Parent Material Effect on Soil Organic Carbon Concentration under Primeval European Beech Forests at a Regional Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Analyses
2.2. Statistical Analyses
3. Results
3.1. Soil Physical and Chemical Properties
3.2. Soil Organic Carbon Variability
3.3. Differences among Localities
4. Discussion
4.1. Predictive Ability of Soil Classification
4.2. Role of Parent Material
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sposito, G. The Chemistry of Soils, 3rd ed.; Oxford University Press: New York, NY, USA, 2016; p. 272. [Google Scholar]
- James, J.; Harrison, R. The Effect of Harvest on Forest Soil Carbon: A Meta-Analysis. Forests 2016, 7, 308. [Google Scholar] [CrossRef]
- Blaschek, M.; Roudier, P.; Poggio, M.; Hedley, C.B. Prediction of soil available water-holding capacity from visible near-infrared reflectance spectra. Sci. Rep. 2019, 9, 12833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lal, R.; Delgado, J.A.; Groffman, P.M.; Millar, N.; Dell, C.; Rotz, A. Management to mitigate and adapt to climate change. J. Soil Water Conserv. 2011, 66, 276–285. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Beyond COP 21: Potential and challenges of the “4 per Thousand” initiative. J. Soil Water Conserv. 2016, 71, 20A–25A. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Nunes, L.J.; Meireles, C.I.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M. Forest Contribution to Climate Change Mitigation: Management Oriented to Carbon Capture and Storage. Climate 2020, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.; Augusto, L.; Cécillon, L.; Ferreira, G.W.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.-P.; et al. Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Warren, K.L.; Ashton, M.S. Change in soil and forest floor carbon after shelterwood harvests in a New England oak-hardwood forest, USA. Int. J. For. Res. 2014, 2014, 27236. [Google Scholar] [CrossRef]
- Haas, J.; Schack-Kirchner, H.; Lang, F. Modeling soil erosion after mechanized logging operations on steep terrain in the Northern Black Forest, Germany. Eur. J. For. Res. 2020, 139, 549–565. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.D.; Harrison, R.B. The Case for Digging Deeper: Soil Organic Carbon Storage, Dynamics, and Controls in Our Changing World. Soil Syst. 2019, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pei, J.; Pendall, E.; Reich, P.B.; Noh, N.J.; Li, B.; Fang, C.; Nie, M. Rising temperature may trigger deep soil carbon loss across forest ecosystems. Adv. Sci. 2020, 7, 2001242. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 2015, 5, 15991. [Google Scholar] [CrossRef]
- Moffat, A.J. Indicators of soil quality for UK forestry. Forestry 2003, 76, 547–568. [Google Scholar] [CrossRef] [Green Version]
- Perie, C.; Ouimet, R. Organic carbon, organic matter and bulk density relationships in boreal forest soils. Can. J. Soil Sci. 2008, 88, 315–325. [Google Scholar] [CrossRef]
- Montréal Process. Criteria and Indicators for the Conservation and Sustainable Management of Temperate and Boreal Forests. 2015. Available online: https://montrealprocess.org/documents/publications/techreports/MontrealProcessSeptember2015.pdf (accessed on 3 September 2020).
- Schleuss, P.M.; Heitkamp, F.; Leuschner, C.; Fender, A.C.; Jungkunst, H.F. Higher subsoil carbon storage in species-rich than species-poor temperate forests. Environ. Res. Lett. 2014, 9, 014007. [Google Scholar] [CrossRef]
- Pandey, D. Carbon Stocks of World Heritage Forest Sites; UNESCO, World Heritage Centre: Paris, France, 2012; p. 9. [Google Scholar]
- Bauhus, J.; Khanna, P.K.; Hopmans, P.; Weston, C. Is soil carbon a useful indicator of sustainable forest soil management? A case study from native eucalypt forests of south-eastern Australia. For. Ecol. Manag. 2002, 171, 59–74. [Google Scholar] [CrossRef]
- UNESCO. Ancient and Primeval Beech Forests of the Carpathians and Other Regions of Europe. Available online: https://whc.unesco.org/en/list/1133/ (accessed on 12 September 2020).
- Tabaku, V.; Meyer, P. Lückenmuster albanischer und mitteleuropäischer Buchenwälder unterschiedlicher Nutzungsintensität. Forstarchiv 1999, 70, 87–97. [Google Scholar]
- Meyer, P.; Tabaku, V.; Burghard, V.L. Die Struktur albanischer Rotbuchen-Urwälder—Ableitungen für eine naturnahe Buchenwirtschaft. Forstwiss. Centralbl. 2003, 122, 47–58. [Google Scholar] [CrossRef]
- Markgraf, F. Aus den südosteuropäischen Urwäldern. I. Die Wälder Albaniens. Z. For. Jagdwes 1931, 63, 1–19. [Google Scholar]
- Jovanović, I.; Dragišić, A.; Ostojić, D.; Krsteski, B. Beech forests as world heritage in aspect to the next extension of the ancient and primeval beech forests of the Carpathians and other regions of Europe world heritage site. Zaštita Prirode 2019, 69, 15–32. [Google Scholar] [CrossRef]
- Walter, H. Vegetation of the Earth in Relation to Climate and the Eco-Physiological Conditions; Springer: New York, NY, USA, 1973; p. 237. [Google Scholar]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Newcomb, C.J.; Qafoku, N.P.; Grate, J.W.; Bailey, V.L.; De Yoreo, J.J. Developing a molecular picture of soil organic matter-mineral interactions by quantifying organo-mineral binding. Nat. Commun. 2017, 8, 396. [Google Scholar]
- Yang, S.; Jansen, B.; Kalbitz, K.; Chunga Castro, F.O.; van Hall, R.L.; Cammeraat, E.L.H. Lithology controlled soil organic carbon stabilization in an alpine grassland of the Peruvian Andes. Environ. Earth Sci. 2020, 79, 66. [Google Scholar] [CrossRef] [Green Version]
- Zinn, Y.L.; Lal, R.; Bigham, J.M.; Resck, D.V.S. Edaphic Controls on Soil Organic Carbon Retention in the Brazilian Cerrado: Texture and Mineralogy. Soil Sci. Soc. Am. J. 2007, 71, 1204–1214. [Google Scholar] [CrossRef]
- Angst, G.; Messinger, J.; Greiner, M.; Häusler, W.; Hertel, D.; Kirfel, K.; Kögel-Knabner, I.; Leuschner, C.; Rethemeyer, J.; Mueller, C.W. Soil organic carbon stocks in topsoil and subsoil controlled by parent material, carbon input in the rhizosphere, and microbial-derived compounds. Soil Biol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Barré, P.; Fernandez-Ugalde, O.; Virto, I.; Velde, B.; Chenu, C. Impact of phyllosilicate mineralogy on organic carbon stabilization in soils: Incomplete knowledge and exciting prospects. Geoderma 2014, 235–236, 382–395. [Google Scholar] [CrossRef]
- Sanchez, F.G. 1998: Soil organic matter and soil productivity: Searching for the missing link. In The Productivity and Sustainability of Southern Ecosystems in a Changing Environment; Mickler, R.A., Fox, S., Eds.; Springer: New York, NY, USA, 1998; pp. 543–556. [Google Scholar]
- Grigal, D.F.; Vance, E.D. Influence of soil organic matter on forest productivity. N. Z. J. For. Sci. 2000, 30, 169–205. [Google Scholar]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil Organic Carbon Storage as a Key Function of Soils—A Review of Drivers and Indicators at Various Scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Baritz, R.; Seufert, G.; Montanarella, L.; Van Ranst, E. Carbon concentrations and stocks in forest soils of Europe. For. Ecol. Manag. 2010, 260, 262–277. [Google Scholar] [CrossRef]
- Leuschner, C.; Meier, I.C.; Hertel, D. On the niche breadth of Fagus sylvatica: Soil nutrient status in 50 Central European beech stands on a broad range of bedrock types. Ann. For. Sci. 2006, 63, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech—A review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Udvardy, M.D.F. A Classification of the Biogeographical Provinces of the World; International Union for Conservation of Nature and Natural Resources: Morges, Switzerland, 1975; Volume 8. [Google Scholar]
- Faško, P.; Šťastný, P. Average Annual Precipitation. In Landscape Atlas of the Slovak Republic, 1st ed.; Miklós, L., Hrnčiarová, T., Eds.; Slovak Environmental Agency: Bratislava, Slovakia, 2002; p. 99. [Google Scholar]
- Hamor, F.; Brändli, U.B. The Uholka-Shrokyi Luh Protected Massif—An overview. In Inventory of the Largest Primeval Beech Forest in Europe—A Swiss-Ukrainian Scientific Adventure; Commarmot, B., Brändli, U.-B., Hamor, F., Lavnyy, V., Eds.; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland; Ukrainian National Forestry University: Ľviv, Ukraine; Carpathian Biosphere Reserve: Rakhiv, Ukraine, 2013; pp. 13–17. [Google Scholar]
- Standovár, T.; Kenderes, K. A review of natural stad dynamics in beechwoods of East Central Europe. Appl. Ecol. Environ. Res. 2003, 1, 19–46. [Google Scholar] [CrossRef]
- Tognetti, R.; Lombardi, F.; Lasserre, B.; Cherubini, P.; Marchetti, M. Tree-ring stable isotopes reveal twentieth-century increases in water-use efficiency of Fagus sylvatica and Nothofagus spp. in Italian and Chilean mountains. PLoS ONE 2014, 9, e113136. [Google Scholar] [CrossRef] [Green Version]
- Biely, A.; Bezák, V.; Elečko, M.; Gross, P.; Kaličiak, M.; Konečný, V.; Lexa, J.; Mello, J.; Nemčok, J.; Polák, M.; et al. 1. Geological structure. In Landscape Atlas of the Slovak Republic, 1st ed.; Miklós, L., Hrnčiarová, T., Eds.; Slovak Environmental Agency: Bratislava, Slovakia, 2002; pp. 74–75. [Google Scholar]
- Drößler, L.; von Lüpke, B. Canopy gaps in two virgin beech forest reserves in Slovakia. J. For. Sci. 2005, 51, 446–457. [Google Scholar] [CrossRef] [Green Version]
- Giurgiu, V.; Doniţă, N.; Bândiu, C.; Radu, S.; Cenuşă, R.; Dissescu, R.; Stoiculescu, C.; Biriş, I.A. Les Forêts Vierges de Roumanie; ASBL Forêt Wallone: Louvainla-Neuve, Belgium, 2001; p. 206. [Google Scholar]
- Piovesan, G.; Di Filippo, A.; Alessandrini, A.; Biondi, F.; Schirone, B. Structure, dynamics and dendroecology of an old-growth Fagus forest in the Apennines. J. Veg. Sci. 2005, 16, 13–28. [Google Scholar] [CrossRef]
- Commarmot, B. Structures of virgin and managed beech forests in Uholka (Ukraine) and Sihlwald (Switzerland): A comparative study. For. Snow Landsc. Res. 2005, 79, 45–56. [Google Scholar]
- Saniga, M.; Jaloviar, P.; Kucbel, S.; Vencurik, J. The changes of structure, necromass and the regeneration processes in NNR Havešová. Acta Fac. For. Zvolen 2011, 53, 49–59. [Google Scholar]
- Pittner, J.; Saniga, M. The structure, coarse woody debris volume, and regeneration processes of a beech natural forest in the National Nature Reserve Vtáčnik. Acta Fac. For. Zvolen 2006, 48, 95–109. [Google Scholar]
- IUSS Working Group WRB. World Reference Base or Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2005. [Google Scholar]
- Korpel, S. Die Urwälder der Westkarpaten; Gustav Fischer: Stuttgart, Germany, 1995; p. 310. [Google Scholar]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2019, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fieldes, M.; Perrott, K.W. The nature of allophane in soils. Part 3. Rapid field and laboratory test for allophane. N. Z. J. Sci. 1966, 9, 623–629. [Google Scholar]
- Alexander, E.B. Volume estimates of coarse fragments in soils: A combination of visual and weighing procedures. J. Soil Water Conserv. 1982, 37, 62–63. [Google Scholar]
- Daddow, R.L.; Warrington, G.E. Growth-Limiting Soil Bulk Densities as Influenced by Soil Texture; In WSDG Technical Report; USDA Forest Service: Fort Collins, CO, USA, 1983; p. 17. [Google Scholar]
- Day, R.W.; Quinn, G.P. Comparisons of treatments after an analysis of variance in ecology. Ecol. Monogr. 1989, 59, 433–463. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014; Available online: http://www.biostathandbook.com/ (accessed on 12 September 2020).
- Skovsgaard, J.P.; Vanclay, J.K. Forest site productivity: A review of the evolution of dendrometric concepts for even-aged stands. Forestry 2008, 81, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Zaiontz, C. Real Statistics Using Excel. 2020. Available online: http://www.real-statistics.com/ (accessed on 12 December 2020).
- Curtis, R.O.; Post, B.W. Estimating bulk density from organic matter content in some Vermont forest soils. Soil Sci. Soc. Am. Proc. 1964, 28, 285–286. [Google Scholar] [CrossRef]
- Grüneberg, E.; Schöning, I.; Kalko, E.K.V.; Weisser, W.W. Regional organic carbon stock variability: A comparison between depth increments and soil horizons. Geoderma 2010, 155, 426–433. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Zanini, M.; Dowgiallo, G.; Burrascano, S. Multiscale heterogeneity of topsoil properties in southern European old-growth forests. Eur. J. For. Res. 2015, 134, 911–925. [Google Scholar] [CrossRef]
- Merino, A.; Real, C.; Rodriguez-Guitian, M.A. Nutrient status of managed and natural forest fragments of Fagus sylvatica in southern Europe. For. Ecol. Manag. 2008, 255, 3691–3699. [Google Scholar] [CrossRef]
- Cremer, M.; Kern, N.V.; Prietzel, J. Soil organic carbon and nitrogen stocks under pure and mixed stands of European beech, Douglas fir and Norway spruce. For. Ecol. Manag. 2016, 367, 30–40. [Google Scholar] [CrossRef]
- Schrumpf, M.; Schulze, E.D.; Kaiser, K.; Schumacher, J. How accurately can soil organic carbon stocks and stock changes be quantified by soil inventories? Biogeosci. Discuss. 2011, 8, 723–769. [Google Scholar]
- Chesworth, W. Encyclopedia of Soil Science; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2008; p. 902. [Google Scholar]
- Dahlgren, R.A.; Saigusa, M.; Ugolini, F.C. The nature, properties and management of volcanic soils. Adv. Agron. 2004, 82, 113–182. [Google Scholar]
- Oades, J.M. The retention of organic matter in soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, E.P. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef] [Green Version]
- Homolák, M.; Kriaková, E.; Pichler, V.; Gömöryová, E.; Bebej, J. Isolating the soil type effect on the organic carbon content in a Rendzic Leptosol and an andosol on alimestone plateau with andesite protrusions. Geoderma 2017, 302, 1–5. [Google Scholar] [CrossRef]
- Sandén, T.; Lair, G.J.; van Leeuwen, J.P.; Gísladóttir, G.; Bloem, J.; Ragnarsdóttir, K.V.; Steffens, M.; Blum, W.E. Soil aggregation and soil organic matter in conventionally and organically farmed Austrian Chernozems. Die Bodenkult. J. Land Manag. Food Environ. 2017, 68, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.M.; Drury, C.F.; Reynolds, W.D.; Yang, J.Y. How do changes in bulk soil organic carbon content affect carbon concentrations in individual soil particle fractions? Sci. Rep. 2016, 6, 27173. [Google Scholar] [CrossRef] [Green Version]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Kramer, M.G.; Chadwick, O.A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale. Nat. Clim. Chang. 2018, 8, 1104–1108. [Google Scholar] [CrossRef]
- Eusterhues, K.; Rumpel, C.; Kogel-Knabner, I. Organo-mineral associations in sandy acid forest soils: Importance of specific surface area, iron oxides and micropores. Eur. J. Soil Sci. 2005, 56, 753–763. [Google Scholar] [CrossRef]
- Moni, C.; Chabbi, A.; Nunan, N.; Rumpel, C.; Chenu, C. Spatial dependance of organic carbon–metal relationship Fe-bearing crystalline secondary minerals: A multi-scale statistical analysis, from horizon to field. Geoderma 2010, 158, 120–127. [Google Scholar] [CrossRef]
- Vaselli, O.; Buccianti, A.; De Siena, C.; Bini, C.; Coradossi, N.; Angelone, M. Geochemical characterization of ophiolitic soils in a temperate climate: A multi-variate statistical approach. Geoderma 1997, 75, 117–133. [Google Scholar] [CrossRef]
- Dontsova, K.; Norton, L.D. Effects of exchangeable Ca: Mg ratio on soil clay flocculation, infiltration and erosion. In Sustaining the Global Farm; Stott, D.E., Mohtar, R.H., Steinhardt, G.C., Eds.; USDA-ARS National Soil Erosion Research Laboratory: West Lafayette, IN, USA, 2001; pp. 580–585. [Google Scholar]
- Bani, A.; Echevarria, G.; Montarges-Pelletier, E.; Gjoka, F.; Sulce, S.; Morel, J.L. Pedogenesis and nickel biogeochemistry in a typical Albanian ultramafic toposequence. Environ. Monit. Assess. 2014, 186, 4431–4442. [Google Scholar] [CrossRef] [PubMed]
- Holden, P.A.; Fierer, N. Microbial Processes in the Vadose Zone. Vadose Zone J. 2005, 4, 1–21. [Google Scholar] [CrossRef]
- Xiang, S.-R.; Doyle, A.; Holden, P.A.; Schimel, J.P. Drying and rewetting effects on C and N mineralization and microbial activity in surface and subsurface California grassland soils. Soil Biol. Biochem. 2008, 40, 2281–2289. [Google Scholar] [CrossRef]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Fagus sylvatica and Other Beeches in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; The Publication Office of the European Union: Luxembourg, 2016; pp. 94–96. [Google Scholar]


| Locality | Country | Orographic Unit | Geographical Coordinates | Elevation (m a.s.l.) | Aspect | Slope (°) | MAT (°C) | MAP (mm) | AHM |
|---|---|---|---|---|---|---|---|---|---|
| Vtáčnik | Slovakia | West | 48°37’351’ N, 18°38’700’ E | 1150 | S | 19 | 4 | 1050 | 13.3 |
| Carpathians | |||||||||
| Havešová | Slovakia | East | 49°00’645’ N, 22°19’538’ E | 650 | SE | 20 | 6 | 900 | 17.8 |
| Carpathians | |||||||||
| Uholka | Ukraine | East | 48°16’080’ N, 23°37’341’ E | 800 | SE | 22 | 5.5 | 1300 | 11.9 |
| Carpathians | |||||||||
| Izvoarele Nerei | Romania | South | 45°07.364’ N, 22°04.596’ E | 1250 | SW | 21 | 4 | 1150 | 12.2 |
| Carpathians | |||||||||
| Rajcë | Albania | Northern | 41°09.971’ N, 20°31.847’ E | 1350 | E | 20 | 6 | 1800 | 8.9 |
| Albanides | |||||||||
| Val Cervara | Italy | Central | 41° 49.641’N, 13° 43.933’ E | 1800 | NNW | 23 | 7.2 | 1211 | 14.2 |
| Apennines |
| Locality | Parent Material | Soil | Humus Form | Plant Community | Forest Stand Volume (m3 ha−1) |
|---|---|---|---|---|---|
| Vtáčnik | Andesite | Cambic | Moder | Dentario bulbiferae-Fagetum | 645 |
| Andosol | |||||
| Havešová | Sandstones, claystone (flysch) | Dystric | Moder | Dentario glandulosae-Fagetum | 701 |
| Cambisol | |||||
| Uholka | Sandstones, marlstones (flysch) | Dystric | Moder | Fagetum dentariosum Fagetum asperulosum | 770 |
| Cambisol | |||||
| Izvoarele | Crystalline schists (mica-schists) | Dystric | Moder | Hieracio rotundati-Fagetum | 620 |
| Nerei | Cambisol | ||||
| Rajcë | Serpentinite, gabbro, dolomite | Eutric | Moder | Fagetum asperuletosum | 807 |
| Cambisol | |||||
| Val Cervara | Limestones | Rendzic | Moder | Polysticho-Fagetum | 497 |
| Leptosol |
| Locality | Depth (m) | BD (kg m−3) | SF (m m−3) | Texture (%) | pH | Fe (g kg−1) | CaCO3 | Alp | Mot | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sn | Sl | Cl | H2O | KCl | Cr | Am | |||||||
| Vtáčnik | 0.1 | 870 | 0.11 | 21.6 | 50.9 | 27.5 | 5.05 | 4.55 | 8.82 | 0.24 | A | P | A |
| 0.5 | 1010 | 0.25 | 29.1 | 51.3 | 19.6 | 5.25 | 4.85 | 10.33 | 0.02 | A | P | A | |
| Havešová | 0.1 | 970 | 0.13 | 10.1 | 71.6 | 18.3 | 4.77 | 3.34 | 9.97 | 0.88 | A | A | A |
| 0.5 | 1140 | 0.24 | 15.4 | 62.9 | 21.7 | 5 | 3.43 | 13 | 0.58 | A | A | P | |
| Uholka | 0.1 | 830 | 0.2 | 5.8 | 59.4 | 34.8 | 4.68 | 3.45 | 15.57 | 1.65 | A | A | A |
| 0.5 | 1300 | 0.26 | 9.4 | 58.5 | 32.1 | 4.75 | 3.6 | 15.92 | 2.56 | A | A | A | |
| IzvoareleNerei | 0.1 | 580 | 0.07 | 49.6 | 32.9 | 17.5 | 4.05 | 3.51 | 9.79 | 0.57 | A | A | A |
| 0.5 | 890 | 0.36 | 54 | 27.8 | 18.3 | 4.59 | 4.15 | 10.84 | 0.45 | A | A | A | |
| Rajcë | 0.1 | 1000 | 0.1 | 13.6 | 62.3 | 24.1 | 5.56 | 4.48 | 95.64 | 0.52 | A | A | A |
| 0.5 | 1210 | 0.14 | 5.4 | 40.9 | 53.7 | 6.29 | 5.19 | 161.11 | 3.76 | A | A | A | |
| Val Cervara | 0.1 | 593 | 0.12 | 4.1 | 68.3 | 27.6 | 5.84 | 4.78 | 19.66 | 0.56 | P | A | A |
| 0.5 | 522 | 0.66 | 4.8 | 66.5 | 28.7 | 6.96 | 6.21 | 20.03 | 1.52 | P | A | A | |
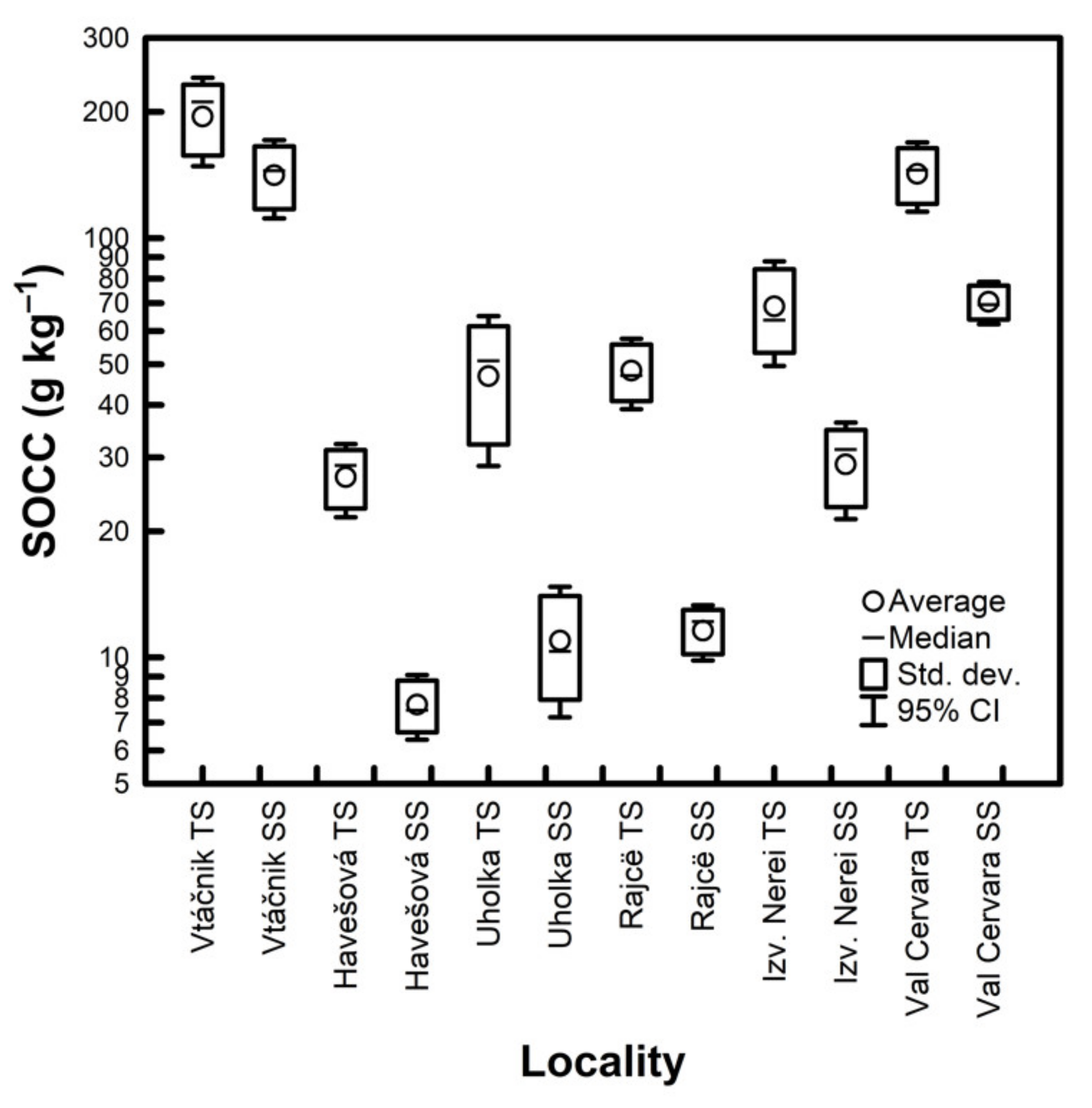
| Locality | SOCC (g kg−1) | CV (%) | C:N Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Profile No | Avg. | TS | SS | ||||||
| 1 | 2 | 3 | 4 | 5 | |||||
| Vtáčnik | 112.18 | 154.99 | 171.96 | 179.26 | 155.41 | 154.76 | 16.82 | 14.8 | 16.6 |
| Havešová | 14.34 | 10.43 | 12.94 | 11.21 | 13.74 | 12.53 | 13.27 | 8.5 | 5 |
| Uholka | 22.08 | 25.75 | 20.5 | 10.98 | 20.51 | 19.96 | 27.35 | 9.3 | 6.4 |
| Izvoarele Nerei | 47.46 | 43.01 | 34.09 | 29.9 | 39.76 | 38.85 | 17.98 | 14.1 | 14.4 |
| Rajcë | 21.17 | 20.11 | 18.76 | 21.37 | 22.48 | 20.78 | 6.77 | 16.8 | 10.7 |
| Val Cervara | 78.88 | 96 | 89.02 | 94.98 | 83.52 | 88.48 | 8.3 | 13.7 | 13.1 |
| Locality and Main Local SOC-Binding Agent | Mean Difference in SOCC (A–B) (g kg−1) | Std. Error | 95% Confidence Interval for MD | p-Value | ||
|---|---|---|---|---|---|---|
| A | B | Lower Bound | Upper Bound | |||
| Vtáčnik (ALP) | Havešová (PHS) | 142.23 | 8.25 | 87.14 | 197.31 | 0.001 |
| Vtáčnik (ALP) | Uholka (PHS+) | 134.8 | 8.41 | 80.69 | 188.91 | 0.001 |
| Vtáčnik (ALP) | Izvoarele Nerei (MS) | 115.91 | 8.52 | 62.36 | 169.47 | 0.002 |
| Vtáčnik (ALP) | Rajcë (FOX) | 133.98 | 8.24 | 78.87 | 189.1 | 0.002 |
| Vtáčnik (ALP) | Val Cervara (CAC) | 66.28 | 8.55 | 12.86 | 119.7 | 0.022 |
| Havešová (PHS) | Uholka (PHS+) | 7.43 | 1.81 | −3.73 | 18.59 | 0.189 |
| Havešová (PHS) | Izvoarele Nerei (MS) | 26.31 | 2.27 | 11.87 | 40.76 | 0.005 |
| Havešová (PHS) | Rajcë (FOX) | 8.24 | 0.69 | 4.66 | 11.83 | 0 |
| Havešová (PHS) | Val Cervara (CAC) | 75.95 | 2.38 | 60.72 | 91.18 | 0 |
| Uholka (PHS+) | Izvoarele Nerei (MS) | 18.88 | 2.8 | 4.18 | 33.59 | 0.014 |
| Uholka (PHS+) | Rajcë (FOX) | 0.81 | 1.78 | −10.45 | 12.08 | 0.999 |
| Uholka (PHS+) | Val Cervara (CAC) | 68.52 | 2.9 | 53.23 | 83.8 | 0 |
| Izvoarele Nerei (MS) | Rajcë (FOX) | 18.07 | 2.25 | 3.53 | 32.6 | 0.023 |
| Izvoarele Nerei (MS) | Val Cervara (CAC) | 49.63 | 3.21 | 33.06 | 66.21 | 0 |
| Rajcë (FOX) | Val Cervara (CAC) | 67.7 | 2.37 | 52.38 | 83.02 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pichler, V.; Gömöryová, E.; Leuschner, C.; Homolák, M.; Abrudan, I.V.; Pichlerová, M.; Střelcová, K.; Di Filippo, A.; Sitko, R. Parent Material Effect on Soil Organic Carbon Concentration under Primeval European Beech Forests at a Regional Scale. Forests 2021, 12, 405. https://doi.org/10.3390/f12040405
Pichler V, Gömöryová E, Leuschner C, Homolák M, Abrudan IV, Pichlerová M, Střelcová K, Di Filippo A, Sitko R. Parent Material Effect on Soil Organic Carbon Concentration under Primeval European Beech Forests at a Regional Scale. Forests. 2021; 12(4):405. https://doi.org/10.3390/f12040405
Chicago/Turabian StylePichler, Viliam, Erika Gömöryová, Christoph Leuschner, Marián Homolák, Ioan Vasile Abrudan, Magdaléna Pichlerová, Katarína Střelcová, Alfredo Di Filippo, and Roman Sitko. 2021. "Parent Material Effect on Soil Organic Carbon Concentration under Primeval European Beech Forests at a Regional Scale" Forests 12, no. 4: 405. https://doi.org/10.3390/f12040405
APA StylePichler, V., Gömöryová, E., Leuschner, C., Homolák, M., Abrudan, I. V., Pichlerová, M., Střelcová, K., Di Filippo, A., & Sitko, R. (2021). Parent Material Effect on Soil Organic Carbon Concentration under Primeval European Beech Forests at a Regional Scale. Forests, 12(4), 405. https://doi.org/10.3390/f12040405










