Regeneration of Riparian and Maritime Pine Forests after a Large Wildfire on the Largest Public Forest of Portugal
Abstract
:1. Introduction
- Are there differences in plant composition between understory vegetation of pine stands and riparian ecosystems after the fire?
- Does fire severity affect the regeneration potential of pine stands and riparian forests?
- Does the age of pines significantly influence the regeneration of pine stands?
2. Materials and Methods
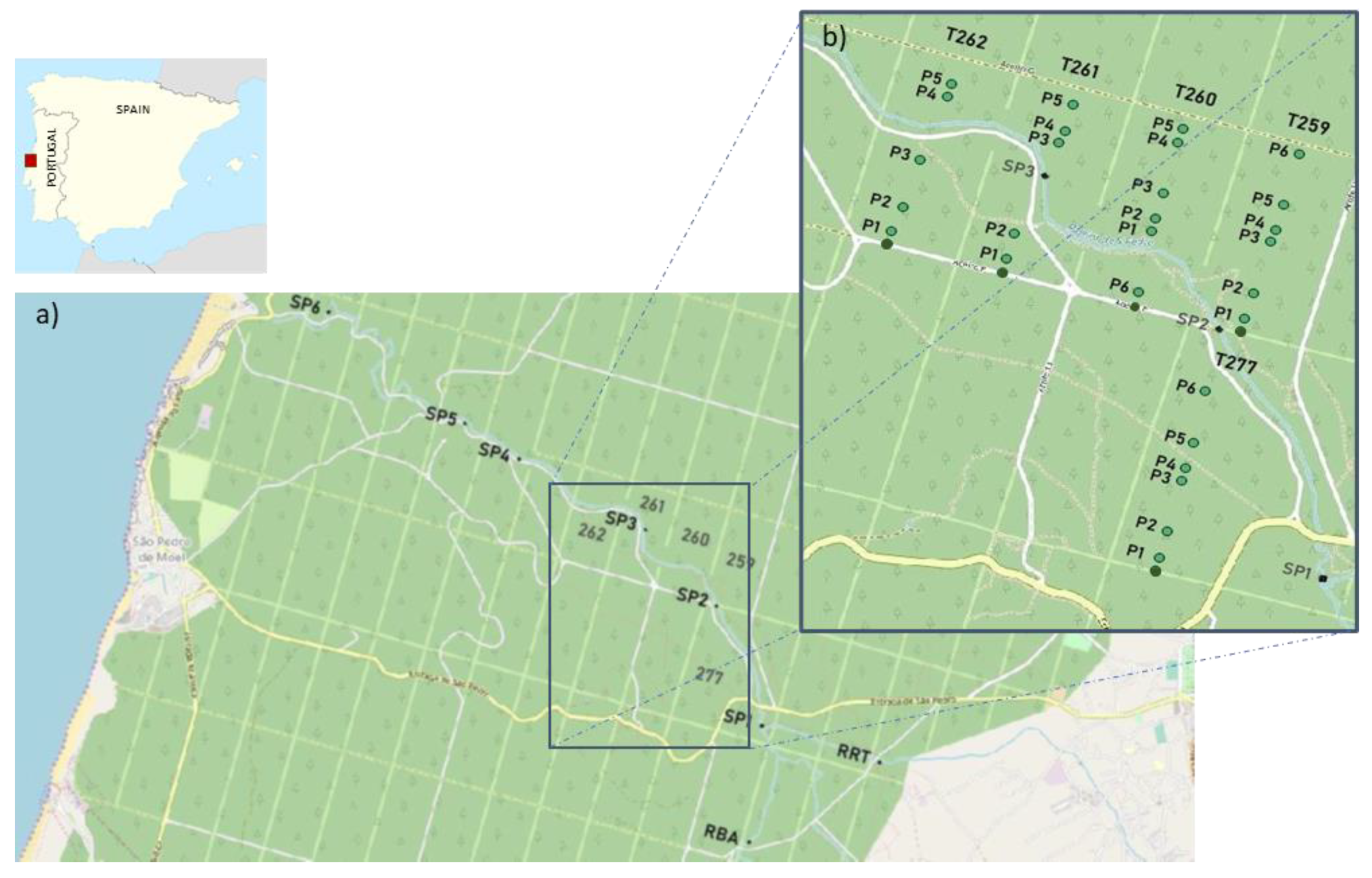
2.1. Study Area
2.2. Sampling Design and Surveys
2.3. Fire Severity and Age of Pine Stands
2.4. Statistical Analysis
3. Results
3.1. Floristic Composition
3.1.1. Overview
3.1.2. Differences in Understory Vegetation between the Burnt Pine Stands and São Pedro River
3.2. Effect of Age and Fire Severity on Natural Regeneration of Pine Stands
3.3. Effect of Fire Severity on the Species Composition on São Pedro River
3.4. Spatial and Temporal Differences on Natural Regeneration on São Pedro River
4. Discussion
4.1. Regeneration Potential of MNL Pine Stands
4.2. Regeneration Potential of Understory Vegetation in Pine Stands and São Pedro River
4.3. Limitations and Future Research
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFFIS. European Forest Fire Information System—Estimates per Country. Available online: https://effis.jrc.ec.europa.eu/static/effis.statistics.portal/effis-estimates/EU (accessed on 2 February 2021).
- ICNF. 10° Relatório Provisório de Incêndios Florestais-2017; RIF 10/2017; Instituto de Conservação da Natureza e das Florestas: Lisboa, Portugal, 2017. [Google Scholar]
- Pinto, A.A. O Pinhal do Rei—Subsídios; Oficina José de Oliveira Junior: Alcobaça, Portugal, 1938. [Google Scholar]
- AFN. Plano de Gestão Florestal da Mata Nacional de Leiria; Unidade de Gestão do Centro Litoral, Ministério da Agricultura, do Desenvolvimento Rural e das Pescas: Lisboa, Portugal, 2010; p. 189. [Google Scholar]
- Botequim, B.; Fernandes, P.M.; Garcia-Gonzalo, J.; Silva, A.; Borges, J.G. Coupling fire behaviour modelling and stand characteristics to assess and mitigate fire hazard in a maritime pine landscape in Portugal. Eur. J. For. Res. 2017, 136, 527–542. [Google Scholar] [CrossRef]
- Castro, A.C.M.; Nunes, A.; Sousa, A.; Lourenço, L. Mapping the causes of forest fires in Portugal by clustering analysis. Geosciences 2020, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Clerici, N.; Paracchini, M.L.; Maes, J. Land-cover change dynamics and insights into ecosystem services in European stream riparian zones. Ecohydrol. Hydrobiol. 2014, 14, 107–120. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Bentz, J.; Silva, J.M.N.; Fonseca, A.L.; Swart, R.; Santos, F.D.; Penha-Lopes, G. Adaptation to climate change at local level in Europe: An overview. Environ. Sci. Policy 2018, 86, 38–63. [Google Scholar] [CrossRef]
- Buhk, C.; Götzenberger, L.; Karsten, W.; Gómez, P.S.; Hensen, I.; Wesche, K. Post-fire regeneration in a Mediterranean pine forest with historically low fire frequency. Acta Oecol. 2006, 30, 288–298. [Google Scholar] [CrossRef]
- Marais, K.E.; Pratt, R.B.; Jacobs, S.M.; Jacobsen, A.L.; Esler, K.J. Postfire regeneration of resprouting mountain fynbos shrubs: Differentiating obligate resprouters and facultative seeders. Plant Ecol. 2014, 215, 195–208. [Google Scholar] [CrossRef]
- Águas, A.; Larcombe, M.J.; Matias, H.; Deus, E.; Potts, B.M.; Rego, F.C.; Silva, J.S. Understanding the naturalization of Eucalyptus globulus in Portugal: A comparison with Australian plantations. Eur. J. For. Res. 2017, 136, 433–446. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Epicormic Resprouting in Fire-Prone Ecosystems. Trends Plant Sci. 2017, 22, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Soares, P.; Calado, N.; Carneiro, S. Manual de Boas Práticas Para o Pinheiro-Bravo; Centro Pinus: Lisboa, Portugal, 2020; p. 31. [Google Scholar]
- Reyes, O.; Casal, M. Effect of high temperatures on cone opening and on the release and viability of Pinus pinaster and Pinus radiata seeds in NW Spain. Ann. For. Sci. 2002, 59, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.M.; Rigolot, E. The fire ecology and management of maritime pine (Pinus pinaster Ait). For. Ecol. Manag. 2007, 241, 1–13. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire intensity, fire severity and burn severity: A brief review and suggested usage. Int. J. Wildland Fire 2009, 18, 116–126. [Google Scholar] [CrossRef]
- Maia, P.; Pausas, J.G.; Vasques, A.; Keiser, J.J. Fire severity as a key factor in post-fire regeneration of Pinus pinaster (Ait.) in Central Portugal. Ann. For. Sci. 2012, 69, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Monteiro-Henriques, T.; Fernandes, P.M. Regeneration of Native Forest Species in Mainland Portugal: Identifying Main Drivers. Forests 2018, 9, 694. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.F.; Bento, J.S.; Rego, F. Regeneration of Pinus pinaster forests after wildfire. In Proceedings of the Third international Symposium on Fire Ecology, Freiburg, Germany, 16–20 May 1989; SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 71–75. [Google Scholar]
- Santos, L.; Capelo, J.; Tavares, M. Germination Patterns of Soil Seed Banks in Relation to Fire in Portuguese Littoral Pine Forest Vegetation. Fire Ecol. 2010, 6, 1–15. [Google Scholar] [CrossRef]
- Urza, A.K.; Weisberg, P.J.; Chambers, J.C.; Dhaemers, J.M.; Board, D. Post-fire vegetation response at the woodland–shrubland interface is mediated by the pre-fire community. Ecosphere 2017, 8, e01851. [Google Scholar] [CrossRef]
- Espírito-Santo, M.D.; Capelo, J.H. Ten years of observations after wildfire on permanent plots in Central Mediterranean Portugal. In Fire Management and Landscape Ecology; Trabaud, L., Ed.; International Association of Wildland Fire: Fairfield, WA, USA, 1998; pp. 87–101. [Google Scholar]
- Silva, J.S.; Vaz, P.; Moreira, F.; Catry, F.; Rego, F.C. Wildfires as a major driver of landscape dynamics in three fire-prone areas of Portugal. Landsc. Urban Plan. 2011, 101, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Riis, T.; Kelly-Quinn, M.; Aguiar, F.C.; Manolaki, P.; Bruno, D.; Bejarano, M.D.; Clerici, N.; Fernandes, M.R.; Franco, J.C.; Pettit, N.; et al. Global Overview of Ecosystem Services Provided by Riparian Vegetation. BioScience 2020, 70, 501–514. [Google Scholar] [CrossRef]
- Ferreira, T.; Rivaes, R.; Branco, P.; Santos, J.L.; Catry, F.; Aguiar, F.; Segurado, P.; Fabião, A.; Santos, J.M.; Abrantes, N.; et al. Recuperação de Ecossistemas Aquáticos e Ripícolas; Comissão Científica do Programa de Recuperação das Matas Litorais, ICNF: Lisboa, Portugal, 2018. [Google Scholar]
- Bixby, R.J.; Cooper, S.D.; Gresswell, R.E.; Brown, L.E.; Dahm, C.N.; Dwire, K.A. Fire effects on aquatic ecosystems: An assessment of the current state of the science. Freshw. Sci. 2015, 34, 1340–1350. [Google Scholar] [CrossRef]
- Pettit, N.E.; Naiman, R.J. Fire in the Riparian Zone: Characteristics and Ecological Consequences. Ecosystems 2007, 10, 673–687. [Google Scholar] [CrossRef]
- Pettit, N.E.; Naiman, R.J. Postfire response of flood-regenerating riparian vegetation in a semi-arid landscape. Ecology 2007, 88, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.I.D.B.F. Factores Facilitadores da Invasibilidade de Acacia dealbata em Função do Uso do Solo. Master’s Thesis, University of Lisbon, Lisbon, Portugal, 2017. [Google Scholar]
- Vaz, P.G.; Merten, E.C.; Warren, D.R.; Durscher, K.; Tapp, M.; Robinson, C.T.; Rego, F.C.; Pinto, P. Fire meets inland water via burned wood: And then what? Freshw. Sci. 2015, 34, 1468–1481. [Google Scholar] [CrossRef] [Green Version]
- Madeira, M.; Fabião, A.; Páscoa, F.; Magalhães, M.C.; Cameira, M.C.; Ribeiro, C. Carbono e nutrientes na biomassa aérea arbórea, vegetação sob coberto e solo numa cronossequência de povoamento de pinhal bravo. Rev Ciênc Agrár 2009, 32, 154–170. [Google Scholar]
- Monteiro, F.M.G.; Marques, P.J.P.; Madeira, M. Are Podzols dominant in sand formations of the Portuguese Litoral? The case of the Leiria National Forest. Rev. Ciênc. Agrár. 2015, 38, 455–472. [Google Scholar] [CrossRef]
- Grebner, D.L.; Bettinger, P.; Siry, J.P. Common forestry practices. In Introduction to Forestry and Natural Resources; Grebner, D.L., Bettinger, P., Siry, J.P., Eds.; Academic Press, Elsevier Science: London, UK, 2013; pp. 255–285. [Google Scholar]
- MAGRAMA. Protocolo de muestreo y laboratorio de macrófitos en ríos. ML-R-M-2015. Centro de Publicaciones, Secretaría General Técnica Ministerio de Agricultura, Alimentación y Medio Ambiente (MAGRAMA). Madrid. 2015. Available online: https://www.miteco.gob.es/es/agua/temas/estado-y-calidad-de-las-aguas/ml_r_m_2015_protocolodemuestreoylaboratoriodemacrofitosenrios_def_tcm30-175290.pdf (accessed on 29 March 2021).
- Franco, J.A. Nova Flora de Portugal: Continente e Açores; Vol.1 Lycopodiaceae-Umbelliferae; Author Edition: Lisbon, Portugal, 1971. [Google Scholar]
- Franco, J.A. Nova Flora de Portugal: Continente e Açores; Vol.2 Clethraceae-Compositae; Author Edition: Lisbon, Portugal, 1984. [Google Scholar]
- Franco, J.A.; Rocha-Afonso, M. Nova Flora de Portugal: Continente e Açores; Vol.3(I) Alismataceae-Iridaceae; Escolar Editora: Lisbon, Portugal, 1994. [Google Scholar]
- Franco, J.A.; Rocha-Afonso, M. Nova Flora de Portugal: Continente e Açores; Vol.3(II) Gramineae; Escolar Editora: Lisbon, Portugal, 1998. [Google Scholar]
- Franco, J.A.; Rocha-Afonso, M. Nova Flora de Portugal: Continente e Açores; Vol.3(III) Juncaceae-Orchidaceae; Escolar Editora: Lisbon, Portugal, 2003. [Google Scholar]
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, D.F.; West, C.J. Naturalization and invasion of alien plants—concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Rodrigues, A.C.M. Regeneração pós-fogo da vegetação na Ribeira de São Pedro e em Povoamentos de pinheiro bravo adjacentes. Master of Science in Management and Conservation of Natural Resources, School of Agriculture, University of Lisbon, Lisbon, Portugal. 2019. Available online: http://hdl.handle.net/10400.5/18369 (accessed on 2 February 2021).
- Bayer, A.P.A. Biomass Forest Modelling Using UAV LiDAR Data under Fire Effect. Master’s Thesis, University of Lisbon, Lisbon, Portugal, 2019. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Marques, P.J.P. Os Solos da Mata Nacional de Leiria: Características e Classificação. Master’s Thesis, University of Lisbon, Lisbon, Portugal, 2010. Available online: http://hdl.handle.net/10400.5/2461 (accessed on 10 December 2020).
- Almeida, A.F.; Capelo, J.; Mesquita, S. Mata Nacional de Leiria: Indicadores Fitoecológicos. Silva Lusit. 2002, 10, 195–200. [Google Scholar]
- Rodrigo, A.; Retana, J.; Picó, X. Direct regeneration is not the only response of Mediterranean forests to large fires. Ecology 2004, 85, 716–729. [Google Scholar] [CrossRef]
- Calvo, L.; Santalla, S.; Valbuena, L.; Marcos, E.; Tárrega, R.; Luis-Calabui, E. Post-fire natural regeneration of a Pinus pinaster forest in NW Spain. Plant Ecol. 2008, 197, 81–90. [Google Scholar] [CrossRef]
- Parra, A.; Moreno, J.M. Post-fire environments are favourable for plant functioning of seeder and resprouter Mediterranean shrubs, even under drought. New Phytol. 2017, 214, 1118–1131. [Google Scholar] [CrossRef] [Green Version]
- Peterson, D.W.; Dodson, E.K.; Harrod, R.J. Post-fire logging reduces surface woody fuels up to four decades following wildfire. For. Ecol. Manag. 2015, 338, 84–91. [Google Scholar] [CrossRef]
- Arán, D.; García-Duro, J.; Cruz, O.; Casal, M.; Reyes, O. Understanding biological characteristics of Acacia melanoxylon in relation to fire to implement control measurements. Ann. For. Sci. 2017, 74, 61. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Rodríguez, J.; González, L.; Lourenzo, P. Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Ann. For. Sci. 2017, 74, 55. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.; Figueiredo, A.; Graça, M.A.S.; Marchante, E.; Pereira, A. Invasion of temperate deciduous broadleaf forests by N-fixing tree species—consequences for stream ecosystems. Biol. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Dwire, K.A.; Kauffman, J.B. Fire and riparian ecosystems in landscapes of western USA. For. Ecol. Manag. 2003, 178, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Catry, F.X.; Pausas, J.G.; Moreira, M.; Fernandes, P.M.; Rego, F. Post-fire response variability in Mediterranean Basin tree species in Portugal. Int. J. Wildland Fire 2013, 22, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Filotas, E.; Parrott, L.; Burton, P.J.; Chazdon, R.L.; Coates, K.D.; Coll, L.; Haeussler, S.; Martin, K.; Nocentini, S.; Puettmann, K.J.; et al. Viewing forests through the lens of complex systems science. Ecosphere 2014, 5, 1–23. [Google Scholar] [CrossRef]
- Harris, H.E.; Baxter, C.V.; Davis, J.M. Debris flows amplify effects of wildfire on magnitude and composition of tributary subsidies to mainstem habitats. Freshw. Sci. 2015, 34, 1457–1467. [Google Scholar] [CrossRef]
- Maia, P.; Keizer, J.; Vasques, A.; Abrantes, N.; Roxo, L.; Fernandes, P.; Ferreira, A.; Moreira, F. Post-fire plant diversity and abundance in pine and eucalypt stands in Portugal: Effects of biogeography, topography, forest type and post-fire management. For. Ecol. Manag. 2014, 334, 154–162. [Google Scholar] [CrossRef]
- Paula, S.; Arianoutsou, M.; Kazanis, D.; Tavsanoglu, Ç.; Lloret, F.; Buhk, C.; Ojeda, F.; Luna, B.; Moreno, J.M.; Rodrigo, A.; et al. Fire-related traits for plant species of the Mediterranean Basin. Ecology 2009, 90, 1420. [Google Scholar] [CrossRef] [Green Version]
- Pausas, J.G. Generalized fire response strategies in plants and animals. Oikos 2019, 128, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Viana-Soto, A.; Aguado, I.; Martínez, S. Assessment of Post-Fire Vegetation Recovery Using Fire Severity and Geographical Data in the Mediterranean Region (Spain). Environments 2017, 4, 90. [Google Scholar] [CrossRef] [Green Version]
- Rego, F.; Moreno, J.; Vallejo, V.R.; Xanthopoulos, G. Forest Fires—Sparking policies in the EU. In Directorate-General for Research and Innovation Climate Action and Resource Efficiency; Faivre, N., Ed.; Publication of the EU: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]




| Pine Stands (Burnt) | São Pedro River_Burnt | São Pedro River_Unburnt | |||
|---|---|---|---|---|---|
| Species | IV | Species | IV | Species | IV |
| Pteridium aquilinum | 67.9 | Pteridium aquilinum | 20.2 | Alnus glutinosa | 7.6 |
| Dittrichia viscosa | 12.9 | Fumaria capreolata | 11.7 | Hedera hibernica | 7.2 |
| Pinus pinaster | 12.8 | Acacia melanoxylon * | 8.8 | Pteridium aquilinum | 6.8 |
| Corynephorus macrantherus | 11.1 | Apium nodiflorum | 8.4 | Laurus nobilis | 4.5 |
| Phillyrea angustifolia | 10.6 | Eucalyptus globulus | 7.3 | Rubus ulmifolius | 4.4 |
| Cistus salvifolius | 10.4 | Rubus ulmifolius | 5.7 | Acacia melanoxylon * | 3.5 |
| Halimium calycinum | 10.2 | Geranium robertianum | 5.6 | Carex pendula | 2.3 |
| Ulex europaeus | 8.2 | Alnus glutinosa | 3.8 | Ruscus aculeatus | 1.9 |
| Stauracanthus genistoides | 6.9 | Oenanthe crocata | 3.3 | Sparganium erectum | 1.5 |
| Cytisus scoparius | 6.1 | Robinia pseudoacacia * | 3.3 | Acacia dealbata * | 0.9 |
| Erica arborea | 5.9 | Fumaria officinalis | 2.7 | Solanum nigrum | 0.9 |
| Lotus subbiflorus | 4.3 | Rumex crispus | 2.5 | Brachypodium sylvaticum | 0.9 |
| Scilla monophyllos | 3.5 | Sonchus asper | 2.5 | Acer pseudoplatanus | 0.8 |
| Tuberaria guttata | 3.4 | Ruscus aculeatus | 2.1 | Osmunda regalis | 0.8 |
| Conyza bonariensis * | 2.9 | Tamus communis | 1.9 | Equisetum arvense | 0.7 |
| Pine Seedlings | Class of Age of Pines | |
|---|---|---|
| <25 y (n = 11) | >60 y (n = 17) | |
| Mean ± SD | 7.6 ± 6.4 | 96.6 ± 85.2 |
| Range of variation (minimum–maximum) | 3–21 | 16–318 |
| Management units (number of seedlings/ha) | ||
| T259 | - | 71,020 |
| T260 | 3265 | 150,041 |
| T261 | 4082 | 28,299 |
| T262 | 10,612 | 26,667 |
| T277 | 5986 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, F.C.; Rodrigues, C.; Pina, J.P.; Soares, P. Regeneration of Riparian and Maritime Pine Forests after a Large Wildfire on the Largest Public Forest of Portugal. Forests 2021, 12, 477. https://doi.org/10.3390/f12040477
Aguiar FC, Rodrigues C, Pina JP, Soares P. Regeneration of Riparian and Maritime Pine Forests after a Large Wildfire on the Largest Public Forest of Portugal. Forests. 2021; 12(4):477. https://doi.org/10.3390/f12040477
Chicago/Turabian StyleAguiar, Francisca C., Carolina Rodrigues, João P. Pina, and Paula Soares. 2021. "Regeneration of Riparian and Maritime Pine Forests after a Large Wildfire on the Largest Public Forest of Portugal" Forests 12, no. 4: 477. https://doi.org/10.3390/f12040477






