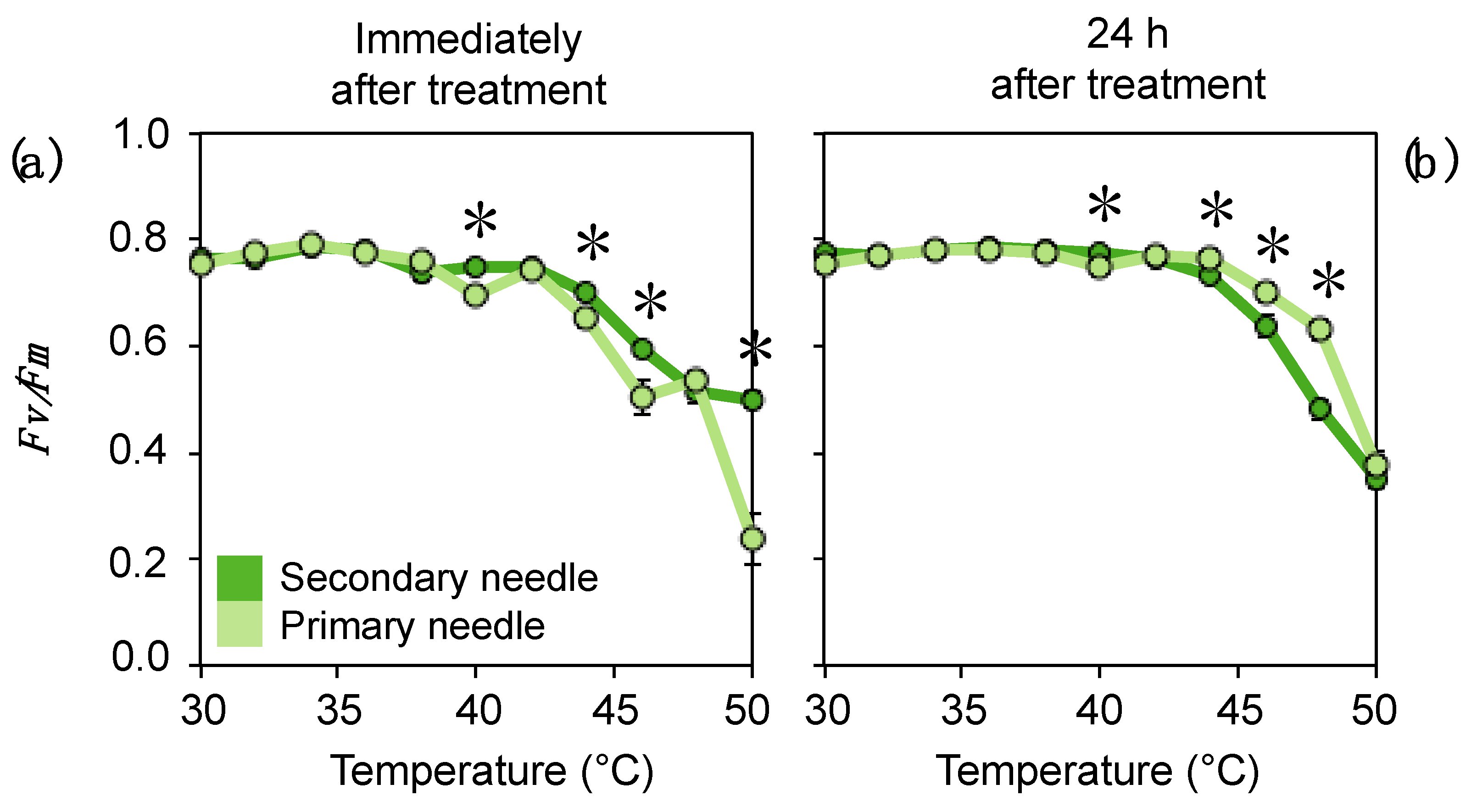
3.4. Tolerance to Extreme Temperatures
4. Discussion
4.1. Study Frame
4.2. Anatomical and Physiological Traits Related to Photosynthesis
4.3. Water Relations and Stress Tolerance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goebel, K. Ueber die Jugendzustände der Pflanzen. Flora 1889, 72, 1–44. [Google Scholar]
- Zotz, G.; Wilhelm, K.; Becker, A. Heteroblasty—A review. Bot. Rev. 2011, 77, 109–151. [Google Scholar] [CrossRef]
- Nakayama, H.; Sinha, N.R.; Kimura, S. How do plants and phytohormones accomplish heterophylly, leaf phenotypic plasticity, in response to environmental cues. Front. Plant Sci. 2017, 8, 1717. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, H.; Nakayama, N.; Nakamasu, A.; Sinha, N.; Kimura, S. Toward elucidating the mechanisms that regulate heterophylly. Plant Morphol. 2012, 24, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Gould, K. Leaf heteroblasty in Pseudopanax crassifolius: Functional signifciance of leaf morphology and anatomy. Ann. Bot. 1993, 71, 61–70. [Google Scholar] [CrossRef]
- Climent, J.; San-Martín, R.; Chambel, M.R.; Mutke, S. Ontogenetic differentiation between Mediterranean and Eurasian pines (sect. Pinus) at the seedling stage. Trees Struct. Funct. 2011, 25, 175–186. [Google Scholar] [CrossRef]
- Kuusk, V.; Niinemets, Ü.; Valladares, F. A major trade-off between structural and photosynthetic investments operative across plant and needle ages in three Mediterranean pines. Tree Physiol. 2018, 38, 543–547. [Google Scholar] [CrossRef] [Green Version]
- Nowak-Dyjeta, K.; Giertych, M.J.; Thomas, P.; Iszkuło, G. Males and females of Juniperus communis L. and Taxus baccata L. show different seasonal patterns of nitrogen and carbon content in needles. Acta Physiol. Plant. 2017, 39, 191. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.L.; Xu, X.H.; Li, X.G.; Li, Y.L.; Guy, R.D.; Chen, H.P. Photoprotection in heteromorphic leaves of savin juniper (Juniperus sabina L.). Photosynthetica 2019, 57, 780–787. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Jach, M.E.; Ceulemans, R. Stomatal density and needle anatomy of Scots pine (Pinus sylvestris) are affected by elevated CO2. New Phytol. 2001, 150, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Climent, J.; Dantas, A.K.; Alia, R.; Majada, J. Clonal variation for shoot ontogenetic heteroblasty in maritime pine (Pinus pinaster Ait.). Trees Struct. Funct. 2013, 27, 1813–1819. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Sanz, M.; Cervera, M.T.; Pinto, E.; Aranda, I.; Cadahía, E. Leaf metabolic response to water deficit in Pinus pinaster Ait. relies upon ontogeny and genotype. Environ. Exp. Bot. 2017, 140, 41–55. [Google Scholar] [CrossRef]
- Mediavilla, S.; Herranz, M.; González-Zurdo, P.; Escudero, A. Ontogenetic transition in leaf traits: A new cost associated with the increase in leaf longevity. J. Plant Ecol. 2014, 7, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Boddi, S.; Bonzi, L.M.; Calamassi, R. Structure and ultrastructure of Pinus halepensis primary needles. Flora 2002, 197, 10–23. [Google Scholar] [CrossRef]
- Lester, D.T. Developmental patterns of axillary meristematic activity in seedlings of Pinus. Bot. Gaz. 1968, 129, 206–210. [Google Scholar] [CrossRef]
- Calamassi, R.; Falusi, M.; Principe, M. Relations between apical structure and growth patterns in Pinus halepensis Mill. seedlings. G. Bot. Ital. 1988, 122, 321–338. [Google Scholar] [CrossRef]
- Climent, J.; Chambel, M.R.; López, R.; Mutke, S.; Alía, R.; Gil, L. Population divergence for heteroblasty in the Canary Island pine (Pinus canariensis, Pinaceae). Am. J. Bot. 2006, 93, 840–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, M.S.; Zellnig, G.; Stabentheiner, E.; Peters, J.; Morales, D.; Grill, D. Structure and ultrastructure of Pinus canariensis needles. Flora 2000, 195, 228–235. [Google Scholar] [CrossRef]
- Ferrer-Gallego, P.P.; Boisset, F. Correct type designation of the endemic Canary pine Pinus canariensis (Pinaceae). Taxon 2018, 67, 581–585. [Google Scholar] [CrossRef]
- Climent, J.; Tapias, R.; Pardos, J.A.; Gil, L. Fire adaptations in the Canary Islands pine (Pinus canariensis). Plant Ecol. 2004, 171, 185–196. [Google Scholar] [CrossRef]
- Brito, P.; Lorenzo, J.R.; González-Rodríguez, Á.M.; Morales, D.; Wieser, G.; Jimenez, M.S. Canopy transpiration of a Pinus canariensis forest at the tree line: Implications for its distribution under predicted climate warming. Eur. J. For. Res. 2014, 133, 491–500. [Google Scholar] [CrossRef]
- del Arco Aguilar, M.J.; Rodríguez Delgado, O. Vegetation of the Canary Islands; Springer: Cham, Switzerland, 2018; ISBN 9783319772547. [Google Scholar]
- Fernández-Palacios, J.M.; Nicolás, J.P. Altitudinal pattern of vegetation variation on Tenerife. J. Veg. Sci. 1995, 6, 183–190. [Google Scholar] [CrossRef]
- Miranda, J.C.; Rodríguez-Calcerrada, J.; Pita, P.; Saurer, M.; Oleksyn, J.; Gil, L. Carbohydrate dynamics in a resprouting species after severe aboveground perturbations. Eur. J. For. Res. 2020, 139, 841–852. [Google Scholar] [CrossRef]
- Brito, P.; Lorenzo, J.R.; González-Rodríguez, Á.M.; Morales, D.; Wieser, G.; Jiménez, M.S. Canopy transpiration of a semi arid Pinus canariensis forest at a treeline ecotone in two hydrologically contrasting years. Agric. For. Meteorol. 2015, 201, 120–127. [Google Scholar] [CrossRef]
- Zellnig, G.; Peters, J.; Jiménez, M.S.; Morales, D.; Grill, D.; Perktold, A. Three-dimensional reconstruction of the stomatal complex in Pinus canariensis needles using serial sections. Plant Biol. 2002, 4, 70–76. [Google Scholar] [CrossRef]
- Grill, D.; Tausz, M.; Pöllinger, U.; Jiménez, M.; Morales, D. Effects of drought on needle anatomy of Pinus canariensis. Flora 2004, 199, 85–89. [Google Scholar] [CrossRef]
- Stabentheiner, E.; Pfeifhofer, H.W.; Peters, J.; Jiménez, M.S.; Morales, D.; Grill, D. Different surface characteristics of primary and secondary needles of Pinus canariensis. Flora 2004, 199, 90–99. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; Aranda, I. Metabolic response to elevated CO2 levels in Pinus pinaster Aiton needles in an ontogenetic and genotypic-dependent way. Plant Physiol. Biochem. 2018, 132, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Climent, J.; Silva, F.C.E.; Chambel, M.R.; Pardos, M.; Almeida, M.H. Freezing injury in primary and secondary needles of Mediterranean pine species of contrasting ecological niches. Ann. For. Sci. 2009, 66, 407. [Google Scholar] [CrossRef] [Green Version]
- Pardos, M.; Climent, J.; Almeida, H.; Calama, R. The role of developmental stage in frost tolerance of Pinus pinea L. seedlings and saplings. Ann. For. Sci. 2014, 71, 551–562. [Google Scholar] [CrossRef]
- Ruffault, J.; Curt, T.; Moron, V.; Trigo, R.M.; Mouillot, F.; Koutsias, N.; Pimont, F.; Martin-StPaul, N.; Barbero, R.; Dupuy, J.L.; et al. Increased likelihood of heat-induced large wildfires in the Mediterranean Basin. Sci. Rep. 2020, 10, 13790. [Google Scholar] [CrossRef]
- Perry, J. The Pines of Mexico and Central America; Timber Press Inc.: Portland, OR, USA, 1991. [Google Scholar]
- Burns, R.; Honkala, B. Silvics of North America. Vol 1. Agriculture Handbook (Washington); USDA Forest Service: Washington, DC, USA, 1990.
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- White, A.J.; Critchley, C. Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth. Res. 1999, 59, 63–72. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [Green Version]
- Saroussi, S.; Beer, S. Alpha and quantum yield of aquatic plants derived from PAM fluorometry: Uses and misuses. Aquat. Bot. 2007, 86, 89–92. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Lukjanova, A.; Turnbull, M.H.; Sparrow, A.D. Plasticity in mesophyll volume fraction modulates light-acclimation in needle photosynthesis in two pines. Tree Physiol. 2007, 27, 1137–1151. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.; Morales, D.; Soledad Jiménez, M. Gas exchange characteristics of Pinus canariensis needles in a forest stand on Tenerife, Canary Islands. Trees Struct. Funct. 2003, 17, 492–500. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosyntehtic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Fernández-Marín, B.; López-pozo, M.; Perera-castro, A.V.; Arzac, M.I.; Sáenz-ceniceros, A.; Colesie, C.; Ríos, A.D.L.; Sancho, L.G.; Pintado, A.; Laza, J.M. Symbiosis at its limits: Ecophysiological consequences of lichenization in the genus Prasiola in Antarctica. Ann. Bot. 2019, 124, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.T.; Rice, S.K. Photosynthesis in Bryophytes and Early Land Plants; Springer: Dordrecht, The Netherlands, 2014; Volume 37. [Google Scholar] [CrossRef]
- López-Pozo, M.; Flexas, J.; Gulías, J.; Carriquí, M.; Nadal, M.; Perera-Castro, A.; Clemente-Moreno, M.; Gago, J.; Núñez-Olivera, E.; Martínez-Abaigar, J.; et al. A field portable method for semi-quantitative estimation of dehydration tolerance of photosynthetic tissues across distantly related land plants. Physiol. Plant. 2019, 167, 540–555. [Google Scholar] [CrossRef] [Green Version]
- Perera-Castro, A.; Brito, P.; González-Rodríguez, A. Changes in thermic limits and acclimation assessment for an alpine plant by chlorophyll fluorescence analysis: Fv/Fm vs. Rfd. Photosynthetica 2018, 56, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Anderegg, L.D.; Berner, L.T.; Badgley, G.; Sethi, M.L.; Law, B.E.; HilleRisLambers, J. Within-species patterns challenge our understanding of the leaf economics spectrum. Ecol. Lett. 2018, 21, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Lizarazo, K.; Fernández-Marín, B.; Becerril, J.M.; García-Plazaola, J.I. Ageing and irradiance enhance vitamin E content in green edible tissues from crop plants. J. Sci. Food Agric. 2010, 90, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Azuma, W.; Ishii, H.R.; Masaki, T. Height-related variations of leaf traits reflect strategies for maintaining photosynthetic and hydraulic homeostasis in mature and old Pinus densiflora trees. Oecologia 2019, 189, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Palmroth, S.; Maier, C.A.; Domec, J.C.; Oren, R. Anatomical changes with needle length are correlated with leaf structural and physiological traits across five Pinus species. Plant Cell Environ. 2019, 42, 1690–1704. [Google Scholar] [CrossRef]
- González-Rodríguez, Á.M.; Brito, P.; Lorenzo, J.R.; Jiménez, M.S. Photosynthetic performance in Pinus canariensis at semiarid treeline: Phenotype variability to cope with stressful environment. Forests 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Kuusk, V.; Niinemets, Ü.; Valladares, F. Structural controls on photosynthetic capacity through juvenile-to-adult transition and needle ageing in Mediterranean pines. Funct. Ecol. 2018, 32, 1479–1491. [Google Scholar] [CrossRef]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Fernández-Marín, B.; Ferrio, J.P.; Alday, J.G.; Hoch, G.; Landais, D.; Milcu, A.; Tissue, D.T.; Voltas, J.; Gessler, A.; et al. Endogenous circadian rhythms in pigment composition induce changes in photochemical efficiency in plant canopies. Plant Cell Environ. 2017, 40, 1153–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Marín, B.; Atherton, J.; Olascoaga, B.; Kolari, P.; Porcar-Castell, A.; García-Plazaola, J.I. When the sun never sets: Daily changes in pigment composition in three subarctic woody plants during the summer solstice. Trees Struct. Funct. 2018, 32, 615–630. [Google Scholar] [CrossRef] [Green Version]
- Tausz, M.; Wonisch, A.; Peters, J.; Jiménez, M.S.; Morales, D.; Grill, D. Short-term changes in free radical scavengers and chloroplast pigments in Pinus canariensis needles as affected by mild drought stress. J. Plant Physiol. 2001, 158, 213–219. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Garcia-Plazaola, J.I.; Nichol, C.J.; Kolari, P.; Olascoaga, B.; Kuusinen, N.; Fernández-Marín, B.; Pulkkinen, M.; Juurola, E.; Nikinmaa, E. Physiology of the seasonal relationship between the photochemical reflectance index and photosynthetic light use efficiency. Oecologia 2012, 170, 313–323. [Google Scholar] [CrossRef]
- Morais, M.C.; Cabral, J.A.; Gonçalves, B. Seasonal variation in the leaf physiology of co-occurring invasive (Hakea sericea) and native (Pinus pinaster) woody species in a Mediterranean-type ecosystem. For. Ecol. Manag. 2021, 480, 118662. [Google Scholar] [CrossRef]
- Sofronova, V.E.; Dymova, O.V.; Golovko, T.K.; Chepalov, V.A.; Petrov, K.A. Adaptive changes in pigment complex of Pinus sylvestris needles upon cold acclimation. Russ. J. Plant Physiol. 2016, 63, 433–442. [Google Scholar] [CrossRef]
- Deligöz, A.; Bayar, E.; Genç, M.; Karatepe, Y.; Kirdar, E.; Cankara, F.G. Seasonal and needle age-related variationsin the biochemical characteristics of Pinus nigra subsp. pallasiana (Lamb.) Holmboe. J. For. Sci. 2018, 64, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Cape, J.N. Contact angles of water droplets on needles of Scots Pine (Pinus sylvestris) growing in polluted atmospheres. New Phytol. 1983, 93, 293–299. [Google Scholar] [CrossRef]
- Leyton, L.; Armitage, I.P. Cuticle structure and water relations of the needles of Pinus radiata (D. Don). New Phytol. 1968, 67, 31–38. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Reum Han, A.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Climent, J.M.; Aranda, I.; Alonso, J.; Pardos, J.A.; Gil, L. Developmental constraints limit the response of Canary Island pine seedlings to combined shade and drought. For. Ecol. Manag. 2006, 231, 164–168. [Google Scholar] [CrossRef]
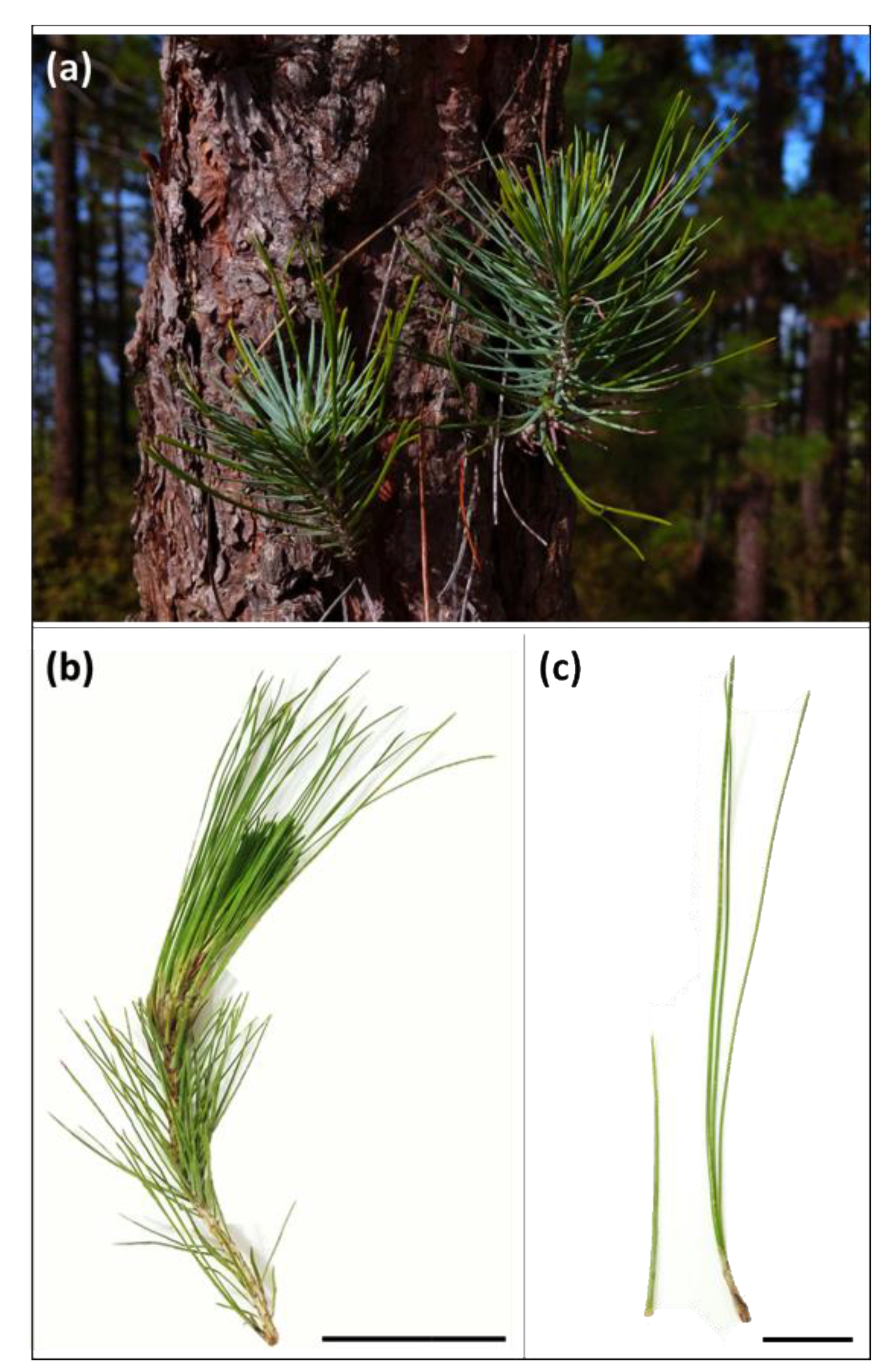
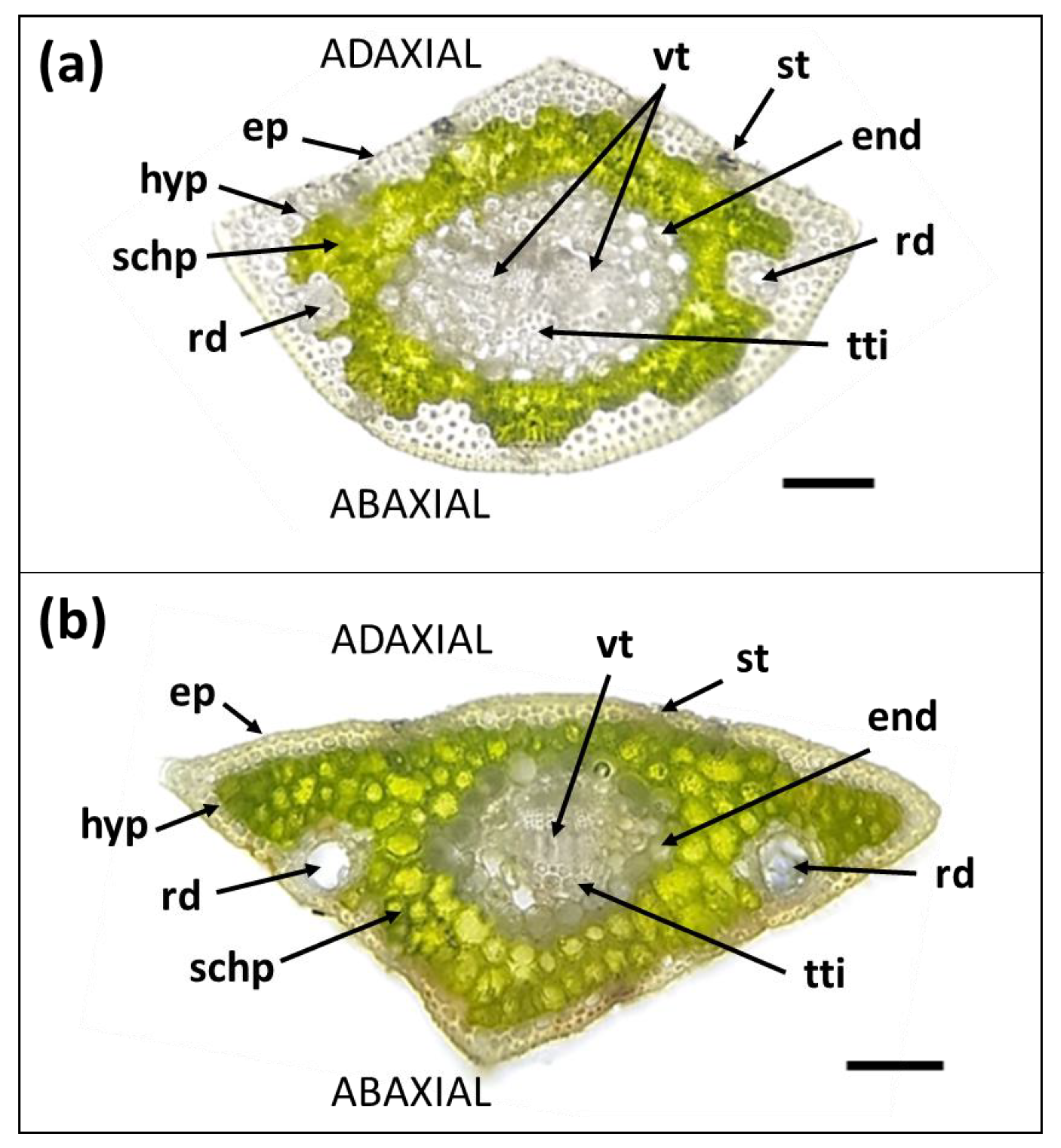
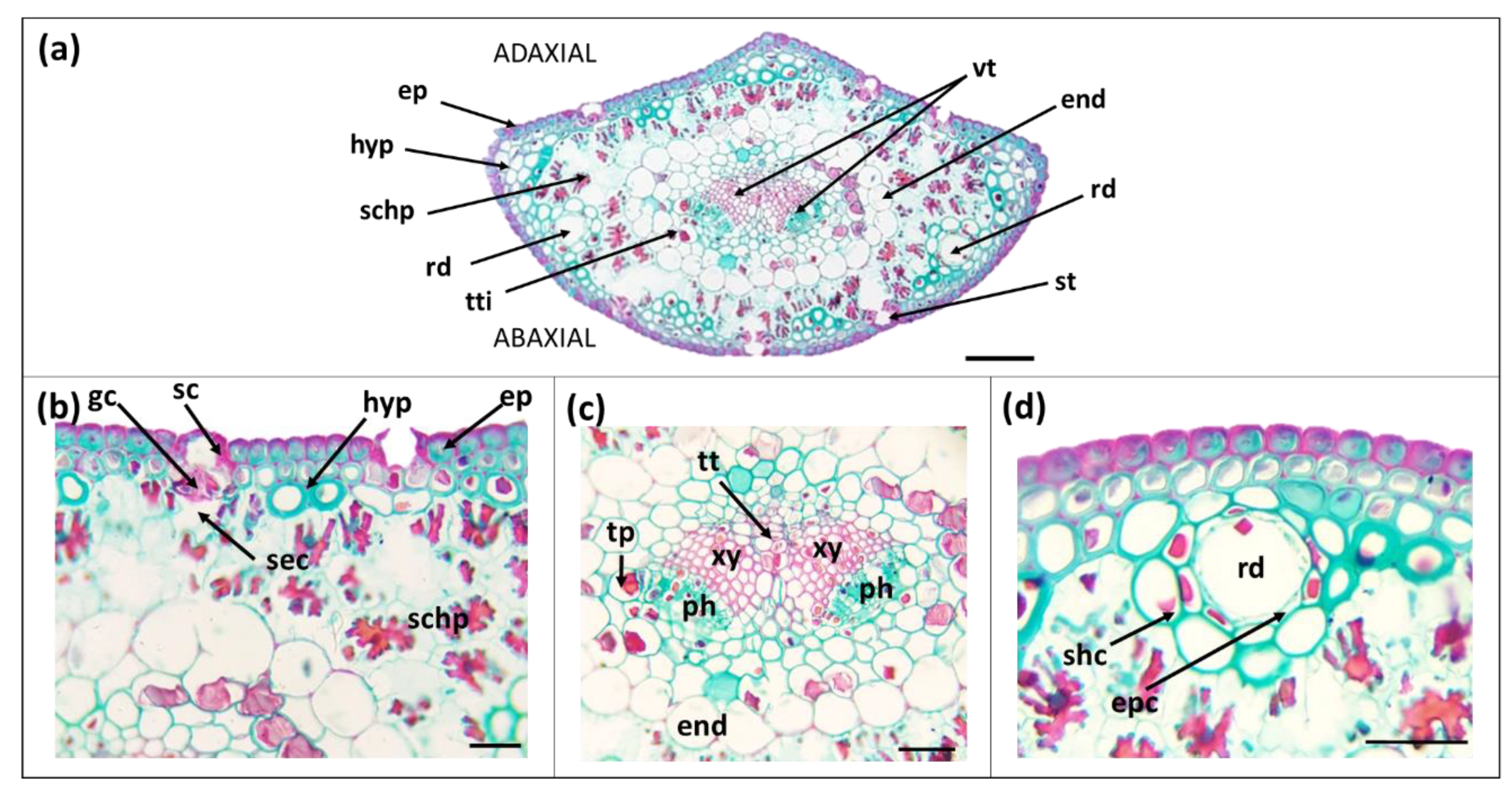
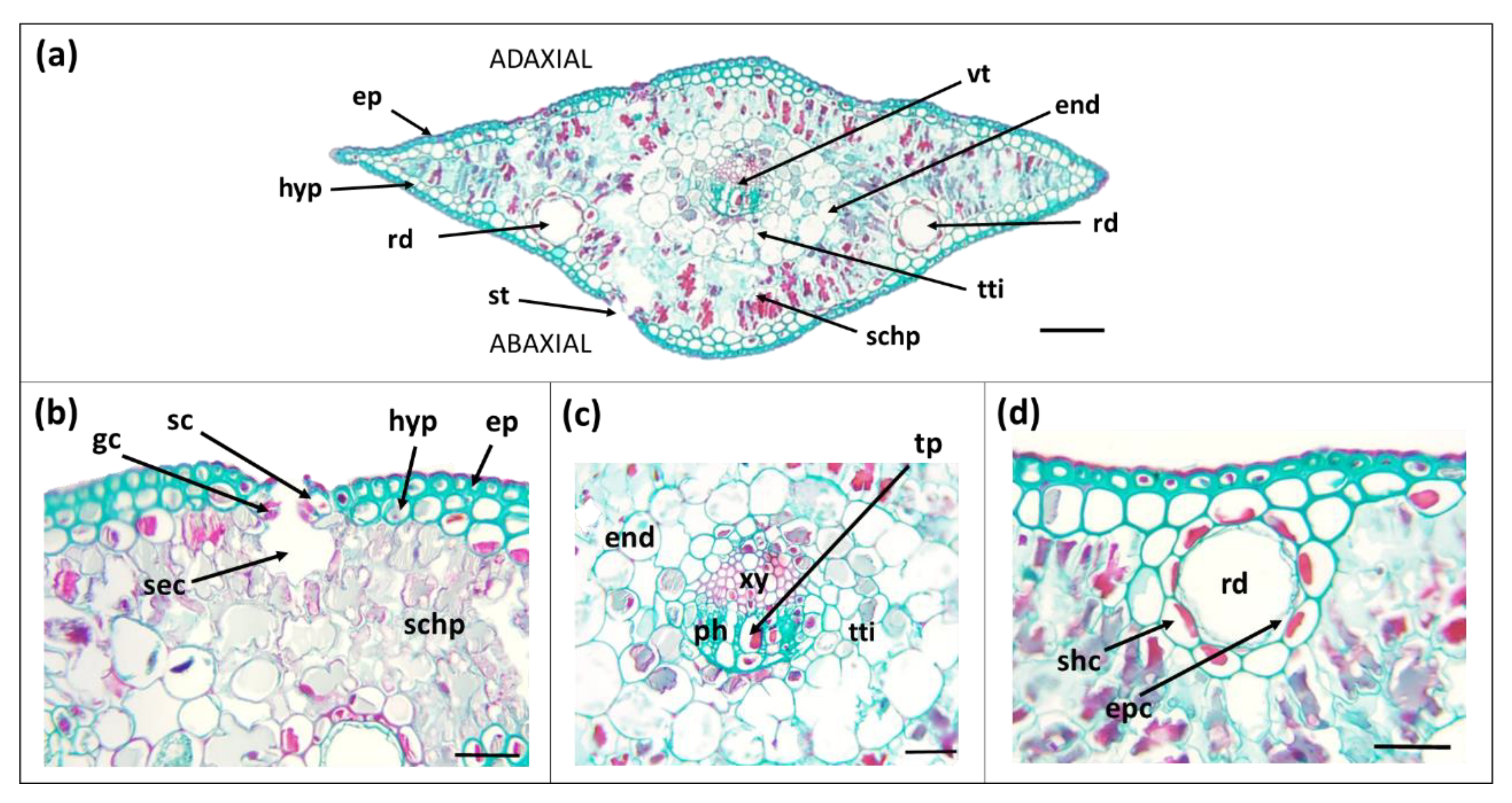
| Needle Part | Parameter | Secondary Needles | Primary Needles |
|---|---|---|---|
| General traits | Needles per brachyblast (Nº) | 3 | 1 |
| Internode length among needle insertions/brachyblasts (cm) | 0.13 ± 0.01 b | 0.22 ± 0.01 a | |
| Leaf length (cm) | 11.5 ± 0.4 b | 4.36 ± 0.10 a | |
| Leaf width (mm) | 0.408 ± 0.002 b | 0.573 ± 0.006 a | |
| Leaf thickness (mm) | 0.231 ± 0.002 a | 0.232 ± 0.001 a | |
| Leaf water content at turgor (gH2O g−1 DW) | 1.39 ± 0.03 a | 1.36 ± 0.04 a | |
| Leaf DW/FW ratio | 0.418 ± 0.005 a | 0.425 ± 0.008 a | |
| LMA (g m−2) | 181.7 ± 5.9 a | 164.7 ± 5.7 a | |
| CA (°) | 114.2 ± 2.7b | 137.8 ± 2.1a | |
| Stomata | Number of rows on adaxial surface (Nº) | 3 to 5 | 2 to 5 |
| Number of rows on abaxial surface (Nº) | 4 to 5 | 2 to 4 | |
| Density on the adaxial surface (Nº mm−2) | 39.8 ± 1.2 a | 41.5 ± 1.7 a | |
| Density on the abaxial surface (Nº mm−2) | 51.4 ± 1.4 b | 36.9 ± 1.9 a |
| Needle Part | Parameter | Secondary Needles | Primary Needles |
|---|---|---|---|
| Whole needle | Cross-section area (cm2) | 0.32 ± 0.01 b | 0.25 ± 0.01 a |
| Cuticle | Thickness (adaxial side) (µm) | 0.532 ± 0.034 b | 0.906 ± 0.058 a |
| Hypodermis | Layers (Nº) | 1 to 5 | 1 to 2 |
| Cells diameter (µm) | 6.13 ± 0.18 b | 9.20 ± 0.40 a | |
| Hypodermis (%) | 22.9 ± 0.4 b | 16.5 ± 0.2 a | |
| Mesophyll | Spongy parenchyma (%) | 33.9 ± 0.4 b | 46.9 ± 0.4 a |
| Endodermis | Cell diameter (µm) | 16.9 ± 0.5 b | 21.1 ± 0.9 a |
| Vascular bundle | Transfusion tissue (%) | 8.63 ± 0.12 b | 13.9 ± 0.2 a |
| Vascular bundles (Nº) | 2 | 1 | |
| Vascular tissue (%) | 4.63 ± 0.07 b | 2.14 ± 0.03 a | |
| Xylem (%) | 3.28 ± 0.06 b | 1.34 ± 0.02 a | |
| Phloem (%) | 1.35 ± 0.03 b | 0.806 ± 0.014 a | |
| Resin ducts | Number per needle | 2 | 2 |
| Position | under hypodermis | under hypodermis | |
| Resin ducts lumen area (µm2) | 327 ± 9 b | 669 ± 10 a | |
| Resin ducts diameter (µm) | 40.5 ± 0.7 b | 47.7 ± 0.7 a | |
| Sheath-cell layers (Nº) | 2 | 1 | |
| Sheath-cell diameter (µm) | 9.47 ± 0.54 b | 12.97 ± 0.38 a |
| Needle Age | Fv/Fm Freezing Treatment (−10 °C) | ||
|---|---|---|---|
| Initial | Post-Treatment | 24 h Recovery Post-Treatment | |
| Secondary | 0.763 ± 0.010 | 0.744 ± 0.014 | 0.743 ± 0.013 |
| Primary | 0.785 ± 0.006 | 0.771 ± 0.007 | 0.765 ± 0.008 |
| F | 3.59 | 3.00 | 2.15 |
| p | 0.076 | 0.103 | 0.162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Marín, B.; Ruiz-Medina, M.A.; Miranda, J.C.; González-Rodríguez, Á.M. Coexistent Heteroblastic Needles of Adult Pinus canariensis C.Sm. ex DC. in Buch Trees Differ Structurally and Physiologically. Forests 2021, 12, 341. https://doi.org/10.3390/f12030341
Fernández-Marín B, Ruiz-Medina MA, Miranda JC, González-Rodríguez ÁM. Coexistent Heteroblastic Needles of Adult Pinus canariensis C.Sm. ex DC. in Buch Trees Differ Structurally and Physiologically. Forests. 2021; 12(3):341. https://doi.org/10.3390/f12030341
Chicago/Turabian StyleFernández-Marín, Beatriz, Marcos Adrián Ruiz-Medina, José Carlos Miranda, and Águeda María González-Rodríguez. 2021. "Coexistent Heteroblastic Needles of Adult Pinus canariensis C.Sm. ex DC. in Buch Trees Differ Structurally and Physiologically" Forests 12, no. 3: 341. https://doi.org/10.3390/f12030341
APA StyleFernández-Marín, B., Ruiz-Medina, M. A., Miranda, J. C., & González-Rodríguez, Á. M. (2021). Coexistent Heteroblastic Needles of Adult Pinus canariensis C.Sm. ex DC. in Buch Trees Differ Structurally and Physiologically. Forests, 12(3), 341. https://doi.org/10.3390/f12030341






