Effects of Soil Moisture and Temperature on Microbial Regulation of Methane Fluxes in a Poplar Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling and Laboratory Incubation Experimental Design
2.3. Soil Analysis after Incubation
2.4. Gas Sampling and Analysis
2.5. DNA Extraction, Polymerase Chain Reaction Amplification, and Illumina MiSeq Sequencing
2.6. Data Analysis
3. Results
3.1. Variation in Soil CH4 Fluxes
3.2. Variability in Soil Chemical Properties and Response of Experimental Treatments
3.3. Microbial α-Diversity and Species Composition Patterns
3.4. Correlation of Soil Chemical Properties and Microbial Community Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, K.; Dobbie, K.; Ball, B.; Bakken, L.; Sitaula, B.; Hansen, S.; Brumme, R.; Borken, W.; Christensen, S.; Priemé, A.; et al. Oxidation of atmospheric methane in Northern European soils, comparison with other ecosystems, and uncertainties in the global terrestrial sink. Glob. Chang. Biol. 2000, 6, 791–803. [Google Scholar] [CrossRef]
- Wang, Z.P.; Ineson, P. Methane oxidation in a temperate coniferous forest soil: Effects of inorganic N. Soil Biol. Biochem. 2003, 35, 427–433. [Google Scholar] [CrossRef]
- Aronson, E.L.; Helliker, B.R. Methane flux in non-wetland soils in response to nitrogen addition: A meta-analysis. Ecology 2010, 91, 3242–3251. [Google Scholar] [CrossRef]
- Chan, A.S.K.; Parkin, T.B. Methane oxidation and production activity in soils from natural and agricultural ecosystems. J. Environ. Qual. 2001, 30, 1896–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Fischer, J.C.; Hedin, L.O. Separating methane production and consumption with a field-based isotope pool dilution technique. Glob. Biogeochem. Cycles 2002, 16, 8-1–8-13. [Google Scholar] [CrossRef]
- Shoemaker, J.K.; Keenan, T.F.; Hollinger, D.Y.; Richardson, A.D. Forest ecosystem changes from annual methane source to sink depending on late summer water balance. Geophys. Res. Lett. 2014, 41, 673–679. [Google Scholar] [CrossRef]
- Do Carmo, J.B.; Keller, M.; Dias, J.D.; de Camargo, P.B.; Crill, P. A source of methane from upland forests in the Brazilian Amazon. Geophys. Res. Lett. 2006, 33, L04809. [Google Scholar] [CrossRef] [Green Version]
- Megonigal, J.P.; Guenther, A.B. Methane emissions from upland forest soils and vegetation. Tree Physiol. 2008, 28, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.S.C.; Nazaries, L.; Delgado-Baquerizo, M.; Macdonald, C.A.; Anderson, I.C.; Hobbie, S.E.; Venterea, R.T.; Reich, P.B.; Singh, B.K. Identifying environmental drivers of greenhouse gas emissions under warming and reduced rainfall in boreal-temperate forests. Funct. Ecol. 2017, 31, 2356–2368. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.; Guo, J.; Han, M.; Wang, W.; Peng, C.; Jin, J.; Song, X.; Yu, S. A review of the mechanisms and controlling factors of methane dynamics in forest ecosystems. For. Ecol. Manag. 2020, 455, 117702. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Ciais, P.; Peylin, P.; Huang, Y.; Sitch, S.; Wang, T. The carbon balance of terrestrial ecosystems in China. Nature 2009, 458, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, C.; Wu, J.; Zhou, L.; Lin, Y.; Fu, S. Subtropical plantations are large carbon sinks: Evidence from two monoculture plantations in South China. Agric. For. Meteorol. 2011, 151, 1214–1225. [Google Scholar] [CrossRef]
- Zhu, J.; He, N.; Wang, Q.; Yuan, G.; Wen, D.; Yu, G.; Jia, Y. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Q.; Wang, H.; Wang, W.; Han, S. Shelterbelt poplar forests induced soil changes in deep soil profiles and climates contributed their inter-site variations in dryland regions, northeastern China. Front. Plant Sci. 2019, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Danilović, M.; Raković, D.; Isajev, D.; Antonić, S. Comparative analysis of the assortment structure of poplar clones I-214 and Pannonia. Glas. Sumar. Fak. 2020, 122, 31–46. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, X.; Guan, H.; Zhou, H.; Hua, M.; Wang, X. Climatic and environmental controls on stable isotopes in atmospheric water vapor near the surface observed in Changsha, China. Atmos. Environ. 2018, 189, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res. Atmos. 2004, 109, D17. [Google Scholar] [CrossRef]
- Meixner, F.X. Biogenic emissions of nitric oxide and nitrous oxide from arid and semi-arid land. In Dryland Ecohydrology; Springer: Berlin/Heidelberg, Germany, 2006; pp. 233–255. [Google Scholar]
- Schaufler, G.; Kitzler, B.; Schindlbacher, A.; Skiba, U.; Sutton, M.A.; Zechmeister-Boltenstern, S. Greenhouse gas emissions from European soils under different land use: Effects of soil moisture and temperature. Eur. J. Soil Sci. 2010, 61, 683–696. [Google Scholar] [CrossRef]
- Bowden, R.D.; Newkirk, K.M.; Rullo, G.M. Carbon dioxide and methane fluxes by a forest soil under laboratory-controlled moisture and temperature conditions. Soil Biol. Biochem. 1998, 30, 1591–1597. [Google Scholar] [CrossRef] [Green Version]
- Whalen, S.; Reeburgh, W. Moisture and temperature sensitivity of CH4 oxidation in boreal soils. Soil Biol. Biochem. 1996, 28, 1271–1281. [Google Scholar] [CrossRef] [Green Version]
- Bian, R.; Chen, J.; Li, W.; Sun, Y.; Chai, X.; Wang, H.; Wang, Y.; Zhao, J. Numerical modeling of methane oxidation and emission from landfill cover soil coupling water-heat-gas transfer: Effects of meteorological factors. Process Saf. Environ. Prot. 2021, 146, 647–655. [Google Scholar] [CrossRef]
- Koh, H.-S.; Ochs, C.A.; Yu, K. Hydrologic gradient and vegetation controls on CH4 and CO2 fluxes in a spring-fed forested wetland. Hydrobiologia 2009, 630, 271–286. [Google Scholar] [CrossRef]
- Reddy, K.R.; Yargicoglu, E.N.; Yue, D.; Yaghoubi, P. Enhanced microbial methane oxidation in landfill cover soil amended with biochar. J. Geotech. Geoenviron. 2014, 140, 04014047. [Google Scholar] [CrossRef]
- Spokas, K.A.; Bogner, J.E. Limits and dynamics of methane oxidation in landfill cover soils. Waste Manag. 2011, 31, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S. The quest for atmospheric methane oxidizers in forest soils. Environ. Microbiol. Rep. 2009, 1, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Malghani, S.; Reim, A.; von Fischer, J.; Conrad, R.; Kuebler, K.; Trumbore, S.E. Soil methanotroph abundance and community composition are not influenced by substrate availability in laboratory incubations. Soil Biol. Biochem. 2016, 101, 184–194. [Google Scholar] [CrossRef]
- Sjogersten, S.; Aplin, P.; Gauci, V.; Peacock, M.; Siegenthaler, A.; Turner, B.L. Temperature response of ex-situ greenhouse gas emissions from tropical peatlands: Interactions between forest type and peat moisture conditions. Geoderma 2018, 324, 47–55. [Google Scholar] [CrossRef]
- Wu, X.; Yao, Z.; Brüggemann, N.; Shen, Z.Y.; Wolf, B.; Dannenmann, M.; Zheng, X.; Butterbach-Bahl, K. Effects of soil moisture and temperature on CO2 and CH4 soil–atmosphere exchange of various land use/cover types in a semi-arid grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, W.; Chi, M.; Sun, Y.; An, J.; Yu, N.; Zou, H. Simulating the effects of soil temperature and soil moisture on CO2 and CH4 emissions in rice straw-enriched paddy soil. CATENA 2020, 194, 104677. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial biomass measurements in forest soils: Determination of kC values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biol. Biochem. 1987, 19, 689–696. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, X.; Yu, Y.; Zhang, H.; Chu, H.; Zhu, J. Elevated ground-level O3 negatively influences paddy methanogenic archaeal community. Sci. Rep. 2013, 3, 3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, S.; Knief, C.; Stubner, S.; Conrad, R. Quantitative detection of methanotrophs in soil by novel pmoA-targeted real-time PCR assays. Appl. Environ. Microbiol. 2003, 69, 2423–2429. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Li, L.; Li, L.; Liu, Y.; Ren, Q.; Shi, M.; Liu, J.; Jiang, J.; Ma, H.; Huang, Z.; et al. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: An analysis of the gut microbiota composition. Acta Diabetol. 2019, 56, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hou, S.; Peng, D.; Wang, Y.; Wang, C.; Xu, F.; Xu, H. Response of soil micro-ecology to different levels of cadmium in alkaline soil. Ecotoxicol. Environ. Saf. 2018, 166, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Guo, J.; Wang, W.; Song, X.; Yu, S. Soil Depth Determines the Composition and Diversity of Bacterial and Archaeal Communities in a Poplar Plantation. Forests 2019, 10, 550. [Google Scholar] [CrossRef] [Green Version]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Fetzer, S.; Conrad, R. Effect of redox potential on methanogenesis by Methanosarcina barkeri. Arch. Microbiol. 1993, 160, 108–113. [Google Scholar] [CrossRef]
- Brioukhanov, A.; Netrusov, A.; Sordel, M.; Thauer, R.K.; Shima, S. Protection of Methanosarcina barkeri against oxidative stress: Identification and characterization of an iron superoxide dismutase. Arch. Microbiol. 2000, 174, 213–216. [Google Scholar] [CrossRef]
- von Arnold, K.; Nilsson, M.; Hanell, B.; Weslien, P.; Klemedtsson, L. Fluxes of CO2, CH4 and N2O from drained organic soils in deciduous forests. Soil Biol. Biochem. 2005, 37, 1059–1071. [Google Scholar] [CrossRef]
- Dunfield, P.; Dumont, R.; Moore, T.R. Methane production and consumption in temperate and subarctic peat soils: Response to temperature and pH. Soil Biol. Biochem. 1993, 25, 321–326. [Google Scholar] [CrossRef]
- Wu, X.L.; Chin, K.J.; Conrad, R. Effect of temperature stress on structure and function of the methanogenic archaeal community in a rice field soil. FEMS Microbiol. Ecol. 2002, 39, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bond-Lamberty, B.; Smith, A.P.; Bailey, V. Temperature and moisture effects on greenhouse gas emissions from deep active-layer boreal soils. Biogeosciences 2016, 13, 6669–6681. [Google Scholar] [CrossRef] [Green Version]
- Fang, S.; Lin, D.; Tian, Y.; Hong, S. Thinning intensity affects soil-atmosphere fluxes of greenhouse gases and soil nitrogen mineralization in a lowland poplar plantation. Forests 2016, 7, 141. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.P.; Diem, T.; Quispe, L.P.H.; Cahuana, A.J.; Reay, D.S.; Meir, P.; Teh, Y.A. Drivers of atmospheric methane uptake by montane forest soils in the southern Peruvian Andes. Biogeosciences 2016, 13, 4151–4165. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, G.; Ye, Y. Coastal vegetation invasion increases greenhouse gas emission from wetland soils but also increases soil carbon accumulation. Sci. Total Environ. 2015, 526, 19–28. [Google Scholar] [CrossRef]
- Dou, X.; Zhou, W.; Zhang, Q.; Cheng, X. Greenhouse gas (CO2, CH4, N2O) emissions from soils following afforestation in central China. Atmos. Environ. 2016, 126, 98–106. [Google Scholar] [CrossRef]
- Phillips, R.L.; Whalen, S.C.; Schlesinger, W.H. Influence of atmospheric CO2 enrichment on methane consumption in a temperate forest soil. Glob. Chang. Biol. 2001, 7, 557–563. [Google Scholar] [CrossRef]
- McLain, J.E.T.; Kepler, T.B.; Ahmann, D.M. Belowground factors mediating changes in methane consumption in a forest soil under elevated CO2. Glob. Biogeochem. Cycles 2002, 16, 23-1–23-14. [Google Scholar] [CrossRef]
- Warner, D.L.; Villarreal, S.; McWilliams, K.; Inamdar, S.; Vargas, R. Carbon dioxide and methane fluxes from tree Stems, coarse woody debris, and soils in an upland temperate forest. Ecosystems 2017, 20, 1205–1216. [Google Scholar] [CrossRef]
- McLain, J.E.T.; Ahmann, D.M. Increased moisture and methanogenesis contribute to reduced methane oxidation in elevated CO2 soils. Biol. Fertil. Soils 2008, 44, 623–631. [Google Scholar] [CrossRef]
- Sun, J.; Xia, Z.; He, T.; Dai, W.; Peng, B.; Liu, J.; Gao, D.; Jiang, P.; Han, S.; Bai, E. Ten years of elevated CO2 affects soil greenhouse gas fluxes in an open top chamber experiment. Plant Soil 2017, 420, 435–450. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Treat, C.C.; Waldrop, M.P.; Waddington, J.M.; Harden, J.W.; McGuire, A.D. Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland. J. Geophys. Res. Biogeosci. 2008, 113, G00A10. [Google Scholar] [CrossRef] [Green Version]
- Rinnan, R.; Michelsen, A.; Jonasson, S. Effects of litter addition and warming on soil carbon, nutrient pools and microbial communities in a subarctic heath ecosystem. Appl. Soil Ecol. 2008, 39, 271–281. [Google Scholar] [CrossRef]
- Pedersen, E.P.; Elberling, B.; Michelsen, A. Seasonal variations in methane fluxes in response to summer warming and leaf litter addition in a subarctic heath ecosystem. J. Geophys. Res. Biogeosci. 2017, 122, 2137–2153. [Google Scholar] [CrossRef]
- Rui, Y.; Gleeson, D.B.; Murphy, D.V.; Hoyle, F.C. Response of microbial biomass and CO2-C loss to wetting patterns are temperature dependent in a semi-arid soil. Sci. Rep. 2017, 7, 13032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarate-Valdez, J.L.; Zasoski, R.J.; Lauchli, A.E. Short-term effects of moisture content on soil solution pH and soil Eh. Soil Sci. 2006, 171, 423–431. [Google Scholar]
- Fu, Q.; Yan, J.; Li, H.; Li, T.; Hou, R.; Liu, D.; Ji, Y. Effects of biochar amendment on nitrogen mineralization in black soil with different moisture contents under freeze-thaw cycles. Geoderma 2019, 353, 459–467. [Google Scholar] [CrossRef]
- Kharitonov, S.; Semenov, M.; Sabrekov, A.; Kotsyurbenko, O.; Zhelezova, A.; Schegolkova, N. Microbial Communities in Methane Cycle: Modern Molecular Methods Gain Insights into Their Global Ecology. Environments 2021, 8, 16. [Google Scholar] [CrossRef]
- Espenberg, M.; Truu, M.; Truu, J.; Maddison, M.; Nolvak, H.; Jarveoja, J.; Mander, Ü. Impact of reed canary grass cultivation and mineral fertilisation on the microbial abundance and genetic potential for methane production in residual peat of an abandoned peat extraction area. PLoS ONE 2016, 11, e0163864. [Google Scholar] [CrossRef]
- Frey, B.; Niklaus, P.A.; Kremer, J.; Luescher, P.; Zimmermann, S. Heavy-machinery traffic impacts methane emissions as well as methanogen abundance and community structure in oxic forest soils. Appl. Environ. Microbiol. 2011, 77, 6060–6068. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, J.R.; Levy-Booth, D.; Prescott, C.E.; Grayston, S.J. Microbial and environmental controls of methane fluxes along a soil moisture gradient in a Pacific coastal temperate rainforest. Ecosystems 2016, 19, 1255–1270. [Google Scholar] [CrossRef]
- Martins, C.S.C.; Macdonald, C.A.; Anderson, I.C.; Singh, B.K. Feedback responses of soil greenhouse gas emissions to climate change are modulated by soil characteristics in dryland ecosystems. Soil Biol. Biochem. 2016, 100, 21–32. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E. Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot. 2008, 56, 369–407. [Google Scholar] [CrossRef]
- Luo, G.J.; Kiese, R.; Wolf, B.; Butterbach-Bahl, K. Effects of soil temperature and moisture on methane uptake and nitrous oxide emissions across three different ecosystem types. Biogeosciences 2013, 10, 3205–3219. [Google Scholar] [CrossRef] [Green Version]


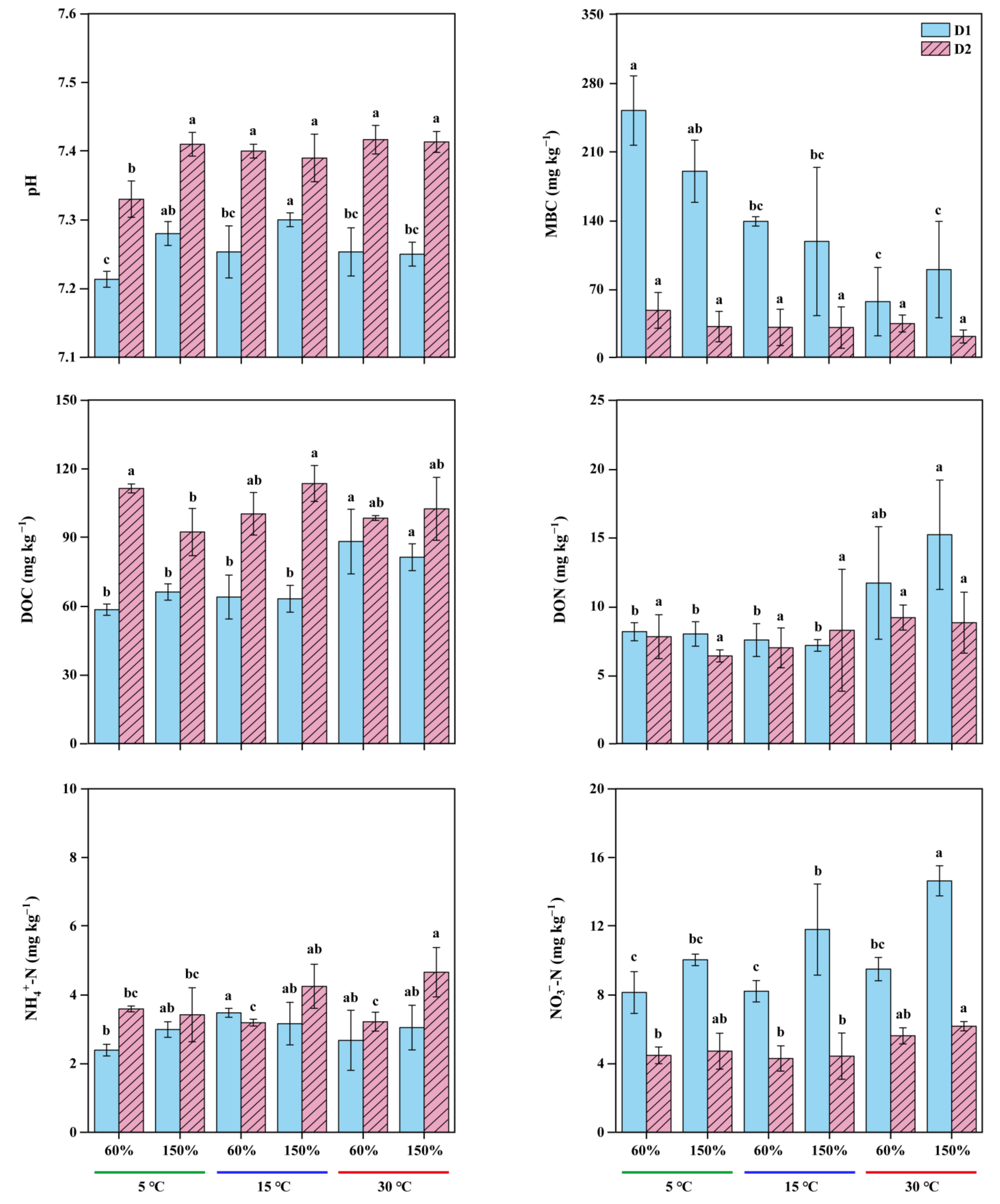
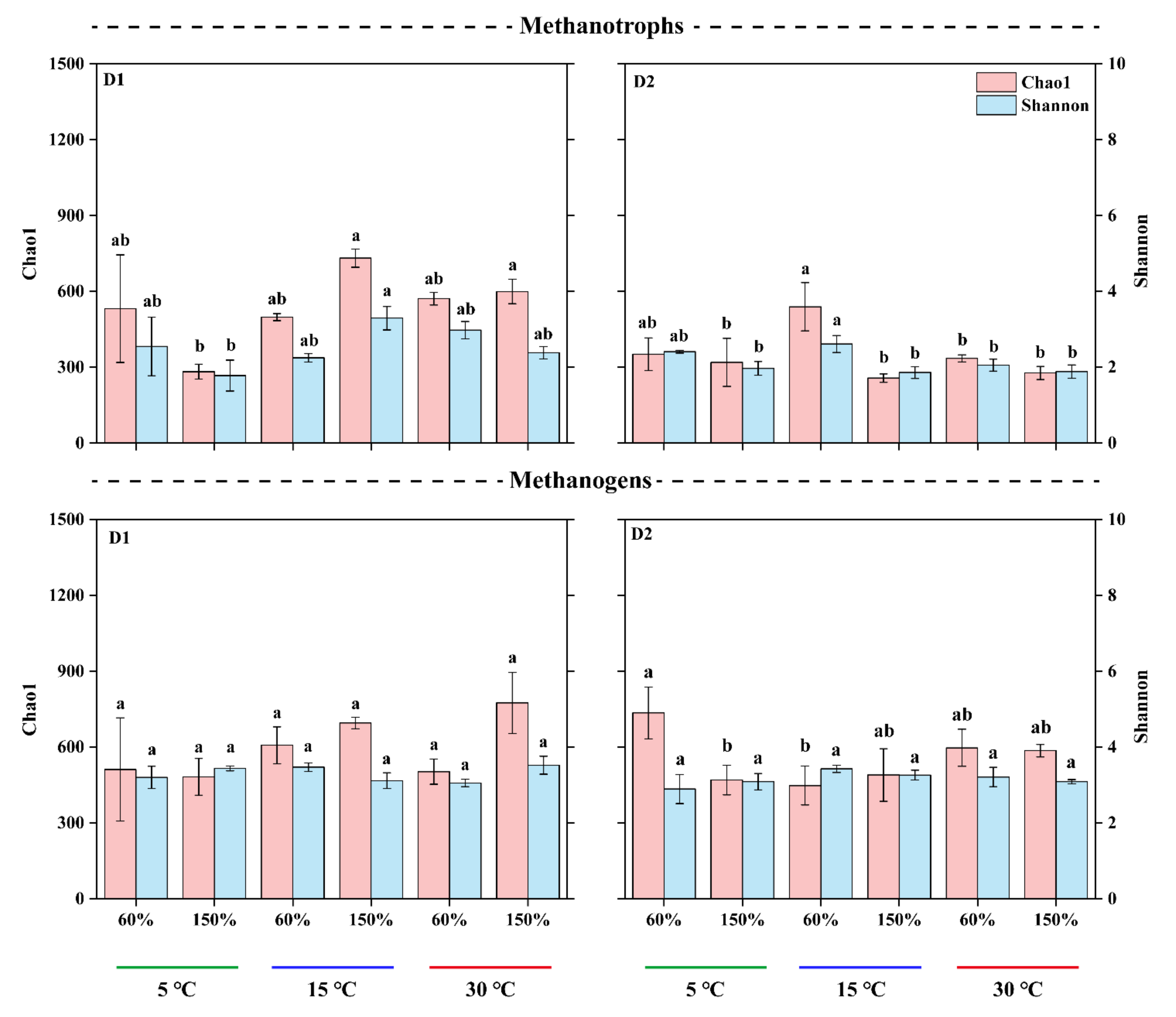
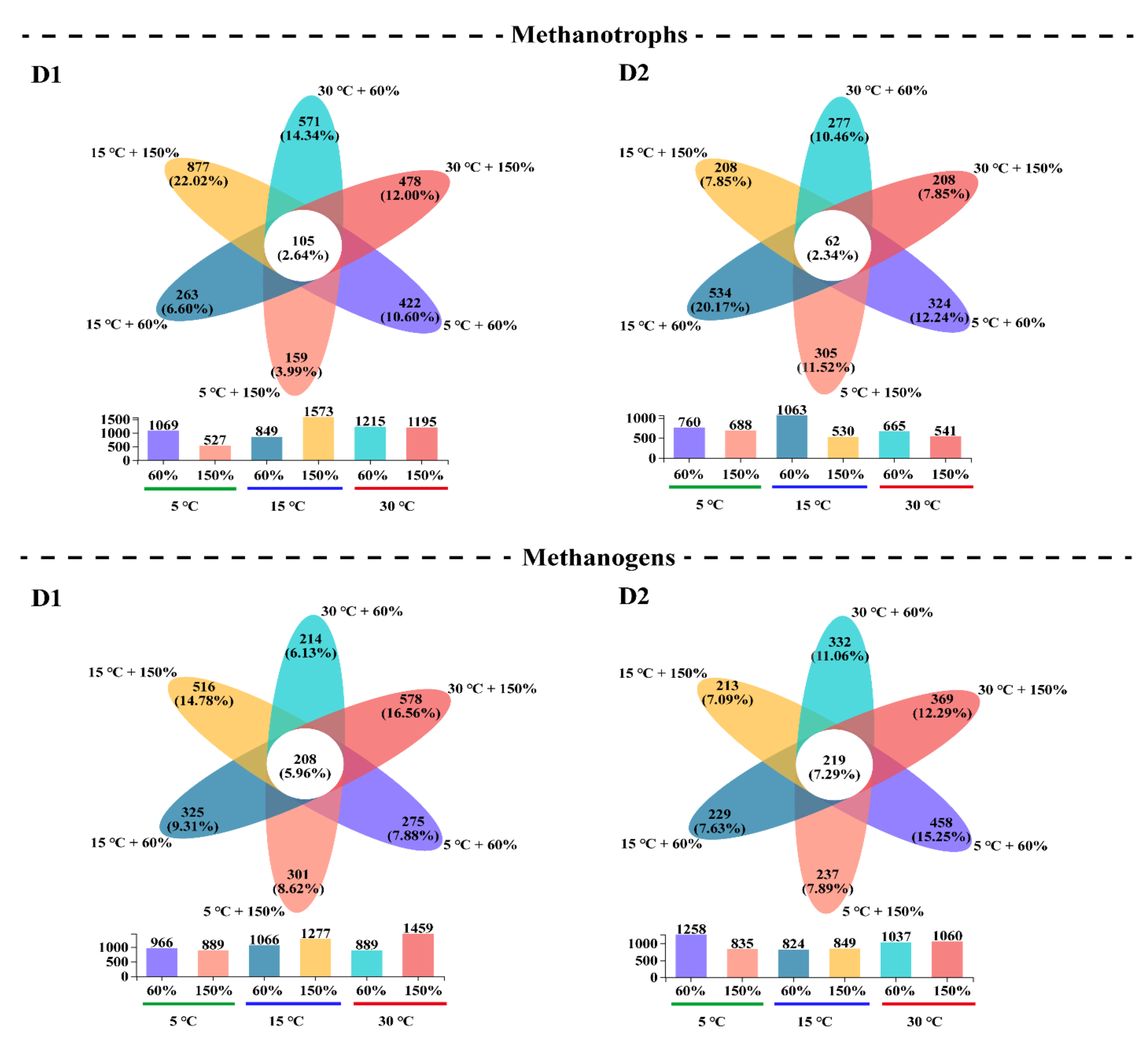
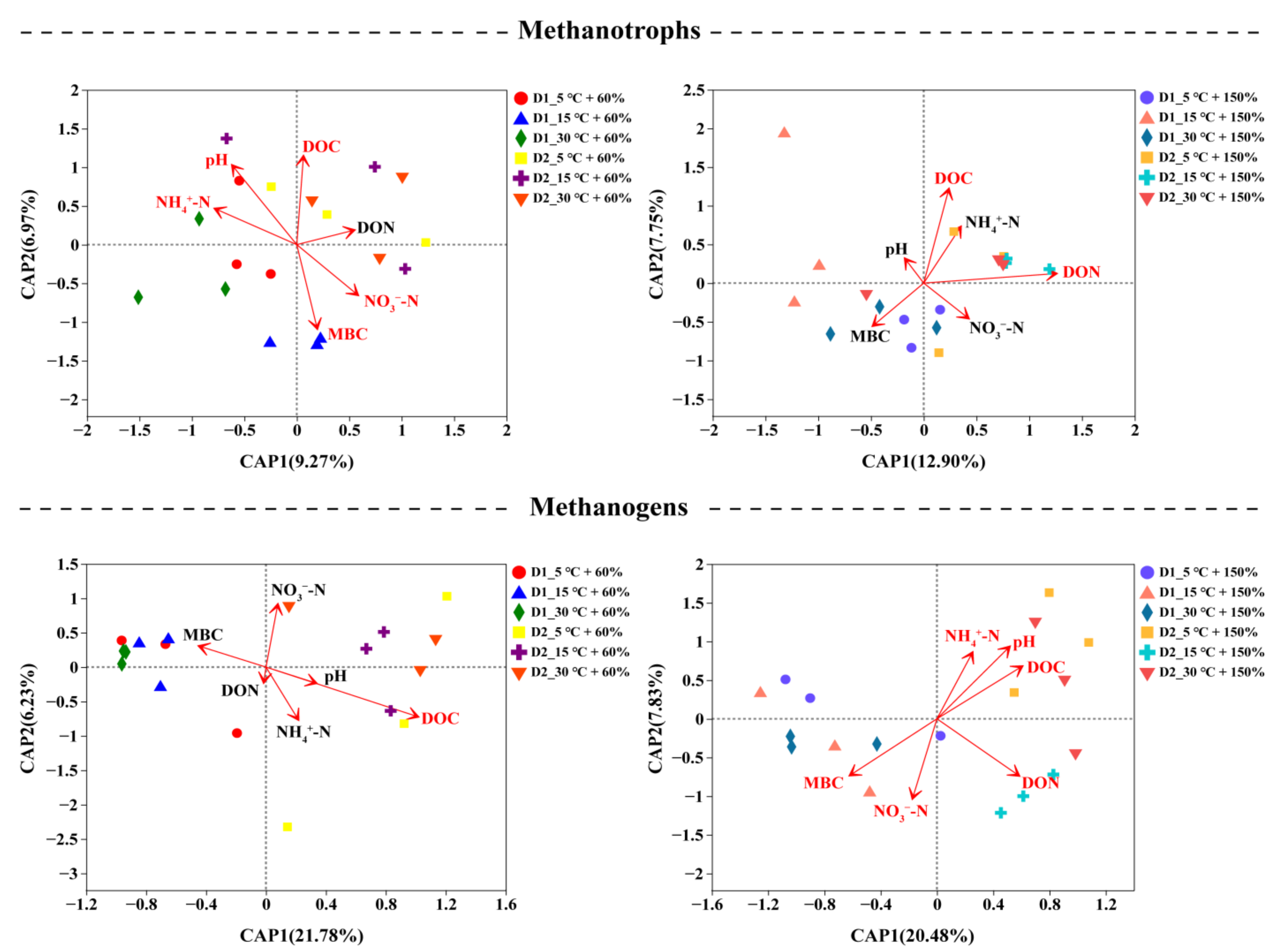
| Depth (cm) | WFPS (%) | CH4 Efflux Rates ± SD (ng g−1 d−1) | Q10 | ||||
|---|---|---|---|---|---|---|---|
| 5 °C | 15 °C | 30 °C | 5–15 °C | 15–30 °C | 5–30 °C | ||
| D1 | 60 | 1.27 ± 0.21 | 1.47 ± 0.10 | 1.95 ± 0.44 | 1.15 | 1.21 | 1.19 |
| 150 | 1.48 ± 0.44 | 1.77 ± 0.30 | 2.18 ± 0.10 | 1.19 | 1.15 | 1.17 | |
| D2 | 60 | 1.18 ± 0.33 | 1.62 ± 0.39 | 2.15 ± 0.60 | 1.37 | 1.21 | 1.27 |
| 150 | 2.09 ± 0.30 | 2.22 ± 0.44 | 2.34 ± 0.58 | 1.06 | 1.04 | 1.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Guo, J.; Malghani, S.; Han, M.; Cao, P.; Sun, J.; Xu, X.; Xu, X.; Wang, W. Effects of Soil Moisture and Temperature on Microbial Regulation of Methane Fluxes in a Poplar Plantation. Forests 2021, 12, 407. https://doi.org/10.3390/f12040407
Feng H, Guo J, Malghani S, Han M, Cao P, Sun J, Xu X, Xu X, Wang W. Effects of Soil Moisture and Temperature on Microbial Regulation of Methane Fluxes in a Poplar Plantation. Forests. 2021; 12(4):407. https://doi.org/10.3390/f12040407
Chicago/Turabian StyleFeng, Huili, Jiahuan Guo, Saadatullah Malghani, Menghua Han, Penghe Cao, Jiejie Sun, Xuan Xu, Xia Xu, and Weifeng Wang. 2021. "Effects of Soil Moisture and Temperature on Microbial Regulation of Methane Fluxes in a Poplar Plantation" Forests 12, no. 4: 407. https://doi.org/10.3390/f12040407
APA StyleFeng, H., Guo, J., Malghani, S., Han, M., Cao, P., Sun, J., Xu, X., Xu, X., & Wang, W. (2021). Effects of Soil Moisture and Temperature on Microbial Regulation of Methane Fluxes in a Poplar Plantation. Forests, 12(4), 407. https://doi.org/10.3390/f12040407








