Disentangling the Ecological Determinants of Species and Functional Trait Diversity in Herb-Layer Plant Communities in European Temperate Forests
Abstract
:1. Introduction
2. Materials and Methods
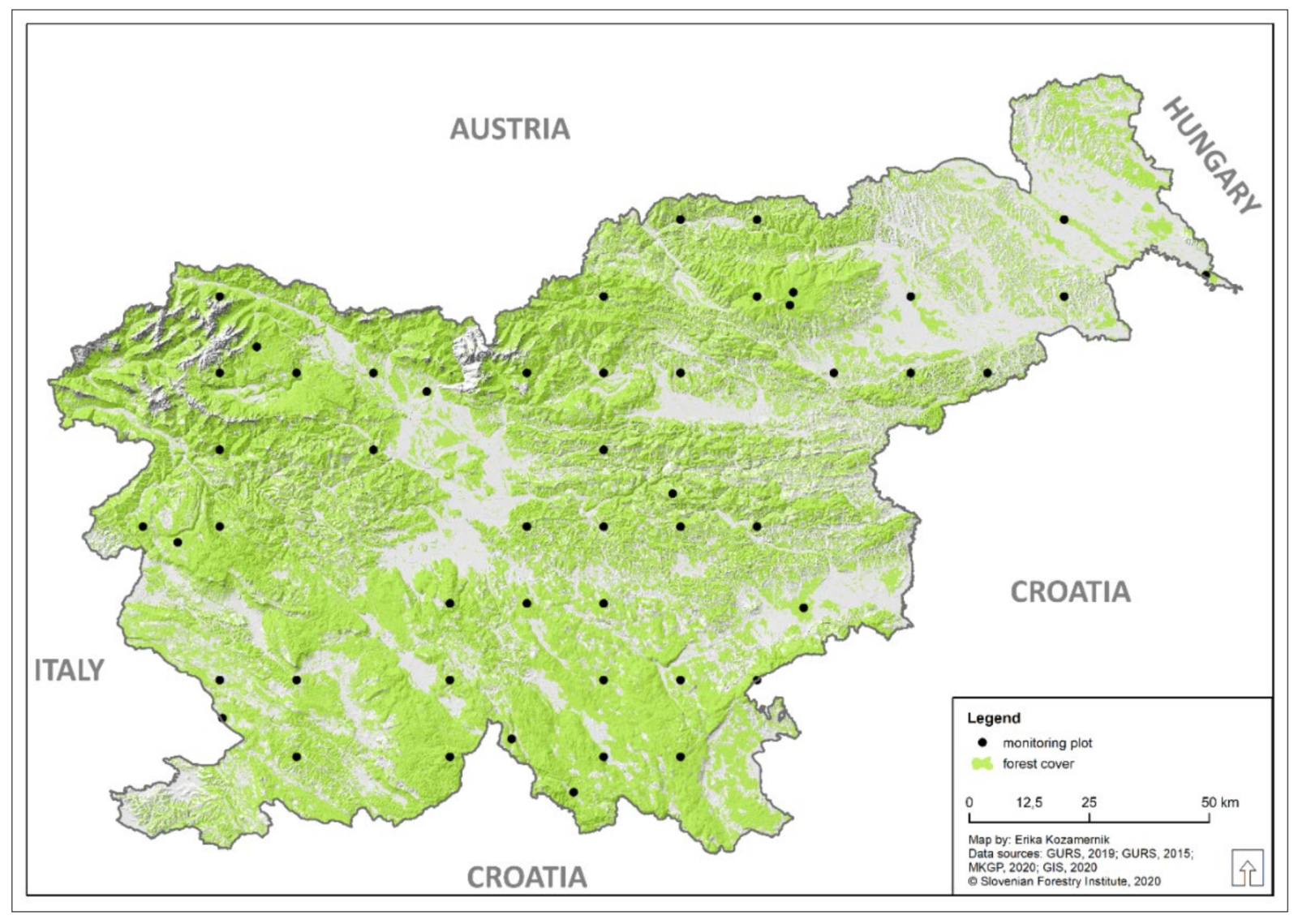
2.1. Study Area, Vegetation Sampling and Data Collection
2.2. Explanatory Variables (Predictors)
2.2.1. Soil Properties
2.2.2. Climatic Parameters
2.2.3. Forest Stand Characteristics
2.2.4. Topographic and Structural Factors
2.3. Response Variables
2.4. Statistical Analyses
3. Results
3.1. Herb-Layer Vegetation Status across Monitoring Plots
3.2. Ecological Predictors of Taxonomic and Functional Response Variables
3.2.1. Herb-Layer Species Richness
3.2.2. Herb-Layer Cover
3.2.3. Herb-Layer Species Evenness
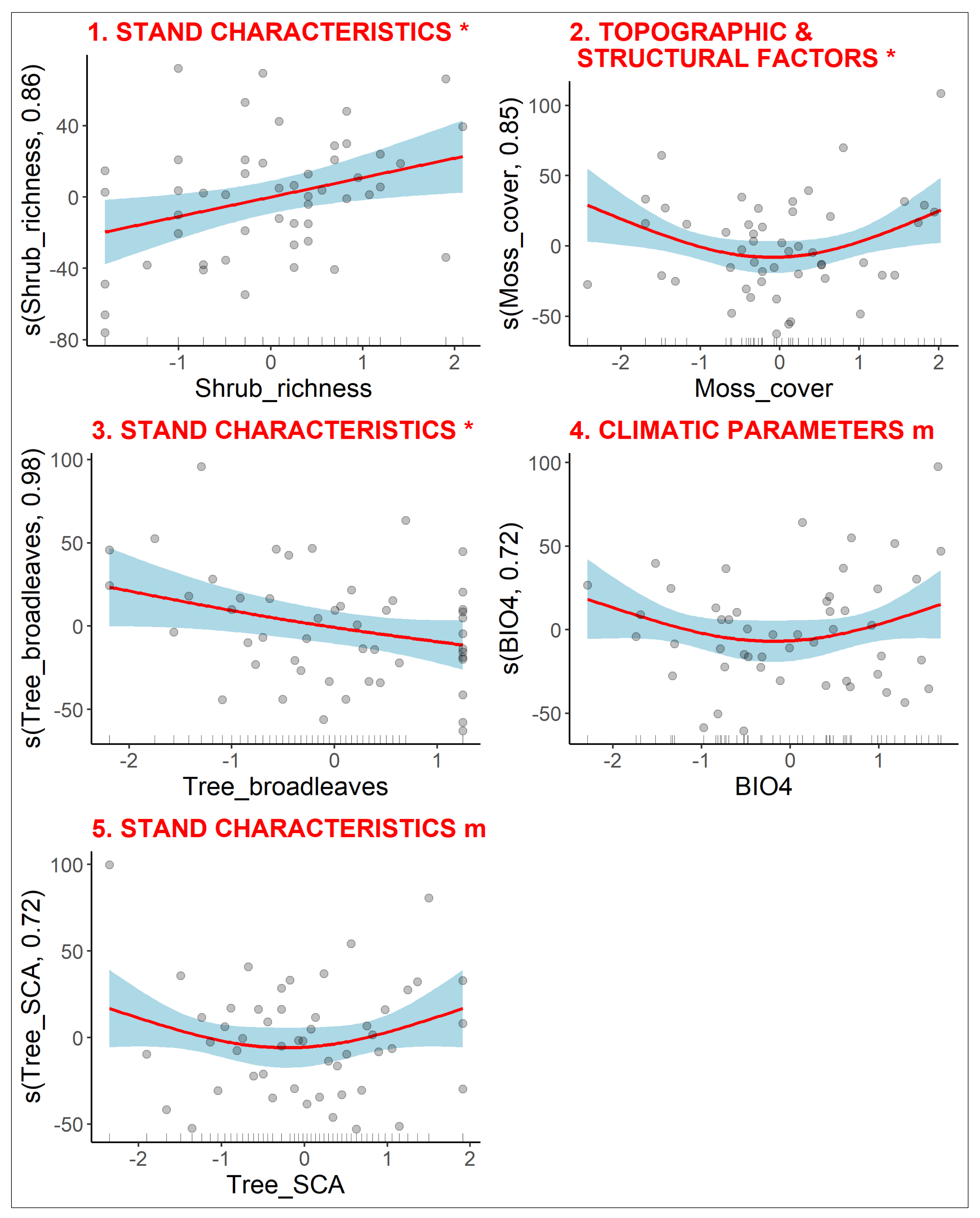
3.2.4. Diversity of Clonal Traits
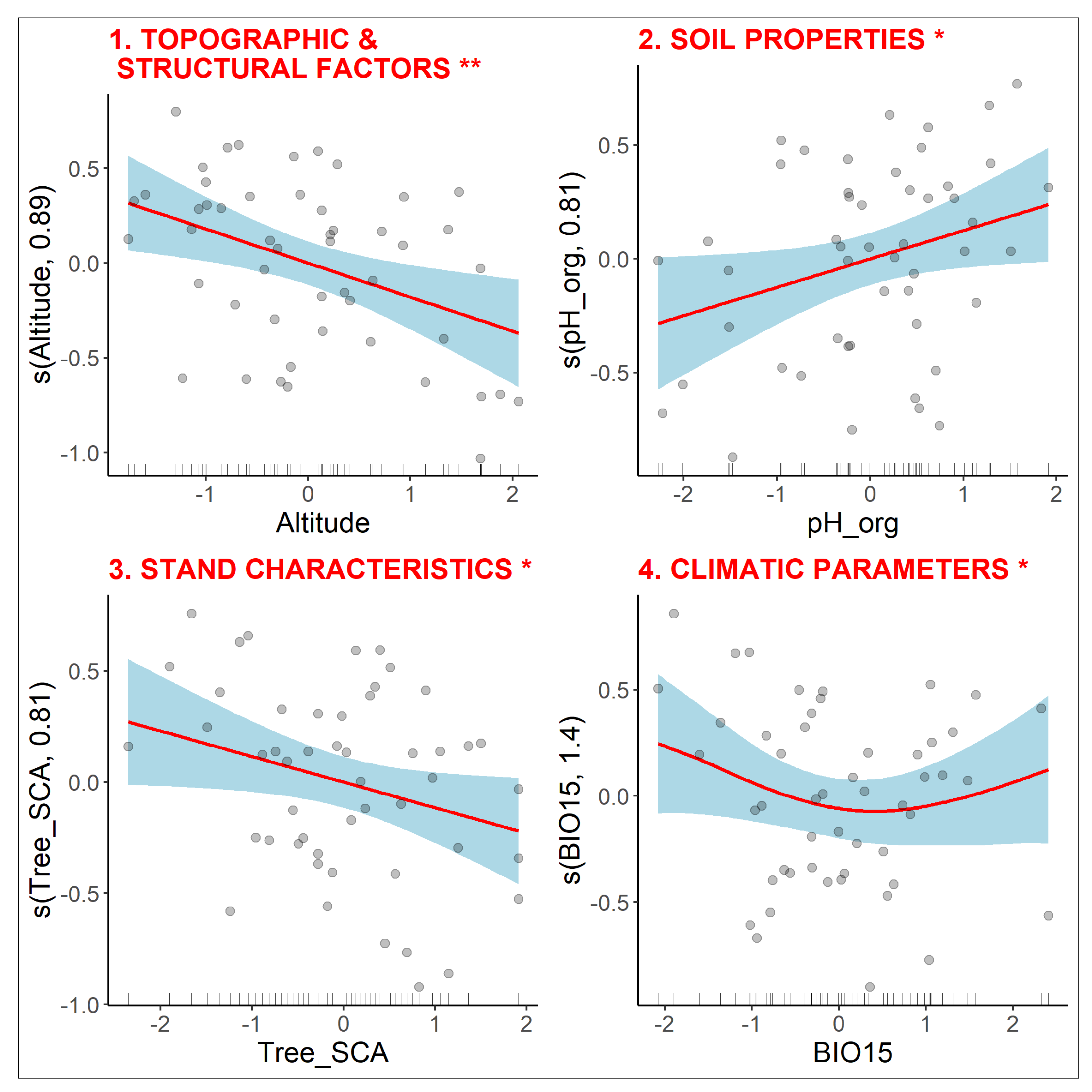
3.2.5. Diversity of Bud Bank Traits
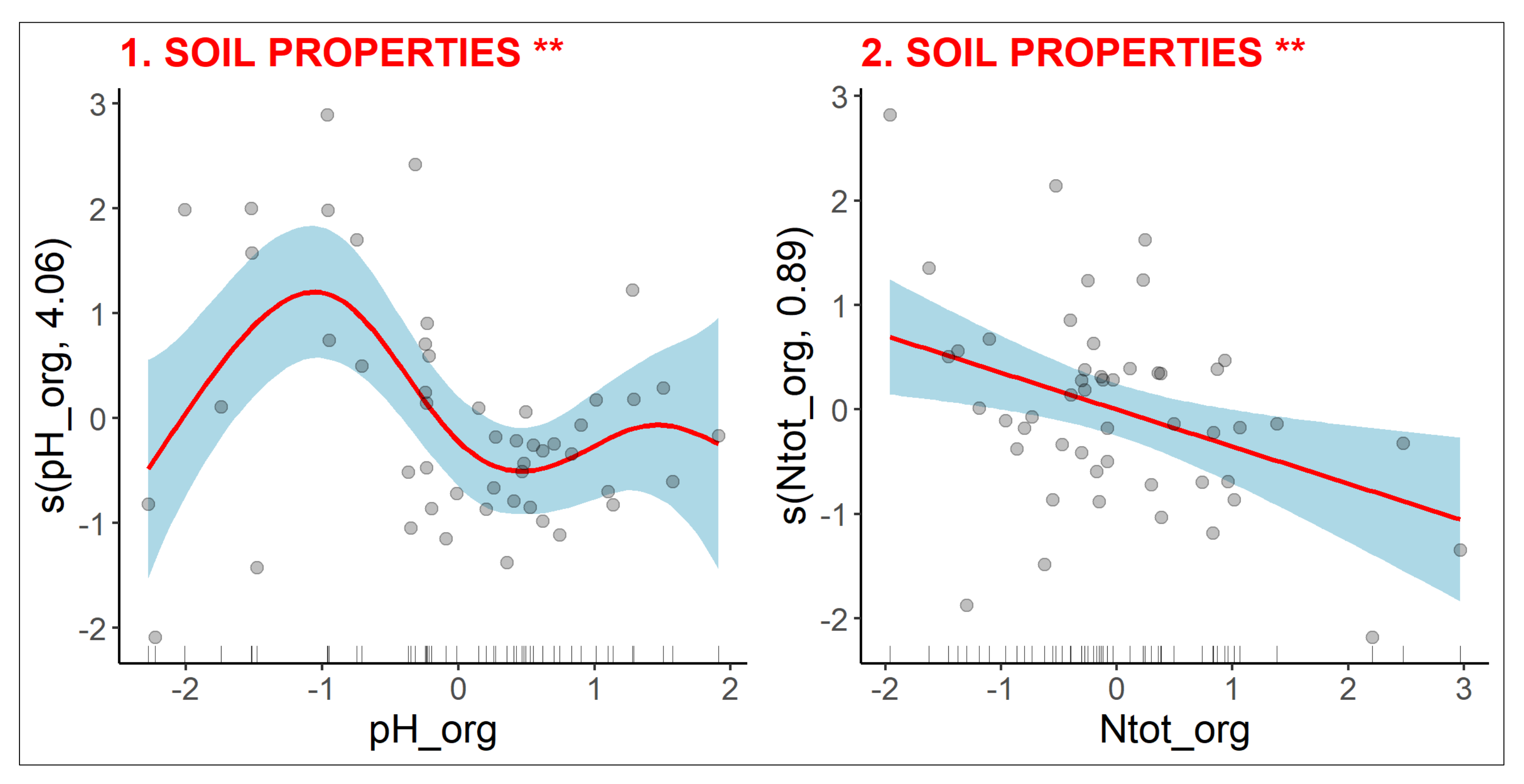
3.2.6. Diversity of Leaf-Height-Seed Traits
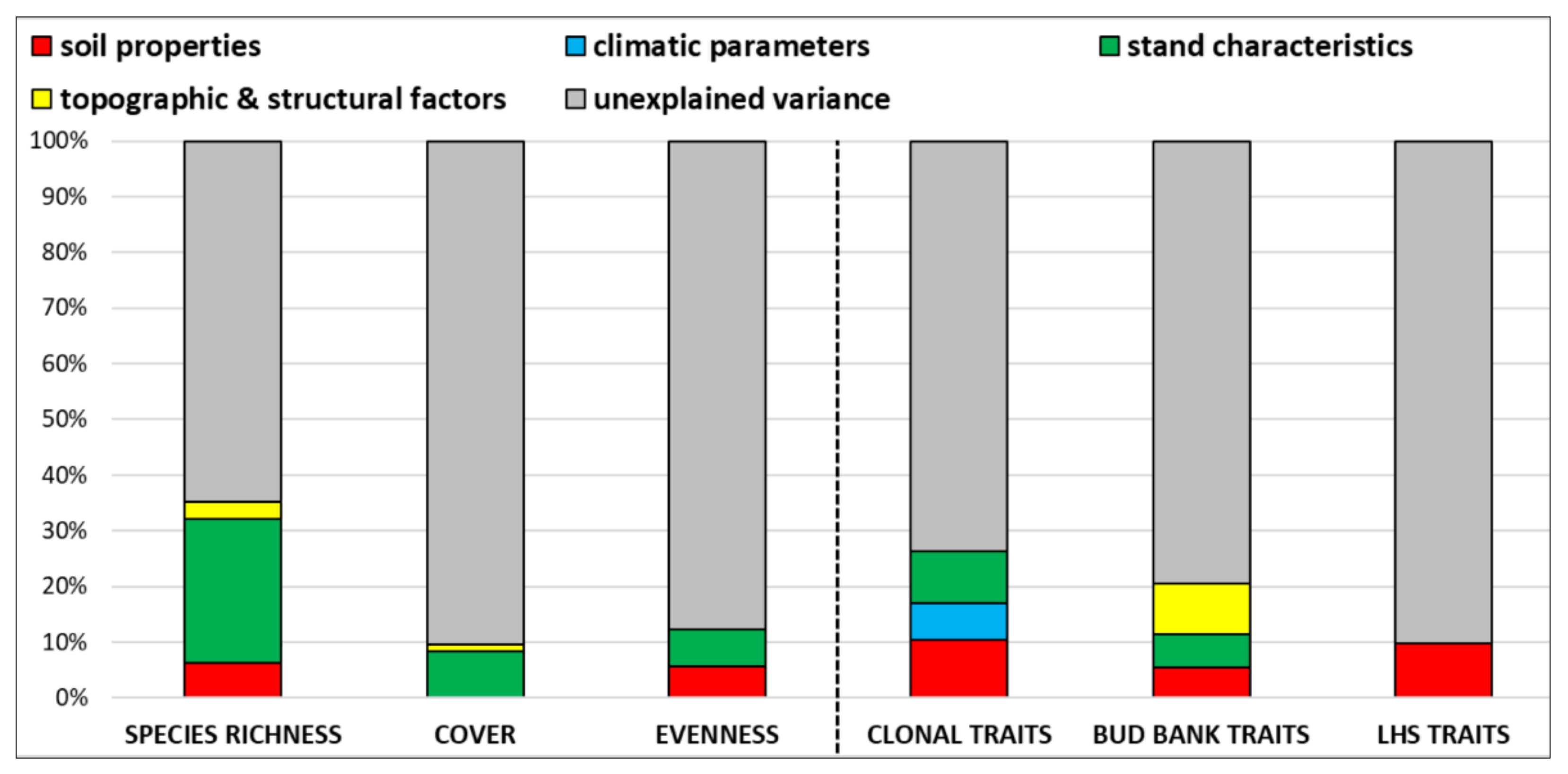
3.3. Variance Partitioning
4. Discussion
4.1. Species–Environment Relationships
4.2. Trait–Environment Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Maarel, E. Vegetation Ecology; Blackwell Publishing: Oxford, UK, 2005; 408p. [Google Scholar]
- Zhang, Y.; Chen, H.Y.H.; Taylor, A. Multiple drivers of plant diversity in forest ecosystems. Glob. Ecol. Biogeogr. 2014, 23, 885–893. [Google Scholar] [CrossRef]
- Sabatini, F.M.; Jiménez-Alfaro, B.; Burrascano, S.; Blasi, C. Drivers of herb-layer species diversity in two unmanaged temperate forest in northern Spain. Community Ecol. 2014, 15, 147–157. [Google Scholar] [CrossRef]
- Maes, S.L.; Perring, M.P.; Depauw, L.; Bernhardt-Römermann, M.; Blondeel, H.; Brūmelis, G.; Brunet, J.; Decocq, G.; den Ouden, J.; Govaert, S.; et al. Plant functional trait response to environmental drivers across European temperate forest understorey communities. Plant Biol. 2020, 22, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, T.; Valdés, A.; Verheyen, K.; Perring, M.P.; Bernhardt-Römermann, M.; Andrieu, E.; Brunet, J.; Cousins, S.A.O.; Deconchat, M.; De Smedt, P.; et al. Functional trait variation of forest understory plant communities across Europe. Basic Appl. Ecol. 2019, 34, 1–14. [Google Scholar] [CrossRef]
- Weigel, R.; Gilles, J.; Klisz, M.; Manthey, M.; Kreyling, J. Forest understory vegetation is more related to soil than to climate towards the cold distribution margin of European beech. J. Veg. Sci. 2019, 30, 746–755. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. Forest Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Petersson, L.; Holmström, E.; Lindbladh, M.; Felton, A. Tree species impact on understory vegetation: Vascular plant communities of Scots pine and Norway spruce managed stands in northern Europe. Forest Ecol. Manag. 2019, 448, 330–345. [Google Scholar] [CrossRef]
- Axmanová, I.; Chytrý, M.; Zelený, D.; Li, C.-F.; Vymazalova, M.; Danihelka, J.; Horsák, M.; Kočí, M.; Kubešová, S.; Lososová, Z.; et al. The species richness-productivity relationship in the herb layer of European deciduous forests. Global Ecol. Biogeogr. 2012, 21, 657–667. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Spasojevic, M.J. The edaphic control of plant diversity. Global Ecol. Biogeogr. 2020, 29, 1634–1650. [Google Scholar] [CrossRef]
- Catorci, A.; Tardella, F.M.; Cutini, M.; Luchetti, L.; Paura, B.; Vitanzi, A. Reproductive traits variation in the herb layer of a submediterranean deciduous forest landscape. Plant Ecol. 2013, 214, 737–749. [Google Scholar] [CrossRef]
- Tardella, F.M.; Postiglione, N.; Bricca, A.; Cutini, M.; Catorci, A. Altitude and aspect filter the herb layer functional structure of sub-Mediterranean forests. Phytocoenologia 2019, 49, 185–198. [Google Scholar] [CrossRef]
- Leuschner, C.; Lendzion, J. Air humidity, soil moisture and soil chemistry as determinants of the herb layer composition in European beech forests. J. Veg. Sci. 2009, 20, 288–298. [Google Scholar] [CrossRef]
- Macek, M.; Kopecký, M.; Wild, J. Maximum air temperature controlled by landscape topography affects plant species composition in temperate forests. Landscape Ecol. 2019, 34, 2541–2556. [Google Scholar] [CrossRef]
- Tinya, F.; Márialigeti, S.; Király, I.; Németh, B.; Ódor, P. The effect of light conditions on herbs, bryophytes and seedlings of temperate mixed forests in Őrség, Western Hungary. Plant Ecol. 2009, 204, 69–81. [Google Scholar] [CrossRef]
- Landuyt, D.; Perring, M.P.; Seidl, R.; Tauber, F.; Verbeeck, H.; Verheyen, K. Modelling understorey dynamics in temperate forests under global change–Challenges and perspectives. Perspect. Plant Ecol. Syst. 2018, 31, 44–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whigham, D.E. Ecology of woodland herbs in temperate deciduous forests. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 583–621. [Google Scholar] [CrossRef] [Green Version]
- Tinya, F.; Márialigeti, S.; Bidló, B.; Ódor, P. Environmental drivers of the forest regeneration in temperate mixed forests. Forest Ecol. Manag. 2019, 433, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Leuschner, C.; Meier, I.C. The ecology of Central European tree species: Trait spectra, functional trade-offs, and ecological classification of adult trees. Perspect. Plant Ecol. 2018, 33, 89–103. [Google Scholar] [CrossRef]
- Valladares, F.; Laanisto, L.; Niinemets, Ü.; Zavala, M.A. Shedding light on shade: Ecological perspectives of understorey plant life. Plant Ecol. Divers. 2016, 9, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Wang, M.; Huang, Z.; Fu, S.; Chen, H.Y.H. Forest Understory Vegetation: Colonization and the Availability and Heterogeneity of Resources. Forests 2019, 10, 944. [Google Scholar] [CrossRef] [Green Version]
- Vockenhuber, E.A.; Scherber, C.; Lagenbruch, C.; Meißner, M.; Seidel, D.; Tscharntke, T. Tree diversity and environmental context predict herb species richness and cover in Germany’s largest connected deciduous forest. Perspect. Plant Ecol. 2011, 13, 111–119. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Landuyt, D.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; Brūmelis, G.; Brunet, J.; Closset-Kopp, D.; Decocq, G.; et al. Evaluating structural and compositional canopy characteristics to predict the light-demand signature of the forest understorey in mixed, semi-natural temperate forests. Appl. Veg. Sci. 2021, 24, e12532. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Landuyt, D.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; Brūmelis, G.; Brunet, J.; Closset-Kopp, D.; Czerepko, J.; et al. Light availability and land-use history drive biodiversity and functional changes in forest herb layer communities. J. Ecol. 2020, 108, 1411–1425. [Google Scholar] [CrossRef]
- Mölder, A.; Streit, M.; Schmidt, W. When beech strikes back: How strict nature conservation reduces herb-layer diversity and productivity in Central European deciduous forests. Forest Ecol. Manag. 2014, 319, 51–61. [Google Scholar] [CrossRef]
- De Vries, W.; Reinds, G.J.; Deelstra, H.D.; Klap, J.M.; Vel, E.M. Intensive Monitoring of Forest Condition in Europe: Technical Report 2000; UN/ECE EC: Brussels, Belgium; Geneva, Switzerland, 2000; p. 193. Available online: https://www.icp-forests.org/pdf/TRLII2000.pdf (accessed on 1 December 2020).
- De Vries, W.; Vel, E.M.; Reinds, G.J.; Deelstra, H.; Klap, J.M.; Leeters, E.E.J.M.; Hendriks, C.M.A.; Kerkvoorden, M.; Landmann, G.; Herkendell, J.; et al. Intensive monitoring of forest ecosystems in Europe. 1. Objectives, set-up and evaluation strategy. For. Ecol. Manag. 2003, 174, 77–95. [Google Scholar] [CrossRef]
- Rautio, P.; Ferretti, M. Monitoring European forests: Results from science, policy, and society. Ann. For. Sci. 2015, 72, 875–876. [Google Scholar] [CrossRef] [Green Version]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Thrippleton, T.; Bugmann, H.; Kramer-Priewasser, K.; Snell, R.S. Herbaceous understorey: An overlooked player in forest landscape dynamics? Ecosystems 2016, 19, 1–15. [Google Scholar] [CrossRef]
- Spicer, M.E.; Mellor, H.; Carson, W.P. Seeing beyond the trees: A comparison of tropical and temperate growth forms and their vertical distribution. Ecology 2020, 101, e02974. [Google Scholar] [CrossRef]
- Landuyt, D.; De Lombaerde, E.; Perring, M.P.; Hertzog, L.R.; Ampoorter, E.; Maes, S.L.; De Frenne, P.; Ma, S.; Proesmans, W.; Blondeel, H.; et al. The functional role of temperate forest understorey vegetation in a changing world. Glob. Change Biol. 2019, 25, 3625–3641. [Google Scholar] [CrossRef] [PubMed]
- Canullo, R.; Starlinger, F.; Granke, O.; Fischer, R.; Aamlid, D.; Neville, P. ICP Forests Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Part VII.1: Assessment of Ground Vegetation; UNECE ICP Forests Programme Coordinating Centre: Hamburg, Germany, 2011; p. 19. Available online: https://www.icp-forests.org/pdf/manual/2016/ICP_Manual_2016_01_part07-1.pdf (accessed on 1 December 2020).
- Thimonier, A.; Kull, P.; Keller, W.; Moser, B.; Wohlgemuth, T. Ground vegetation monitoring in Swiss forests: Comparison of survey methods and implications for trend assessments. Environ. Monit. Assess. 2011, 174, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Seidling, W.; Hamber, L.; Máliš, F.; Salemaa, M.; Kutnar, L.; Czerepko, J.; Kompa, T.; Buriánek, V.; Dupouey, J.-L.; Vodálova, A.; et al. Comparing observer performances in vegetation records by efficiency graphs derived from rarefaction curves. Ecol. Indic. 2020, 109, 105790. [Google Scholar] [CrossRef]
- Dirnböck, T.; Grandin, U.; Bernhardt-Römermann, M.; Beudert, B.; Canullo, R.; Forsius, M.; Grabner, M.-T.; Holmberg, M.; Kleemola, S.; Lundin, L.; et al. Forest floor vegetation response to nitrogen deposition in Europe. Glob. Change Biol. 2014, 20, 429–440. [Google Scholar] [CrossRef]
- van Dobben, H.; de Vries, W. The contribution of nitrogen deposition on the eutrophication signal in understory plant communities of European forests. Ecol. Evol. 2017, 7, 214–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novotný, R.; Buriánek, V.; Šrámek, V.; Hůnová, I.; Skořepova, I.; Zapletal, M.; Lomský, B. Nitrogen deposition and its impact on forest ecosystems in the Czech Republic–change in soil chemistry and ground vegetation. iForest 2016, 10, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Kutnar, L.; Nagel, T.A.; Kermavnar, J. Effects of Disturbance on Understory Vegetation across Slovenian Forest Ecosystems. Forests 2019, 10, 1048. [Google Scholar] [CrossRef] [Green Version]
- Chelli, S.; Simonetti, E.; Wellstein, C.; Campetella, G.; Carnicelli, S.; Andreetta, A.; Giorgini, D.; Puletti, N.; Bartha, S.; Canullo, R. Effects of climate, soil, forest structure and land use on the functional composition of the understorey in Italian forests. J. Veg. Sci. 2019, 30, 1110–1121. [Google Scholar] [CrossRef]
- Chelli, S.; Ottaviani, G.; Simonetti, E.; Wellstein, C.; Canullo, R.; Carnicelli, S.; Andreetta, A.; Puletti, N.; Bartha, S.; Cervellini, M.; et al. Climate is the main driver of clonal and bud bank traits in Italian forest understories. Perspect. Plant Ecol. 2019, 40, 125478. [Google Scholar] [CrossRef] [Green Version]
- Ottaviani, G.; Götzenberger, L.; Bacaro, G.; Chiarucci, A.; de Bello, F.; Marcantonio, M. A multifaceted approach for beech forest conservation: Environmental drivers of understory plant diversity. Flora 2019, 256, 85–91. [Google Scholar] [CrossRef]
- Bricca, A.; Chelli, S.; Canullo, R.; Cutini, M. The legacy of the past logging: How forest structure affects different facets of understory plant diversity in abandoned coppice forests. Diversity 2020, 12, 109. [Google Scholar] [CrossRef] [Green Version]
- Padullés Cubino, J.; Lososová, Z.; Bonari, G.; Agrillo, E.; Attore, F.; Bergmeier, E.; Biurrun, I.; Campos, J.A.; Čarni, A.; Ćuk, M.; et al. Phylogenetic structure of European forest vegetation. J. Biogeogr. 2021. Early view. [Google Scholar] [CrossRef]
- Padullés Cubino, J.; Jiménez-Alfaro, B.; Sabatini, F.M.; Willner, W.; Lososová, Z.; Biurrun, I.; Brunet, J.; Campos, J.A.; Indreica, A.; Jansen, F.; et al. Plant taxonomic and phylogenetic turnover increases toward climatic extremes and depends on historical factors in European beech forests. J. Veg. Sci. 2021, 32, e12977. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Fraser, L.H.; Garris, H.W.; Carlyle, C.N. Predicting plant trait similarity along environmental gradients. Plant Ecol. 2016, 217, 1297–1306. [Google Scholar] [CrossRef]
- Naaf, T.; Wulf, M. Plant community assembly in temperate forests along gradients in soil fertility and disturbance. Acta Oecol. 2012, 39, 101–108. [Google Scholar] [CrossRef]
- Vojtkó, A.E.; Freitag, M.; Bricca, A.; Martello, F.; Compañ, J.M.; Küttim, M.; Kun, R.; de Bello, F.; Klimešová, J.; Götzenberger, L. Clonal vs leaf-height-seed (LHS) traits: Which are filtered more strongly across habitats? Folia Geobot. 2017, 52, 269–281. [Google Scholar] [CrossRef]
- Klimešová, J.; Herben, T. Clonal and bud bank traits: Patterns across temperate plant communities. J. Veg. Sci. 2015, 26, 243–253. [Google Scholar] [CrossRef]
- Klimešová, J.; Tackenberg, O.; Herben, T. Herbs are different: Clonal and bud bank traits can matter more than leaf-height-seed traits. New Phytol. 2016, 210, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutnar, L. Diversity of woody species on forest monitoring plots in Slovenia. GozdVestn 2011, 69, 271–278, (In Slovenian with English Summary). [Google Scholar]
- Urbančič, M.; Kutnar, L.; Kobal, M.; Žlindra, D.; Marinšek, A.; Simončič, P. Soil and vegetation characteristics on Intensive Monitoring Plots of forest ecosystems). GozdVestn 2016, 74, 3–27, (In Slovenian with English Summary). [Google Scholar]
- Kermavnar, J.; Kutnar, L. Patterns of understory community assembly and plant trait-environment relationships in temperate SE European Forests. Diversity 2020, 12, 91. [Google Scholar] [CrossRef] [Green Version]
- WorldClim. Global Climate and Weather Data. 2021. Available online: https://www.worldclim.org/ (accessed on 15 November 2020).
- Slovenia Forest Service. Annual Report of Slovenia Forest Service for Year 2019; Slovenia Forest Service: Ljubljana, Slovenia, 2019. (In Slovenian) [Google Scholar]
- Forest Europe. State of Europe’s Forests 2020. Available online: https://foresteurope.org/publications/ (accessed on 1 December 2020).
- Kutnar, L.; Veselič, Ž.; Dakskobler, I.; Robič, D. Typology of Slovenian forest sites according to ecological and vegetation conditions for the purpose of forest management. GozdVestn 2012, 70, 195–214, (In Slovenian with English Summary). [Google Scholar]
- Bončina, A.; Rozman, A.; Dakskobler, I.; Klopčič, M.; Babij, V.; Poljanec, A. Gozdni rastiščni tipi Slovenije–Vegetacijske, sestojne in upravljavske značilnosti; University of Ljubljana, Biotechnical Faculty, Department of Forestry and Renewable forest resources, Slovenia Forest Service: Ljubljana, Slovenia, 2021. [Google Scholar]
- Barkman, J.J.; Doing, H.S.; Segal, S. Kritische Bemerkungen und Vorschläge zur quantitativen Vegetationsanalyse. Acta Bot. Neerl. 1964, 13, 394–419. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1964–1980; Volume 1–5.
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Martinčič, A.; Wraber, T.; Jogan, N.; Podobnik, A.; Turk, B.; Vreš, B.; Ravnik, V.; Frajman, B.; Strgulc Krajšek, S.; Trčak, B.; et al. Mala Flora Slovenije: Ključ za določanje praprotnic in semenk; Tehniška založba Slovenije: Ljubljana, Slovenia, 2007; p. 967. [Google Scholar]
- WGFB. The BioSoil Forest Biodiversity Field Manual, version 1.0/1.1/1.1a for the field assessment 2006-07; Evaluation of BioSoil Demonstration Project: Forest Biodiversity; JRC–Inst. for Environment and Sustainability, Ed.; Publications Office of the European Union: Luxembourg, 2011; pp. 81–102. [Google Scholar] [CrossRef]
- Urbančič, M.; Kutnar, L.; Kralj, T.; Kobal, M.; Simončič, P. Site characteristics of permanent plots on the Slovenian 16 km × 16 km net. GozdVestn 2009, 67, 17–48, (In Slovenian with English Summary). Available online: https://www.dlib.si/stream/URN:NBN:SI:DOC-OFRDZTK5/76e434ce-10e9-48d5-809b-6b7d367a291f/PDF (accessed on 1 October 2020).
- Bastrup-Birk, A.; Neville, P.; Chirici, G.; Houston, T. The BioSoil—Forest Biodiversity; Field Manual, Ver. 1.0/1.1/1.1a; for the field assessment 2006–07, Forest Focus Demonstration Project; BioSoil, Forest Research: Farnham, UK, 2007; 51p. [Google Scholar]
- ICP Forests. Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests. Part IIIa. Sampling and Analysis of Soil. UN ECE Convention on Long-Range Transboundary Air Pollution. International Co-operative Programme on Assessment and Monitoring of Air Pollution Effects on Forests. Expert Panel on Soil; Forest Soil Co-ordinating Centre, Research Institute for Nature and Forest: Geraardsbergen, Belgium, 2006; 127p. [Google Scholar]
- Maes, S.L.; Blondeel, H.; Perring, M.P.; Depauw, L.; Brūmelis, G.; Brunet, J.; Decocq, G.; den Ouden, J.; Härdtle, W.; Hédl, R.; et al. Litter quality, land-use history, and nitrogen deposition effects on topsoil conditions across European temperate deciduous forests. Forest Ecol. Manag. 2019, 433, 405–418. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climat. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R.J. Global Aridity Index (Global-Aridity) and Global Potential Evapo-Transpiration (Global-PET) Geospatial Database. CGIAR Consortium for Spatial Information. 2009. Published online. Available online: http://www.csi.cgiar.org (accessed on 15 November 2020).
- Hijmans, R.J.; van Etten, J.; Sumner, M.; Cheng, J.; Baston, D.; Bevan, D.; Bivand, R.; Bussetto, L.; Canty, M.; Fasoil, B.; et al. Package “Raster”–Geographic Data Analysis and Modelling; Version 3.4-5; 2020. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 15 November 2020).
- Closset-Kopp, D.; Hattab, T.; Decocq, G. Do drivers of forestry vehicles also drive herb layer changes (1970–2015) in a temperate forest with contrasting habitat and management conditions? J. Ecol. 2019, 107, 1439–1456. [Google Scholar] [CrossRef] [Green Version]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R. The Climate Near the Ground; Harvard University Press: Cambridge, MA, USA, 1966. [Google Scholar]
- Pielou, E.C. Ecological Diversity; Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘vegan’—Community Ecology Package. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 15 November 2020).
- Stirling, G.; Wilsey, B. Empirical Relationships between Species Richness, Evenness, and Proportional Diversity. Am. Nat. 2001, 158, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Campetella, G.; Chelli, S.; Simonetti, E.; Damiani, C.; Bartha, S.; Wellstein, C.; Giorgini, D.; Puletti, N.; Mucina, L.; Cervellini, M.; et al. Plant functional traits are correlated with species persistence in the herb layer of old-growth beech forests. Sci. Rep. 2020, 10, 19253. [Google Scholar] [CrossRef]
- Herben, T.; Klimešová, J.; Chytrý, M. Effects of disturbance frequency and severity on plant traits: An assessment across a temperate flora. Funct. Ecol. 2018, 32, 799–808. [Google Scholar] [CrossRef]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annual Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Chelli, S.; Ottaviani, G.; Simonetti, E.; Campetella, G.; Wellstein, C.; Bartha, S.; Cervellini, M.; Canullo, R. Intraspecific variability of specific leaf area fosters the persistence of understorey specialists across a light availability gradient. Plant Biol. 2021, 23, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Klimešová, J.; de Bello, F. CLO-PLA: The database of clonal and bud bank traits of Central European flora. J. Veg. Sci. 2009, 20, 511–516. [Google Scholar] [CrossRef]
- Klimešová, J.; Danihelka, J.; Chrtek, J.; de Bello, F.; Herben, T. CLO-PLA: A database of clonal and bud bank traits of Central European flora. Ecology 2017, 98, 1179. [Google Scholar] [CrossRef] [Green Version]
- Chytrý, M.; Danihelka, J.; Kaplan, Z.; Wild, J.; Holubová, D.; Novotný, P.; Řezníčková, M.; Rohn, M.; Dřevojan, P.; Grulich, V. Pladias Database of the Czech Flora and Vegetation. Preslia 2021, 93, 1–87. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA traitbase: A database of life-history traits of the Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Botta-Dukát, Z. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Schleuter, D.; Daufresne, M.; Massol, F.; Argiller, C. A user’s guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–871. Available online: https://www.jstor.org/stable/2528823 (accessed on 15 January 2021). [CrossRef]
- De Bello, F.; Botta-Dukát, Z.; Lepš, J.; Fibich, P. Towards a more balanced combination of multiple traits when computing functional differences between species. Methods Ecol. Evol. 2020, 12, 443–448. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. Package “FD”: Measuring Functional Diversity (FD) from Multiple Traits, and Other Tools for Functional Ecology, R Package Version 1.0–12. 2015. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 1 November 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 November 2020).
- Hastie, T.; Tibshirani, R. Generalized Additive Models; Chapman and Hall (CRC Press): London, UK, 1991. [Google Scholar]
- Yee, T.W.; Mitchell, N.D. Generalized additive models in plant ecology. J. Veg. Sci. 1991, 2, 587–602. [Google Scholar] [CrossRef]
- Wood, S. Package ‘mgcv’–Mixed GAM computation Vehicle with Automatic Smoothness Estimation. 2020. Available online: https://cran.r-project.org/web/packages/mgcv/index.html (accessed on 1 December 2020).
- Marra, G.; Wood, S. Practical variable selection for generalized additive models. Comput. Stat. Data An. 2011, 55, 2372–2387. [Google Scholar] [CrossRef]
- Peterson, R.A. Package ‘bestNormalize’–Normalizing Transformation Functions, version 1.6.1. 2020. Available online: https://cran.r-project.org/web/packages/bestNormalize/index.html (accessed on 1 December 2020).
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Pakeman, R.J.; Lepš, J.; Kleyer, M.; Lavorel, S.; Garnier, E.; the VISTA consortium. Relative climatic, edaphic and management controls of plant functional trait signatures. J. Veg. Sci. 2009, 20, 148–159. [Google Scholar] [CrossRef]
- Lee, C.-B.; Chun, J.-H. Environmental drivers of Patterns of Plant Diversity along a Wide Environmental Gradient in Korean Temperate Forests. Forests 2016, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Černý, T.; Doležal, J.; Janeček, Š.; Šrůtek, M.; Valachovič, M.; Petřík, P.; Altman, J.; Bartoš, M.; Song, J.-S. Environmental correlates of plant diversity in Korean temperate forests. Acta Oecol. 2013, 47, 37–45. [Google Scholar] [CrossRef]
- Gilliam, F.S. Excess Nitrogen in Temperate Forest Ecosystems Decrease Herbaceous Layer Diversity and Shifts Control from Soil to Canopy Structure. Forests 2019, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Small, C.J.; McCarthy, B.C. Relationship of understory diversity to soil nitrogen, topographic variation, and stand age in an eastern oak forest, USA. Forest Ecol. Manag. 2005, 217, 229–243. [Google Scholar] [CrossRef]
- Burton, J.I.; Mladenoff, D.J.; Clayton, M.K.; Forrester, J.A. The roles of environmental filtering and colonization in the fine-scale spatial patterning of ground-layer plant communities in north temperate deciduous forests. J. Ecol. 2011, 99, 764–776. [Google Scholar] [CrossRef]
- Simpson, A.H.; Richardson, S.J.; Laughlin, D.C. Soil-climate interactions explain variation in foliar, stem, root and reproductive traits across temperate forests. Global Eol. Biogeogr. 2016, 25, 964–978. [Google Scholar] [CrossRef]
- Márialigeti, S.; Tinya, F.; Bidló, A.; Ódor, P. Environmental drivers of the composition and diversity of the herb layer in mixed temperate forests in Hungary. Plant Ecol. 2016, 217, 549–563. [Google Scholar] [CrossRef] [Green Version]
- Kovács, B.; Tinya, F.; Ódor, P. Stand structural drivers of microclimate in mature temperate mixed forests. Agr. Forest Meteorol. 2017, 234–235, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Bartels, S.F.; Chen, H.Y.H. Interactions between overstorey and understorey vegetation along an overstorey compositional gradient. J. Veg. Sci. 2013, 24, 543–552. [Google Scholar] [CrossRef]
- Koorem, K.; Moora, M. Positive association between understory species richness and a dominant shrub species (Corylus avellana) in a boreonemoral spruce forest. For. Ecol. Manag. 2010, 260, 1407–1413. [Google Scholar] [CrossRef]
- Hofmeister, J.; Hošek, J.; Modrý, J.; Roleček, J. The influence of light and nutrient availability on herb layer species richness in oak-dominated forests in central Bohemia. Plant Ecol. 2009, 205, 57–75. [Google Scholar] [CrossRef]
- Dormann, C.F.; Bagnara, M.; Boch, S.; Hinderling, J.; Janeiro-Otero, A.; Schäfer, D.; Schall, P.; Hartig, F. Plant species richness increases with light availability, but not variability, in temperate forests understorey. BMC Ecol. 2020, 20, 43. [Google Scholar] [CrossRef]
- Neufeld, H.S.; Young, D.R. Ecophysiology of the Herbaceous Layer in Temperate Deciduous Forests. In The Herbaceous Layer in Forests of Eastern North America; Gilliam, F.S., Ed.; Oxford University Press: New York, NY, USA, 2014; pp. 35–95. [Google Scholar]
- Härdtle, W.; von Oheimb, G.; Westphal, C. The effects of light and soil conditions on the species richness of the ground vegetation of deciduous forests in northern Germany (Schleswig-Holstein). Forest Ecol. Manag. 2003, 182, 327–338. [Google Scholar] [CrossRef]
- Ewald, J. The calcareous riddle: Why are there so many calciphilous species in the Central European flora? Folia Geobot. 2003, 38, 357–366. [Google Scholar] [CrossRef]
- Schuster, B.; Diekmann, M. Changes in species density along the soil pH gradient–Evidence from German plant communities. Folia Geobot. 2003, 38, 367–379. [Google Scholar] [CrossRef]
- Costanza, J.K.; Moody, A.; Peet, R.K. Multi-scale environmental heterogeneity as a predictor of plant species richness. Landscape Ecol. 2011, 26, 851–864. [Google Scholar] [CrossRef]
- Gilliam, F.S. Response of herbaceous layer species to canopy and soil variables in a central Appalachian hardwood forest ecosystem. Plant Ecol. 2019, 220, 1131–1138. [Google Scholar] [CrossRef]
- Peppler-Lisbach, C.; Beyer, L.; Menke, N.; Mentges, A. Disentangling the drivers of understorey species richness in eutrophic forest patches. J. Veg. Sci. 2015, 26, 464–479. [Google Scholar] [CrossRef]
- Chazdon, R.L. Sunflecks and their importance to forest understorey plants. Adv. Ecol. Res. 1988, 18, 1–63. [Google Scholar] [CrossRef]
- Zarfos, M.R.; Dovciak, M.; Lawrence, G.B.; McDonnell, T.C.; Sullivan, T.J. Plant richness and composition in hardwood forest understories vary along an acidic deposition and soil-chemical gradient in the northeastern United States. Plant Soil 2019, 438, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Amatangelo, K.L.; Johnson, S.E.; Rogers, D.A.; Waller, D.M. Trait-environment relationships remain strong despite 50 years of trait compositional change in temperate forests. Ecology 2014, 95, 1780–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Liu, J.; Tan, S.; Cadotte, M.W.; Xu, K.; Gao, L.; Li, D. Trait variation and functional diversity maintenance of understory herbaceous species coexisting along an elevational gradient in Yulong Mountain, Southwest China. Plant Divers. 2016, 38, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; ter Braak, C.J.F. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef] [Green Version]



| Variable Name | Unit | Min | Mean | Max | Definition |
|---|---|---|---|---|---|
| Soil Properties | |||||
| pH_org | unitless | 3.1 | 4.8 | 6.2 | pH of the organic layer (Oh, Ol, Of) |
| pH_min | unitless | 3.5 | 4.8 | 7.0 | pH of the upper mineral soil (0–20 cm) |
| Clay | % | 7.9 | 27.5 | 55.4 | Proportion (%) of clay in the mineral soil (0–20 cm) |
| Ntot_org | g/kg | 10.1 | 15.1 | 22.7 | Total nitrogen in the organic layer (Oh, Ol, Of) |
| Ntot_min | g/kg | 1.4 | 4.4 | 23.1 | Total nitrogen in the mineral soil (0–20 cm) |
| Climatic Parameters | |||||
| BIO4 (WorldClim) | CV (%) | 63.1 | 70.7 | 76.3 | Temperature seasonality |
| BIO5 (WorldClim) | °C | 16.3 | 23.2 | 26.6 | Maximum temperature of the warmest month |
| BIO13 (WorldClim) | mm | 90.0 | 167.8 | 304.0 | Precipitation of the wettest month |
| BIO15 (WorldClim) | CV (%) | 18.0 | 24.2 | 37.3 | Precipitation seasonality |
| PET | mm | 746.0 | 878.7 | 1033.0 | Total potential evapotranspiration (annual) |
| Forest Stand Characteristics | |||||
| Tree_richness | n | 1.0 | 4.7 | 10.0 | Number of different tree species in the tree layer |
| Tree_ broadleaves | % | 0.0 | 72.9 | 100.0 | Proportion of broadleaf tree species in the total tree layer cover; a proxy for tree species composition |
| Tree_SCA | unitless | 1.8 | 4.1 | 5.0 | Shade-casting ability of the tree layer; a proxy for light availability in the forest understory |
| Shrub_richness | n | 1 | 8.7 | 25 | Number of different tree and shrub species in the shrub layer |
| Shrub_cover | % | 0.1 | 22.7 | 75.0 | Cover of shrub layer (visually estimated) |
| Topographic and Structural Factors | |||||
| Altitude | m | 160.0 | 650.9 | 1490.0 | Elevation of the monitoring plot |
| Hi | unitless | −0.74 | 0.05 | 0.88 | Heat load index: combining aspect and slope of the monitoring plot (see text for formula) |
| Rockiness | % | 0.0 | 9.6 | 50.0 | Cover of rocks on the surface (visually estimated) |
| Woody debris | % | 1.0 | 5.3 | 15.0 | Cover of woody debris (visually estimated) |
| Moss_cover | % | 0.3 | 7.2 | 56.8 | Cover of mosses and other bryophytes on the forest soil (visually estimated) |
| Functional Trait | Variable Type | % of Missing Values | Definition |
|---|---|---|---|
| Clonal Traits | |||
| Clonality | Binary | 4.6 | Plant ability of clonal reproduction (yes/no) |
| Clonal index | Ordinal | 4.5 | Overall measure of a taxon’s clonal ability |
| Type of clonal growth organ | Categorical | 4.6 | Categories: stolon, turion, stem fragment, budding plant, epigeogenous rhizome, hypogeogenous rhizome, below-ground stem tuber, bulb, root with adventitious buds, root tuber, stolon with tuber |
| Persistence of the clonal growth organ | Ordinal | 3.3 | The lifespan of the physical connection between the parent and offspring shoots |
| Bud Bank Traits | |||
| Bud bank size | Numerical | 5.1 | The number of vegetative buds per shoot, with these buds residing on the below-ground stem-derived organs and root-derived buds of a plant |
| Bud bank depth | Numerical | 4.3 | The depth of buds (including root buds) in relation to the soil surface |
| Leaf-Height-Seed Traits | |||
| Specific leaf area | Numerical | 13.0 | The leaf area per dry weight (g/m2) |
| Plant height | Numerical | 1.9 | The mean distance between the foliage of a plant and the soil surface (m) |
| Seed mass | Numerical | 10.0 | The dry mass without accessories (mg) |
| Variable | Min | Mean | Max |
|---|---|---|---|
| Taxonomy-Based Measure | |||
| Species richness | 8.0 | 44.0 | 81.0 |
| Herb-layer cover (%) | 0.3 | 46.0 | 99.8 |
| Species evenness | 0.16 | 0.69 | 0.86 |
| Trait-Based Measure | |||
| Clonal traits (RaoQ) | 0.01 | 0.70 | 1.31 |
| Bud bank traits (RaoQ) | 0.24 | 1.47 | 2.33 |
| LHS traits (RaoQ) | 0.96 | 2.82 | 5.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kermavnar, J.; Kutnar, L.; Marinšek, A. Disentangling the Ecological Determinants of Species and Functional Trait Diversity in Herb-Layer Plant Communities in European Temperate Forests. Forests 2021, 12, 552. https://doi.org/10.3390/f12050552
Kermavnar J, Kutnar L, Marinšek A. Disentangling the Ecological Determinants of Species and Functional Trait Diversity in Herb-Layer Plant Communities in European Temperate Forests. Forests. 2021; 12(5):552. https://doi.org/10.3390/f12050552
Chicago/Turabian StyleKermavnar, Janez, Lado Kutnar, and Aleksander Marinšek. 2021. "Disentangling the Ecological Determinants of Species and Functional Trait Diversity in Herb-Layer Plant Communities in European Temperate Forests" Forests 12, no. 5: 552. https://doi.org/10.3390/f12050552






