Pollination Contribution Differs among Insects Visiting Cardiocrinum cordatum Flowers
Abstract
:1. Introduction
2. Materials and Methods
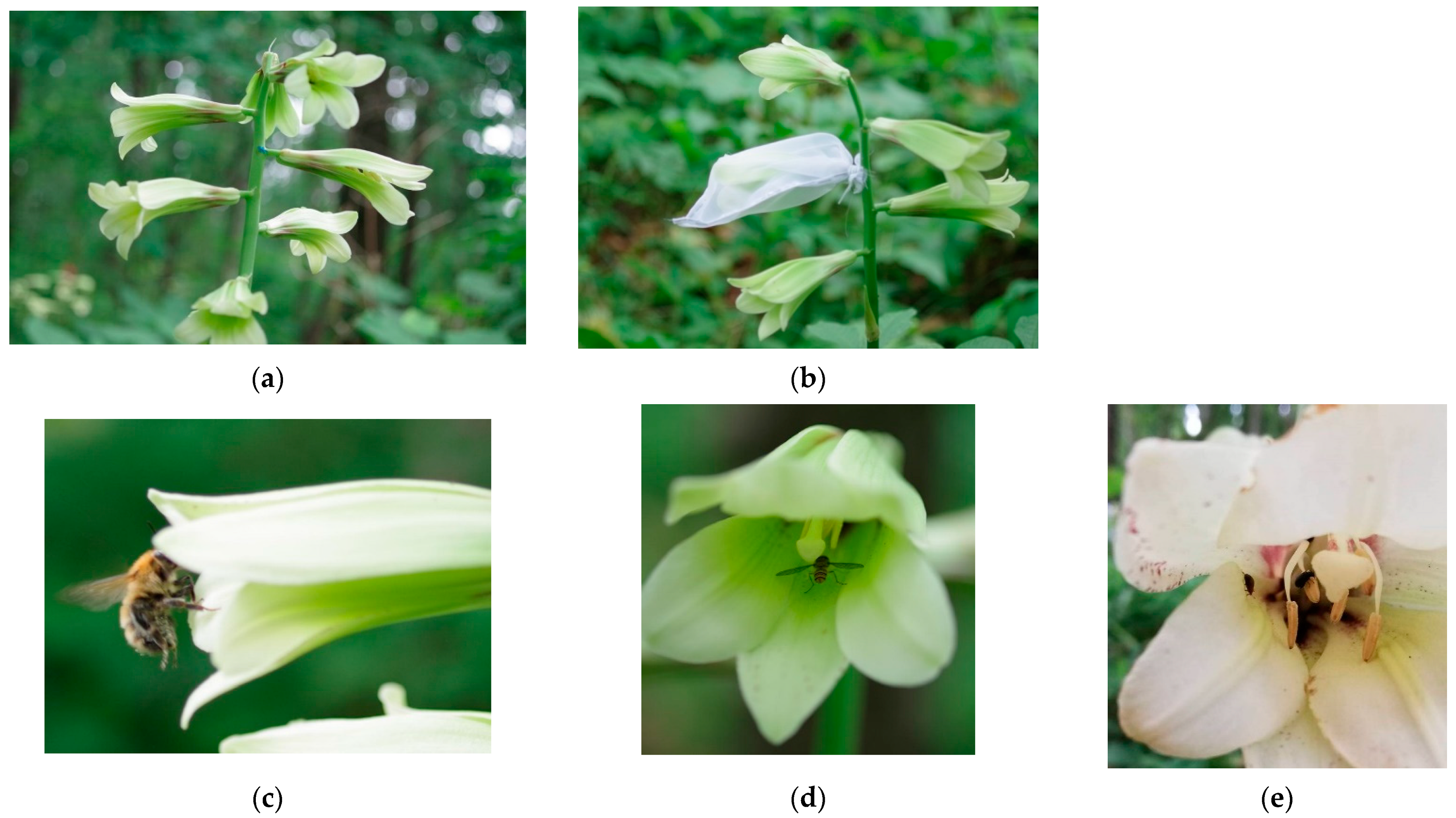
2.1. Study Species
2.2. Study Site
2.3. Monitoring of Flower Visitors
2.4. Pollen Counts
2.5. Pollinator Exclusion Experiment
2.6. Estimating Fruit Set and Seed Number per Fruit
3. Results
3.1. Flower-Visiting Species
3.2. Number of Pollen Grains on the Body Surface
3.3. Pollination Contribution per Single Visit
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collevatti, R.G.; Estolano, R.; Garcia, S.F.; Hay, J.D. Seed abortion in the bat pollinated Neotropical tree species, Caryocar brasiliense (Caryocaraceae). Botany 2009, 87, 1110–1115. [Google Scholar] [CrossRef]
- Kunitake, Y.K.; Hasegawa, M.; Miyashita, T.; Higuchi, H. Role of a seasonally specialist bird Zosterops japonica on pollen transfer and reproductive success of Camellia japonica in a temperate area. Plant Spec. Biol. 2004, 19, 197–201. [Google Scholar] [CrossRef]
- Matsuki, Y.; Tateno, R.; Shibata, M.; Isagi, Y. Pollination efficiencies of flower-visiting insects as determined by direct genetic analysis of pollen origin. Am. J. Bot. 2008, 95, 925–930. [Google Scholar] [CrossRef]
- Nakamura, M.; Nanami, S.; Okuno, S.; Hirota, S.K.; Matsuo, A.; Suyama, Y.; Tokumoto, H.; Yoshihara, S.; Itoh, A. Genetic diversity and structure of apomictic and sexually reproducing Lindera species (Lauraceae) in Japan. Forests 2021, 12, 227. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nikkeshi, A.; Chishiki, A. Identification of effective pollinators of Primula sieboldii E. Morren in a wild habitat in Hiroshima, Japan. Plant Spec. Biol. 2020. [CrossRef]
- Nishizawa, M.; Ohara, M. The role of sexual and vegetative reproduction in the population maintenance of a monocarpic perennial herb, Cardiocrinum cordatum var. glehnii. Plant Spec. Biol. 2018, 33, 289–304. [Google Scholar] [CrossRef]
- Narumi, T.; Ohara, M. Variation in reproductive modes and population genetic structures of a monocarpic perennial herb, Cardiocrinum cordatum, in relation to habitat fragmentation. Plant Spec. Biol. 2018, 33, 248–258. [Google Scholar] [CrossRef]
- Al-Qthanin, R.N.; Alharbi, S.A. Spatial structure and genetic variation of a mangrove species (Avicennia marina (Forssk.) Vierh) in the Farasan Archipelago. Forests 2020, 11, 1287. [Google Scholar] [CrossRef]
- Lu, J.-T.; Qiu, Y.-H.; Lu, J.-B. Effects of landscape fragmentation on genetic diversity of male-biased dioecious plant Pistacia chinensis bunge populations. Forests 2019, 10, 792. [Google Scholar] [CrossRef] [Green Version]
- Collevatti, R.G.; Grattapaglia, D.; Hay, J.D. High resolution microsatellite based analysis of the mating system allows the detection of significant biparental inbreeding in Caryocar brasiliense, an endangered tropical tree species. Heredity 2001, 86, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Geerts, S.; Coetzee, A.; Rebelo, A.G.; Pauw, A. Pollination structures plant and nectar-feeding bird communities in Cape fynbos, South Africa: Implications for the conservation of plant–bird mutualisms. Ecol. Res. 2020, 35, 838–856. [Google Scholar] [CrossRef]
- Herrera, C.M. Components of pollinator “quality”: Comparative analysis of a diverse insect assemblage. Oikos 1987, 50, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Herrera, C.M. Pollinator abundance, morphology, and flower visitation rate: Analysis of the “quantity” component in a plant-pollinator system. Oecologia 1989, 80, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. A general framework for effectiveness concepts in mutualisms. Ecol. Lett. 2017, 20, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Conceição, S.I.R.; Fernandes, J.; Borges da Silva, E.; Caperta, A.D. Reproductive output and insect behavior in hybrids and apomicts from Limonium ovalifolium and L. binervosum complexes (Plumbaginaceae) in an open cross-pollination experiment. Plants 2021, 10, 169. [Google Scholar] [CrossRef]
- Tokumoto, Y.; Matsushita, M.; Kishimoto-Yamada, K.; Nikkeshi, A.; Isogimi, T.; Nakagawa, M. Floral visitors and reproductive success in two sequentially flowering Lindera shrubs (Lauraceae) of central Japan. J. For. Res. 2019, 24, 42–51. [Google Scholar] [CrossRef]
- Yamasaki, E.; Sakai, S. Wind and insect pollination (ambophily) of Mallotus spp. (Euphorbiaceae) in tropical and temperate forests. Aust. J. Bot. 2013, 61, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Giblin, D.E. Variation in floral longevity between populations of Campanula rotundifolia (Campanulaceae) in response to fitness accrual rate manipulation. Am. J. Bot. 2005, 92, 1714–1722. [Google Scholar] [CrossRef]
- Soley, N.M.; Sipes, S.D. Reproductive biology and pollinators of the invasive shrub Autumn olive (Elaeagnus umbellata Thunberg). Plant Spec. Biol. 2020. [CrossRef]
- Hou, S.; Zhao, T.; Yang, D.; Li, Q.; Liang, L.; Wang, G.; Ma, Q. Selection and validation of reference genes for quantitative RT-PCR analysis in Corylus heterophylla Fisch. × Corylus avellana L. Plants 2021, 10, 159. [Google Scholar] [CrossRef]
- Bentrup, G.; Hopwood, J.; Adamson, N.L.; Vaughan, M. Temperate agroforestry systems and insect pollinators: A review. Forests 2019, 10, 981. [Google Scholar] [CrossRef] [Green Version]
- Tran, X.T.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Reduced pollination efficiency compromises some physicochemical qualities in gac (Momordica cochinchinensis Spreng.) fruit. Agronomy 2021, 11, 190. [Google Scholar] [CrossRef]
- Fernández, F.J.; Garay, J.; Móri, T.F.; Csiszár, V.; Varga, Z.; López, I.; Gámez, M.; Cabello, T. Theoretical foundation of the control of pollination by hoverflies in a greenhouse. Agronomy 2021, 11, 167. [Google Scholar] [CrossRef]
- Aizen, M.A.; Aguiar, S.; Biesmeijer, J.C.; Garibaldi, L.A.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Morales, C.L.; Ngo, H.; et al. Global agricultural productivity is threatened by increasing pollinator dependence without a parallel increase in crop diversification. Glob. Chang. Biol. 2019, 25, 3516–3527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusser, S.; Neff, J.L.; Jha, S. Natural land cover drives pollinator abundance and richness, leading to reductions in pollen limitation in cotton agroecosystems. Agr. Ecosyst. Environ. 2016, 226, 33–42. [Google Scholar] [CrossRef] [Green Version]
- McGrady, C.M.; Troyer, R.; Fleischer, S.J. Wild bee visitation rates exceed pollination thresholds in commercial Cucurbita agroecosystems. J. Econ. Entomol. 2020, 113, 562–574. [Google Scholar] [CrossRef]
- Pfister, S.C.; Eckerter, P.W.; Schirmel, J.; Cresswell, J.E.; Entling, M.H. Sensitivity of commercial pumpkin yield to potential decline among different groups of pollinating bees. R. Soc. Open Sci. 2017, 4, 170102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senapathi, D.; Fründ, J.; Albrecht, M.; Garratt, M.P.D.; Kleijn, D.; Pickles, B.J.; Potts, S.G.; An, J.; Andersson, G.K.S.; Bänsch, S.; et al. Wild insect diversity increases inter-annual stability in global crop pollinator communities. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210212. [Google Scholar] [CrossRef]
- Nikkeshi, A.; Inoue, H.; Arai, T.; Kishi, S.; Kamo, T. The bumblebee Bombus ardens ardens (Hymenoptera: Apidae) is the most important pollinator of Oriental persimmon, Diospyros kaki (Ericales: Ebenaceae), in Hiroshima, Japan. Appl. Entomol. Zool. 2019, 54, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, A.L.; Harder, L.D.; Johnson, S.D. Consumptive emasculation: The ecological and evolutionary consequences of pollen theft. Biol. Rev. 2009, 84, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Irwin, R.E.; Bronstein, J.L.; Manson, J.S.; Richardson, L. Nectar robbing: Ecological and evolutionary perspectives. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 271–292. [Google Scholar] [CrossRef]
- Ohara, M.; Narumi, T.; Yoshizane, T.; Okayasu, T.; Masuda, J.; Kawano, S. 7: Cardiocrinum cordatum (Thunb.) Makino (Liliaceae). Plant Spec. Biol. 2006, 21, 201–207. [Google Scholar] [CrossRef]
- Nagamitsu, T.; Tsukuba, S.-A.; Ushirokita, F.; Konno, Y. Foraging habitats and floral resource use by colonies of long- and short-tongued bumble bee species in an agricultural landscape with kabocha squash fields. Appl. Entomol. Zool. 2012, 47, 181–190. [Google Scholar] [CrossRef]
- Konno, Y. Present status of remnant forests in Obihro, eastern Hokkaido, Japan. In Global Perspective in Forest Conservation and Sustainable Agriculture; Obihiro Asia and the Pacific Seminar on Education for Rural Development (OASERD): Obihiro, Japan, 2002; pp. 39–46. [Google Scholar]
- Matsumura, C.; Yokoyama, J.; Washitani, I. Invasion status and potential ecological impacts of an invasive alien bumblebee, Bombus terrestris L. (Hymenoptera: Apidae) naturalized in Southern Hokkaido, Japan. Glob. Environ. Res. 2004, 8, 51–66. [Google Scholar]
- Cao, G.-X.; Kudo, G. Size-dependent sex allocation in a monocarpic perennial herb, Cardiocrinum cordatum (Liliaceae). Plant Ecol. 2008, 194, 99–107. [Google Scholar] [CrossRef]
- Lu, R.-S.; Chen, Y.; Tamaki, I.; Sakaguchi, S.; Ding, Y.-Q.; Takahashi, D.; Li, P.; Isagi, Y.; Chen, J.; Qiu, Y.-X. Pre-quaternary diversification and glacial demographic expansions of Cardiocrinum (Liliaceae) in temperate forest biomes of Sino-Japanese Floristic Region. Mol. Phylogenet. Evol. 2020, 143, 106693. [Google Scholar] [CrossRef]
- Lu, R.-S.; Li, P.; Qiu, Y.-X. The complete chloroplast genomes of three Cardiocrinum (Liliaceae) species: Comparative genomic and phylogenetic analyses. Front. Plant Sci. 2017, 7, 2054. [Google Scholar] [CrossRef]
- Cao, G.X.; Worley, A.C. Life history trade-offs and evidence for hierarchical resource allocation in two monocarpic perennials. Plant Biol. 2013, 15, 158–165. [Google Scholar] [CrossRef]
- Koyama, K.; Hidaka, Y.; Ushio, M. Dynamic scaling in the growth of a non-branching plant, Cardiocrinum cordatum. PLoS ONE 2012, 7, e45317. [Google Scholar] [CrossRef]
- Araki, K.; Shimatani, K.; Nishizawa, M.; Yoshizane, T.; Ohara, M. Growth and survival patterns of Cardiocrinum cordatum var. glehnii (Liliaceae) based on a 13-year monitoring study: Life history characteristics of a monocarpic perennial herb. Botany 2010, 88, 745–752. [Google Scholar] [CrossRef]
- Kondo, T.; Sato, C.; Baskin, J.M.; Baskin, C.C. Post-dispersal embryo development, germination phenology, and seed dormancy in Cardiocrinum cordatum var. glehnii (Liliaceae s. str.), a perennial herb of the broadleaved deciduous forest in Japan. Am. J. Bot. 2006, 93, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hayafune, T.; Utech, F.H.; Ohara, M. Inter-populational variation, but no-annual variation within populations, in terms of reproductive size and genetic structure in a monocarpic perennial herb, Cardiocrinum cordatum var. glehnii. Plant Spec. Biol. 2019, 34, 27–30. [Google Scholar] [CrossRef]
- Hori, K.; Watanabe, T.; Devkota, H.P. Phenolic acid derivatives, flavonoids and other bioactive compounds from the leaves of Cardiocrinum cordatum (Thunb.) Makino (Liliaceae). Plants 2021, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Nishiumi, S.; Hosokawa, K.; Anetai, M.; Shibata, T.; Mukai, R.; Yoshida, K.-i.; Ashida, H. Antagonistic effect of the ainu-selected traditional beneficial plants on the transformation of an aryl hydrocarbon receptor. J. Food Sci. 2012, 77, C420–C429. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: http://www.jma.go.jp (accessed on 14 September 2020).
- Iwabe, R.; Koyama, K.; Komamura, R. Shade avoidance and light foraging of a clonal woody species, Pachysandra terminalis. Plants. under review.
- Takenaka, A. CanopOn 2 ver. 2.03c. 2009. Available online: http://takenaka-akio.org/etc/canopon2/index.html (accessed on 26 September 2020).
- Parker, A.J.; Tran, J.L.; Ison, J.L.; Bai, J.D.K.; Weis, A.E.; Thomson, J.D. Pollen packing affects the function of pollen on corbiculate bees but not non-corbiculate bees. Arthropod-Plant Int. 2015, 9, 197–203. [Google Scholar] [CrossRef]
- Sakai, S. Handbook of Methods in Ecological Research 2: Field Methods in Pollination Ecology; Kyritsu Publishing: Tokyo, Japan, 2015. (In Japanese) [Google Scholar]
- Sutherland, S.D. Why hermaphroditic plants produce many more flowers than fruits: Experimental tests with Agave mckelveyana. Evolution 1987, 41, 750–759. [Google Scholar] [CrossRef]
- Koyama, K.; Tashiro, M. No effect of selective maturation on fruit traits for a bird-dispersed species, Sambucus racemosa. Plants 2021, 10, 376. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kobayashi, S.; Inoue, K.; Kato, M. Evidence of pollen transfer efficiency as the natural selection factor favoring a large corolla of Campanula punctata pollinated by Bombus diversus. Oecologia 1997, 111, 535–542. [Google Scholar] [CrossRef]
- Katayama, E. Studies on the development of the broods of Bombus diversus Smith (Hymenoptera, Apidae): II. Brood development and feeding habits. Kontyu 1966, 34, 8–17. [Google Scholar]
- Darvill, B.; Knight, M.E.; Goulson, D. Use of genetic markers to quantify bumblebee foraging range and nest density. Oikos 2004, 107, 471–478. [Google Scholar] [CrossRef]
- van Rijn, P.C.J.; Wäckers, F.L. Nectar accessibility determines fitness, flower choice and abundance of hoverflies that provide natural pest control. J. Appl. Ecol. 2016, 53, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Inouye, D.W. The effect of proboscis and corolla tube lengths on patterns and rates of flower visitation by bumblebees. Oecologia 1980, 45, 197–201. [Google Scholar] [CrossRef]
- Inoue, M.N.; Yokoyama, J. Competition for flower resources and nest sites between Bombus terrestris (L.) and Japanese native bumblebees. Appl. Entomol. Zool. 2010, 45, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Kato, M. Hana ni Hikiyose Rareru Dobutsu—Hana to Doubutsu no Kyoshinka; Heibonsya: Tokyo, Japan, 1993. (In Japanese) [Google Scholar]
- van Rijn, P.C.J.; Kooijman, J.; Wäckers, F.L. The contribution of floral resources and honeydew to the performance of predatory hoverflies (Diptera: Syrphidae). Biol. Control 2013, 67, 32–38. [Google Scholar] [CrossRef]
- Rostás, M.; Bollmann, F.; Saville, D.; Riedel, M. Ants contribute to pollination but not to reproduction in a rare calcareous grassland forb. PeerJ 2018, 6, e4369. [Google Scholar] [CrossRef] [Green Version]
- Rostás, M.; Tautz, J. Ants as pollinators of plants and the role of floral scents. In All Flesh Is Grass: Plant-Animal Interrelationships; Dubinsky, Z., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 149–161. [Google Scholar] [CrossRef]
- de Vega, C.; Herrera, C.M.; Dötterl, S. Floral volatiles play a key role in specialized ant pollination. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 32–42. [Google Scholar] [CrossRef] [Green Version]
- Collevatti, R.G.; Amara, M.E.C.; Lopes, F.S. Role of pollinators in seed set and a test of pollen limitation hypothesis in the tropical weed Triumfetta semitriloba (Tiliaceae). Rev. Biol. Trop. 1997, 45, 1401–1407. [Google Scholar]


| Flower Age (Day) | 0 | 1 | 2 | 3 | 4 | Total |
|---|---|---|---|---|---|---|
| Bumblebee (Bombus diversus tersatus) | 3.92/3.92 | 1.64/1.64 | 0.78/0.78 | 0 | 0.23/0.23 | 6.57/6.57 |
| Sweat bee (Halictidae sp.) | 0.23/0.23 | 0.68/0.82 | 0.78/0.91 | 0.77/0.92 | 0.46/1.15 | 2.93/4.04 |
| Marmalade hoverfly (Episyrphus balteatus) | 1.15/1.62 | 3.55/4.91 | 1.70/3.13 | 1.08/2.15 | 0.69/0.92 | 8.16/12.73 |
| Ant (Myrmica ruginodis (s.l.)) | 1.15/2.77 | 1.64/4.09 | 1.04/3.39 | 2.46/4.77 | 2.54/4.15 | 8.83/19.17 |
| Leaf beetle (Chrysomelidae sp.) | 0.46/0.46 | 0.27/0.41 | 0.65/0.78 | 0.77/0.77 | 0.46/0.46 | 2.62/2.88 |
| Sap beetle (Nitidulidae sp.) | 0 | 0 | 0 | 0 | 0.00/0.23 | 0.00/0.23 |
| Spider (Araneae sp.) 1 | 0 | 0.00/0.14 | 0 | 0.00/0.15 | 0 | 0.00/0.29 |
| Earwig (Dermaptera sp.) 1 | 0 | 0 | 0 | 0.00/0.15 | 0.00/0.23 | 0.00/0.38 |
| Mosquito (Culicidae sp.) 1,2 | 0.00/0.23 | 0.00/0.55 | 0 | 0.00/0.15 | 0.00/0.23 | 0.00/1.16 |
| Unidentified species 1 | 0 | 0.00/0.14 | 0 | 0.15/0.15 | 0.00/0.46 | 0.15/0.75 |
| Total | 6.92/9.23 | 7.77/12.68 | 4.96/9.00 | 5.23/9.23 | 4.38/8.08 | 29.27/48.22 |
| Flower Age (Day) | 0 | 1 | 2 | 3 | 4 | Total |
|---|---|---|---|---|---|---|
| Bumblebee (Bombus diversus tersatus) | 17/17 | 12/12 | 6/6 | 0/0 | 1/1 | 36/36 |
| Sweat bee (Halictidae sp.) | 1/1 | 5/6 | 6/7 | 5/6 | 2/5 | 19/25 |
| Marmalade hoverfly (Episyrphus balteatus) | 5/7 | 26/36 | 13/24 | 7/14 | 3/4 | 54/85 |
| Ant (Myrmica ruginodis (s.l.)) | 5/12 | 12/30 | 8/26 | 16/31 | 11/18 | 52/117 |
| Leaf beetle (Chrysomelidae sp.) | 2/2 | 2/3 | 5/6 | 5/5 | 2/2 | 16/18 |
| Sap beetle (Nitidulidae sp.) | 0/0 | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 |
| Spider (Araneae sp.) 1 | 0/0 | 0/1 | 0/0 | 0/1 | 0/0 | 0/2 |
| Earwig (Dermaptera sp.) 1 | 0/0 | 0/0 | 0/0 | 0/1 | 0/1 | 0/2 |
| Mosquito (Culicidae sp.) 1,2 | 0/1 | 0/4 | 0/0 | 0/1 | 0/1 | 0/7 |
| Unidentified species 1 | 0/0 | 0/1 | 0/0 | 1/1 | 0/2 | 1/4 |
| Total | 30/40 | 57/93 | 38/69 | 34/60 | 19/35 | 178/297 |
| Species | Total No. of Individual Visitors Captured | No. of Pollens on Each Body Surface | |
|---|---|---|---|
| Mean | SD | ||
| Bumblebee (Bombus diversus tersatus) | 15 | 54,328 | 44,745.5 |
| Sweat bee (Halictidae sp.) | 10 | 25,913 | 25,902.5 |
| Marmalade hoverfly (Episyrphus balteatus) | 15 | 1468.5 | 1813.8 |
| Ant (Myrmica ruginodis (s.l.)) | 4 | 14 | 15.7 |
| Leaf beetle (Chrysomelidae sp.) | 4 | 48.8 | 14.9 |
| Sap beetle (Nitidulidae sp.) | 1 | 0 | - |
| Spider (Araneae sp.) | 1 | 166 | - |
| Earwig (Dermaptera sp.) | 1 | 10 | - |
| Visitor | No. of Flowers Investigated (FL) | No. of Fruits Produced (FR) | Fruit Set (FR/FL) (%) | No. of Fruits Investigated for Seed Counts | Mean No. of Seeds per Fruit | |
|---|---|---|---|---|---|---|
| Mean | SD | |||||
| Uncovered flowers 1 | 84 | 27 | 32.1 | 22 | 516.3 | 93.2 |
| Bumblebee (Bombus diversus tersatus) | 22 | 7 | 31.8 | 7 | 364.6 | 193.4 |
| Sweat bee (Halictidae sp.) | 13 | 3 | 23.1 | 3 | 215.2 | 246.3 |
| Marmalade hoverfly (Episyrphus balteatus) | 22 | 5 | 22.7 | 5 | 194.8 | 259.7 |
| Ant (Myrmica ruginodis (s.l.) | 1 | 1 | 100 | 1 | 78.6 | - |
| Complete pollinator exclusion | 15 | 0 | 0 | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komamura, R.; Koyama, K.; Yamauchi, T.; Konno, Y.; Gu, L. Pollination Contribution Differs among Insects Visiting Cardiocrinum cordatum Flowers. Forests 2021, 12, 452. https://doi.org/10.3390/f12040452
Komamura R, Koyama K, Yamauchi T, Konno Y, Gu L. Pollination Contribution Differs among Insects Visiting Cardiocrinum cordatum Flowers. Forests. 2021; 12(4):452. https://doi.org/10.3390/f12040452
Chicago/Turabian StyleKomamura, Riko, Kohei Koyama, Takeo Yamauchi, Yasuo Konno, and Lingshuang Gu. 2021. "Pollination Contribution Differs among Insects Visiting Cardiocrinum cordatum Flowers" Forests 12, no. 4: 452. https://doi.org/10.3390/f12040452
APA StyleKomamura, R., Koyama, K., Yamauchi, T., Konno, Y., & Gu, L. (2021). Pollination Contribution Differs among Insects Visiting Cardiocrinum cordatum Flowers. Forests, 12(4), 452. https://doi.org/10.3390/f12040452







