Spatial Variation and Temporal Instability in the Growth/Climate Relationship of White Birch (Betula platyphylla Suk) in the Changbai Mountain, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Development of Ring-Width Chronologies
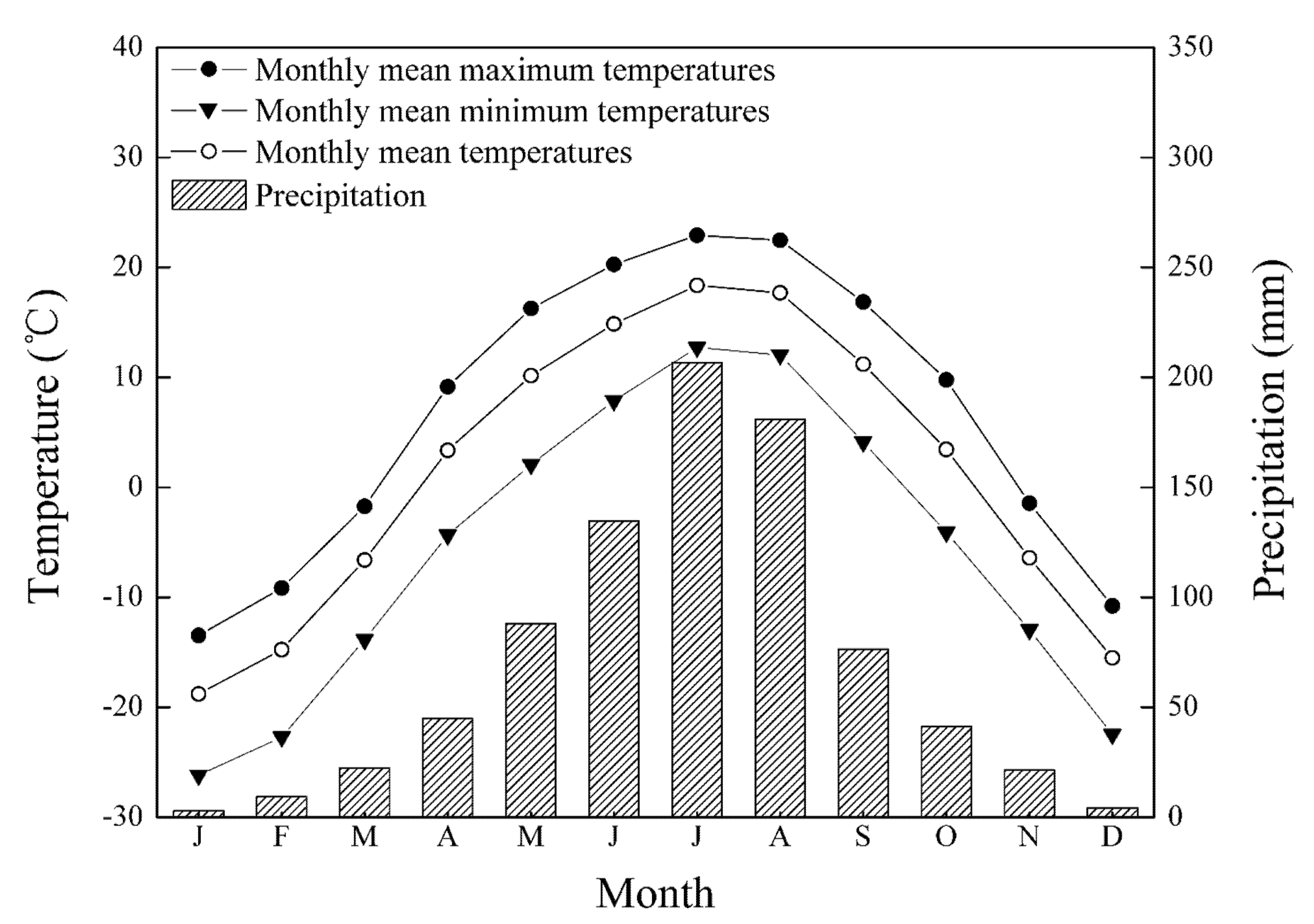
2.3. Climate Data and Climate–Growth Relationships
3. Results
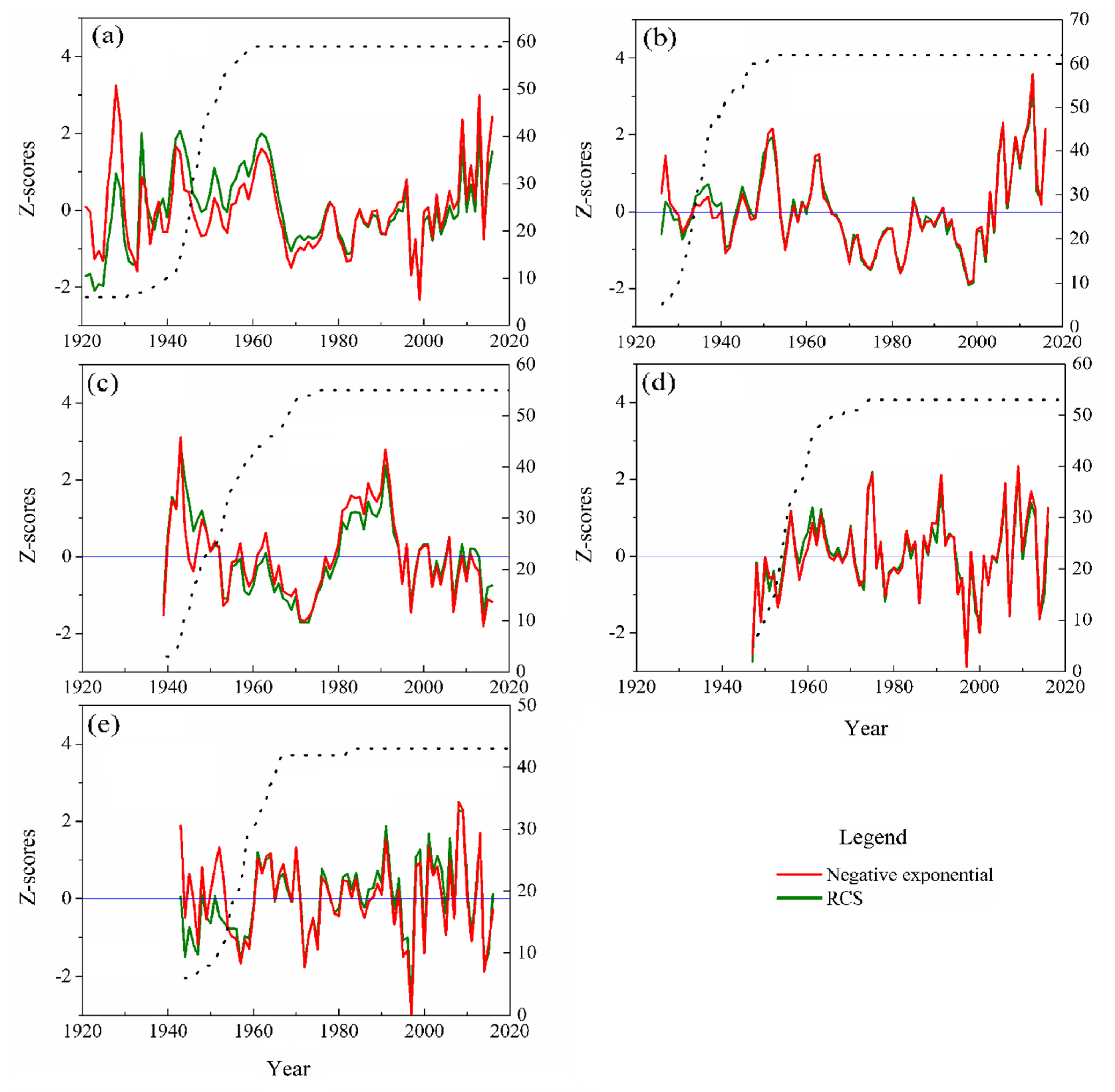
3.1. Standard Chronologies’ Characteristics
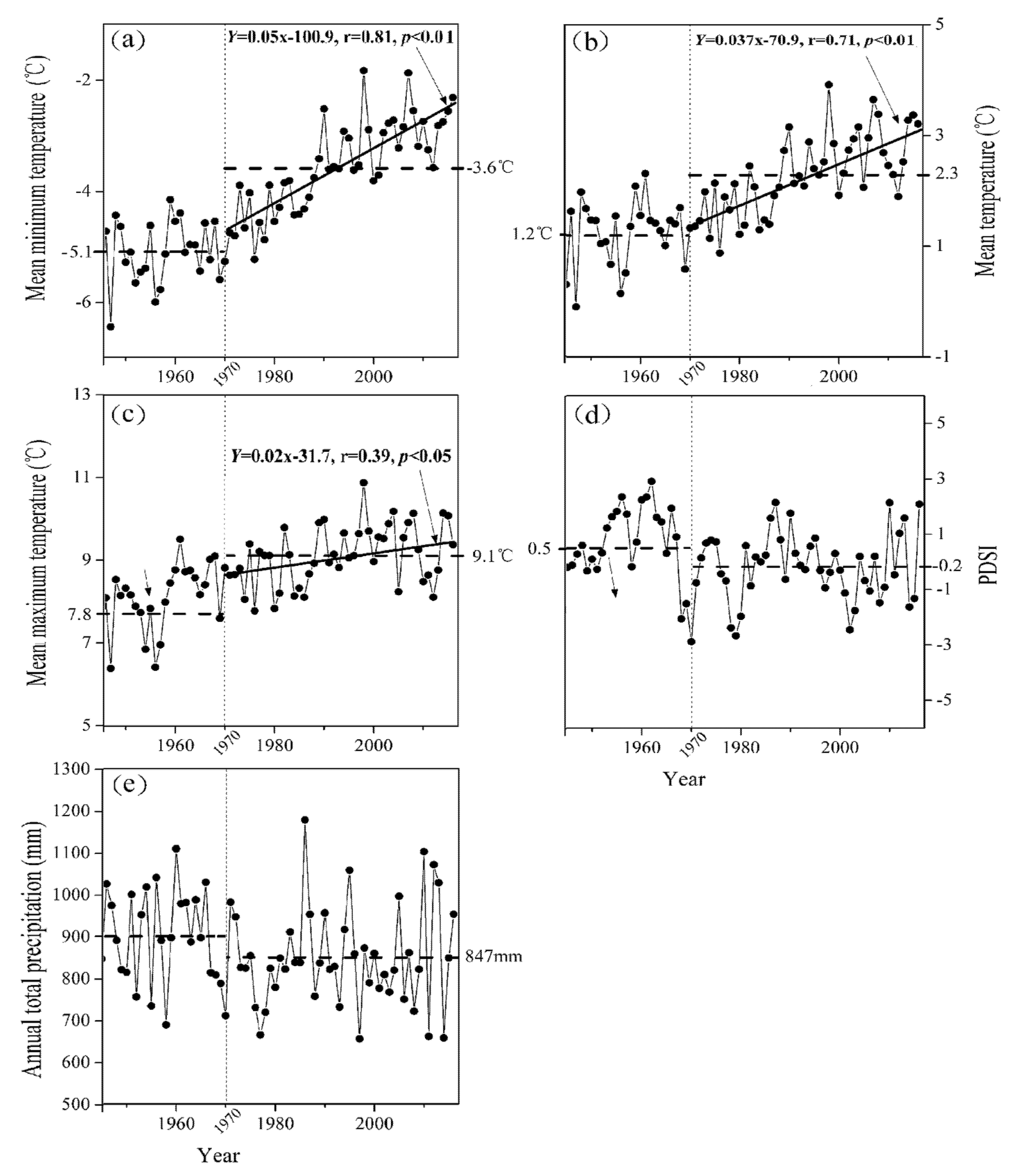
3.2. Climate Variation
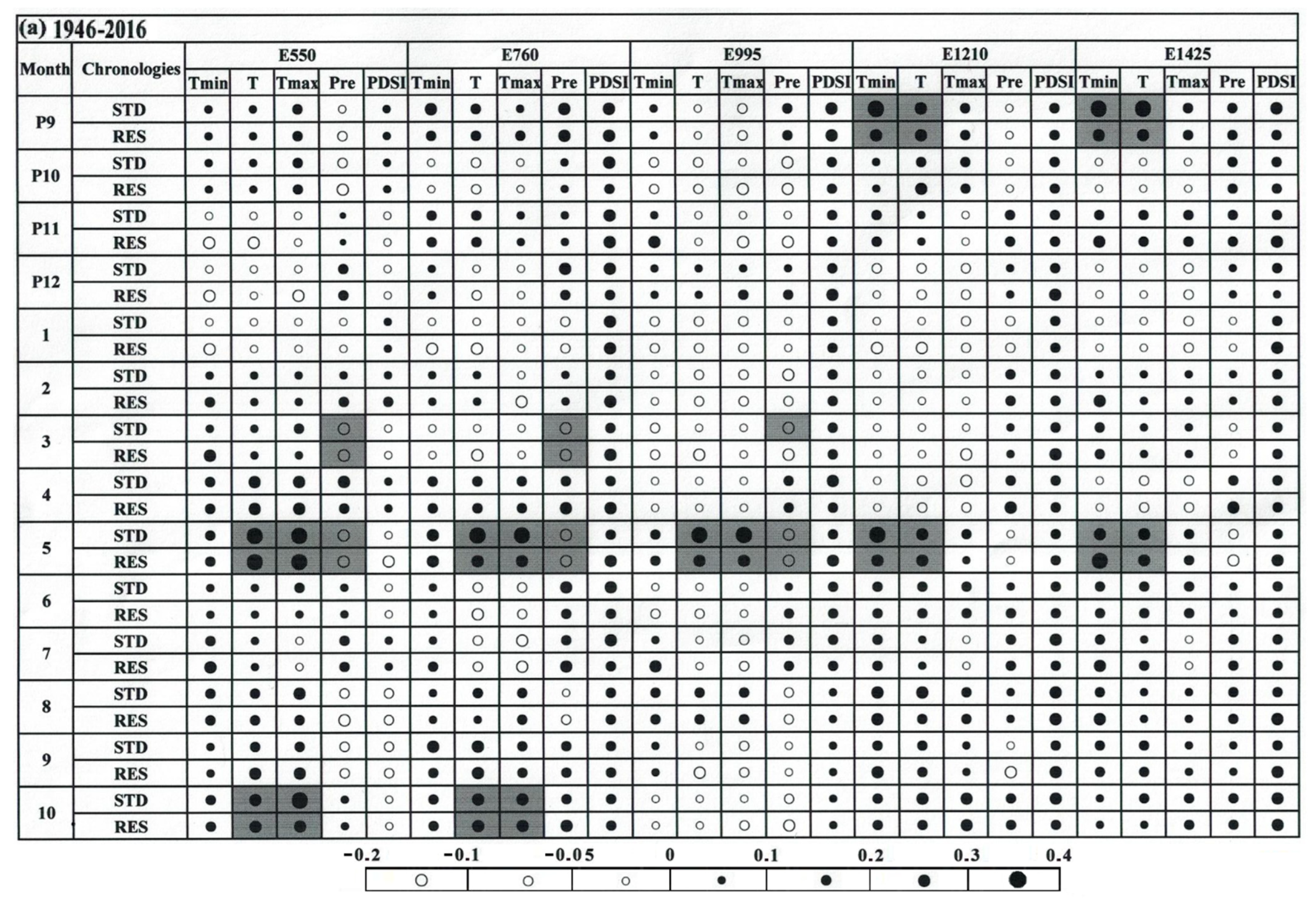
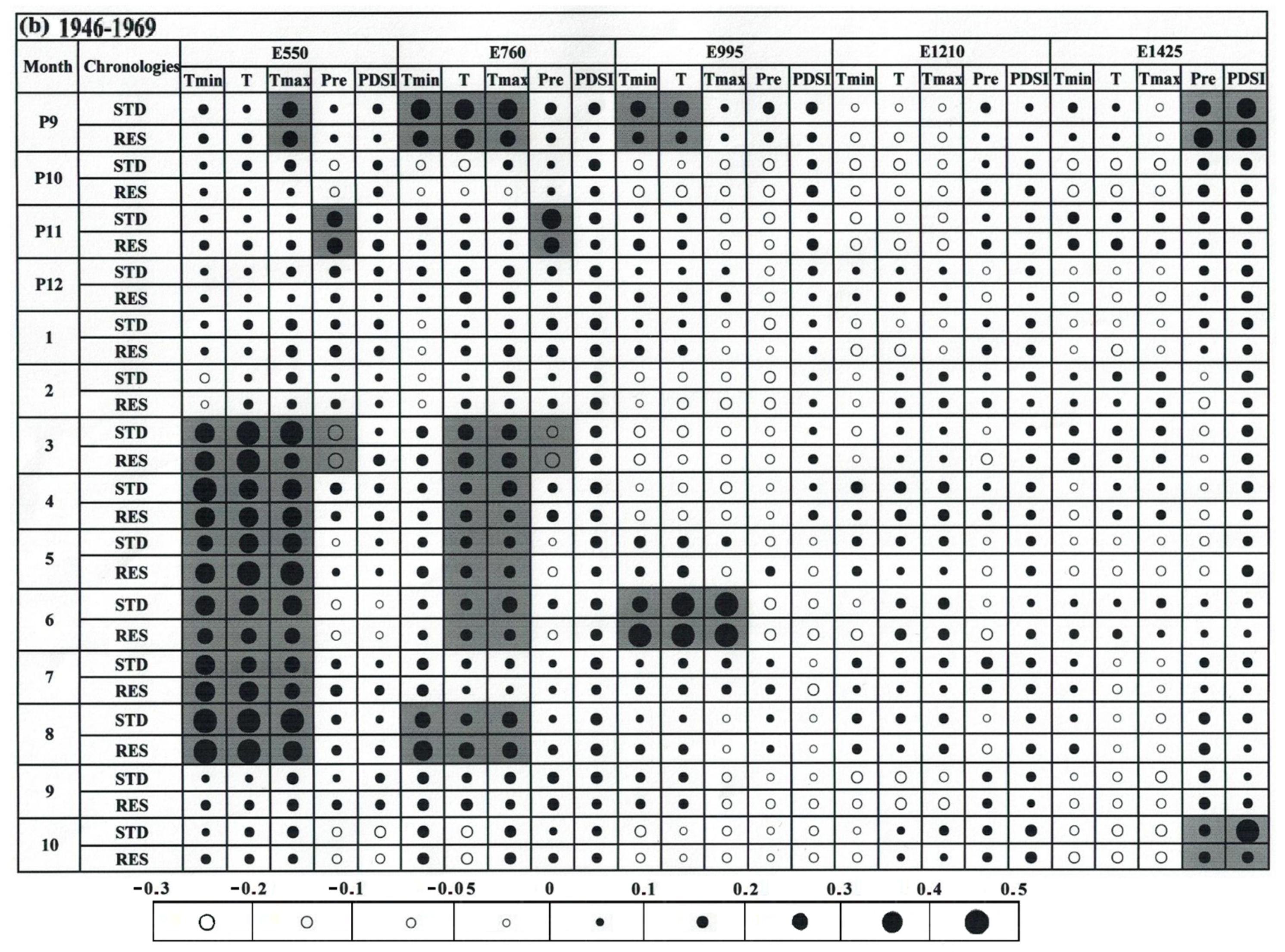
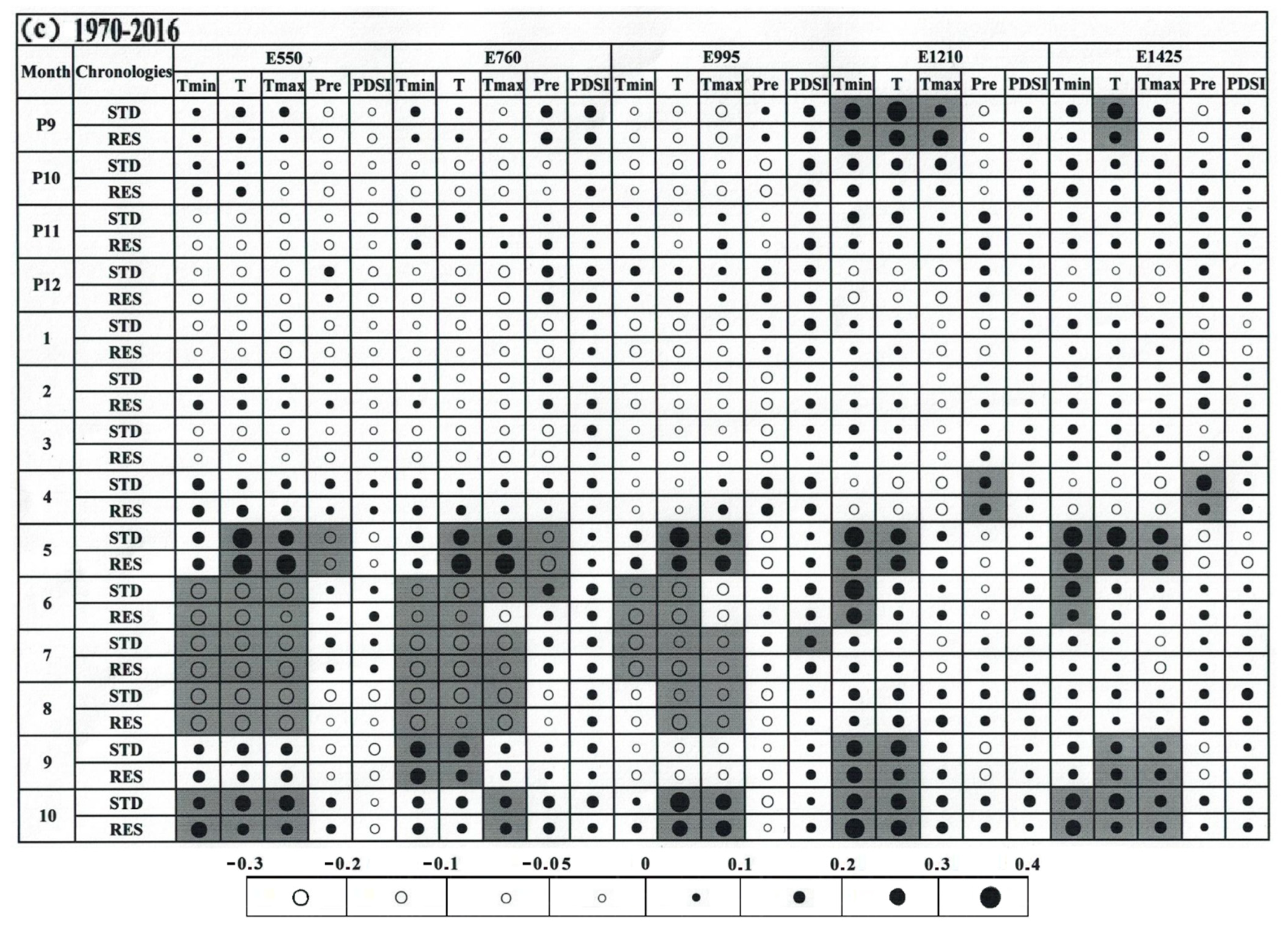
3.3. Climate–Growth Relationship
4. Discussion
4.1. Chronology Evaluation
4.2. The Relationship of Climate/Growth Along an Altitudinal Gradient from 1946 to 2016
4.3. Temporal Stability of the Climate–Growth Relationship
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2014: Mitigation of Climate Change [M/OL]; Cambridge University Press: New York, NY, USA, 2014; Available online: http://www.ipcc.ch/report/ar5/wg3 (accessed on 8 May 2021).
- Chen, P.-Y.; Welsh, C.; Hamann, A. Geographic variation in growth response of Douglas-fir to interannual climate variability and projected climate change. Glob. Chang. Biol. 2010, 16, 3374–3385. [Google Scholar] [CrossRef]
- Huang, J.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Chang. Biol. 2010, 16, 711–731. [Google Scholar] [CrossRef]
- Lin, D.; Xia, J.; Wan, S. Climate warming and biomass accumulation of terrestrial plants: A meta-analysis. New Phytol. 2010, 188, 187–198. [Google Scholar] [CrossRef]
- Mérian, P.; Bontemps, J.-D.; Bergès, L.; Lebourgeois, F. Spatial variation and temporal instability in climate-growth relationships of sessile oak (Quercus petraea [Matt.] Liebl.) under temperate conditions. Plant Ecol. 2011, 212, 1855–1871. [Google Scholar] [CrossRef]
- Liang, J.; Xia, J.; Liu, L.; Wan, S. Global patterns of the responses of leaf-level photosynthesis and respiration in terrestrial plants to experimental warming. J. Plant Ecol. 2013, 6, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Chen, J.; Sun, G.; Chu, H.; Noormets, A.; Ouyang, Z.; John, R.; Wan, S.; Guan, W. Long-term variability and environmental control of the carbon cycle in an oak-dominated temperate forest. For. Ecol. Manag. 2014, 313, 319–328. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Han, S.; Chen, Z.; Setälä, H.; Yu, J.; Zheng, X.; Guo, Y.; Gu, Y. Radial growth response of Larix gmelinii to climate along a latitudinal gradient in the Greater Khingan Mountains, Northeastern China. Forests 2016, 7, 295. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Bai, X.; Chang, Y.; Chen, Z. Increased sensitivity of Dahurian larch radial growth to summer temperature with the rapid warming in Northeast China. Trees 2016, 30, 1799–1806. [Google Scholar] [CrossRef]
- Latreille, A.; Davi, H.; Huard, F.; Pichot, C. Variability of the climate-radial growth relationship among Abies alba trees and populations along altitudinal gradients. For. Ecol. Manag. 2017, 396, 150–159. [Google Scholar] [CrossRef]
- Holz, A.; Hart, S.J.; Williamson, G.J.; Veblen, T.T.; Aravena, J.C. Radial growth response to climate change along the latitudinal range of the world’s southernmost conifer in southern South America. J. Biogeogr. 2018, 45, 1140–1152. [Google Scholar] [CrossRef]
- Liu, B.; Liang, E.; Liu, K.; Camarero, J.J. Species-and elevation-dependent growth responses to climate warming of mountain forests in the Qinling Mountains, Central China. Forests 2018, 9, 248. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Peng, K.; Li, J. Climate-growth response of Chinese white pine (Pinus armandii) at different age groups in the Baiyunshan National Nature Reserve, central China. Dendrochronologia 2018, 49, 102–109. [Google Scholar] [CrossRef]
- Ponocná, T.; Chuman, T.; Rydval, M.; Urban, G.; Migaɬa, K.; Treml, V. Deviations of tree line Norway spruce radial growth from summer temperatures in East-Central Europe. Agric. For. Meteorol. 2018, 253, 62–70. [Google Scholar] [CrossRef]
- Truettner, C.; Anderegg, W.R.; Biondi, F.; Koch, G.W.; Ogle, K.; Schwalm, C.; Litvak, M.E.; Shaw, J.D.; Ziaco, E. Conifer radial growth response to recent seasonal warming and drought from the southwestern USA. For. Ecol. Manag. 2018, 418, 55–62. [Google Scholar] [CrossRef]
- Zhu, L.; Cooper, D.J.; Yang, J.; Zhang, X.; Wang, X. Rapid warming induces the contrasting growth of Yezo spruce (Piceajezoensis var. microsperma) at two elevation gradient sites of Northeast China. Dendrochronologia 2018, 50, 52–63. [Google Scholar] [CrossRef]
- Qian, W.; Qin, A. Spatial-temporal characteristics of temperature variation in China. Meteorol. Atmos. Phys. 2006, 93, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.P.; Wang, H.; Zhang, W. Variation and tendency of surface aridity index from 1960 to 2005 in three provinces of northeast China. Adv. Earth Sci. 2008, 23, 619–662. (In Chinese) [Google Scholar]
- Wang, X.; Zhang, Y.; McRae, D.J. Spatial and age-dependent tree-ring growth responses of Larix gmelinii to climate in NorthEastern China. Trees 2009, 23, 875–885. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Piao, S.; Fang, J. Regional differences in the timing of recent air warming during the past four decades in China. Chin. Sci. Bull. 2010, 55, 1968–1973. [Google Scholar] [CrossRef]
- Yu, D.; Liu, J.; Zhou, L.; Zhou, W.; Fang, X.; Wei, Y.; Jiang, S.; Dai, L. Spatial variation and temporal instability in the climate–growth relationship of Korean pine in the Changbai Mountain region of Northeast China. For. Ecol. Manag. 2013, 300, 96–105. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, C.; Zhang, J.; Han, S.; Coombs, C.; Wang, X.; Wang, J.; Hao, L.; Dong, S. Tree ring width-based January–March mean minimum temperature reconstruction from Larix gmelinii in the Greater Khingan Mountains, China since A.D. 1765. Int. J. Climatol. 2020, 41, E842–E854. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, X.; Zhang, J.; Han, S.; Chen, Z.; Wang, X.; Wang, J.; Hao, L.; Li, G.; Dong, S.; et al. Reconstruction of june–july temperatures based on a 233 year tree-ring of Picea jezoensis var. microsperma. Forests 2019, 10, 416. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Wang, Y.; Zhang, J.; Han, S.; Coombs, C.E.; Escobedo, M.; Wang, J.; Wang, X.; Hao, L.; Li, G.; et al. Tree-ring reconstruction of june–july mean temperatures in the northern Daxing’an Mountains, China. Geochronometria 2020, 47, 13–22. [Google Scholar] [CrossRef]
- Xu, D.; Yan, H. A study of the impacts of climate change on the geographic distribution of Pinus koraiensis in China. Environ. Int. 2001, 27, 201–205. [Google Scholar] [CrossRef]
- Cheng, X.X.; Yan, X.D. Effects of global climate change on forest succession in Daxing’anling of Northeast China. Chin. J. Plant Ecol. 2007, 26, 1277–1284. (In Chinese) [Google Scholar]
- Leng, W.F.; He, H.S.; Bu, R.C.; Hu, Y.M. Sensitivity analysis of the impacts of climate change on potential distribution of three larch (Larix) species in northeastern China. J. Plant Ecol. 2007, 31, 825–833. (In Chinese) [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press: Cambridge, MA, USA, 1976. [Google Scholar]
- Wang, S.W.; Zhao, Z.C.; Tang, G.L. The warming of climate in China. Int. Politics Quart. 2009, 30, 1–11. (In Chinese) [Google Scholar]
- Yu, D.; Wang, S.; Tang, L.; Dai, L.; Wang, Q.; Wang, S. Relationship between tree-ring chronology of Larix olgensis in Changbai Mountains and the climate change. J. Appl. Ecol. (Ying Yong Sheng Tai Xue Bao) 2005, 16, 14–20. (In Chinese) [Google Scholar]
- Yu, D.; Wang, Q.; Wang, G.G.; Dai, L. Dendroclimatic response of Picea jezoensis along an altitudinal gradient in Changbai Mountains. Sci. China Ser. E 2006, 49, 150–159. [Google Scholar] [CrossRef]
- Chen, L.; Wu, S.; Pan, T. Variability of climate-growth relationships along an elevation gradient in the Changbai Mountain, northeastern China. Trees 2011, 25, 1133–1139. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Q.; Wang, Y.; Zhou, W.; Ding, H.; Fang, X.; Jiang, S.; Dai, L. Climatic effects on radial growth of major tree species on Changbai Mountain. Ann. For. Sci. 2011, 68, 921–933. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Shao, X.-M.; Jiang, Y.; Fang, X.-Q.; Wu, S.-H. The impacts of climate change on the radial growth of Pinus koraiensis along elevations of Changbai Mountain in northeastern China. For. Ecol. Manag. 2013, 289, 333–340. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Gao, L. Climatic response of Betula ermanii along an altitudinal gradient in the northern slope of Changbai Mountain, China. Dendrobiology 2013, 70, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Yin, Y.H.; Zhao, D.S.; Yuan, Q.Z.; Wu, S.H. Climate response of tree growth along an altitudinal gradient in the Changbai Mountains, Northeast China. Acta Ecol. Sin. 2014, 34, 1568–1574. (In Chinese) [Google Scholar]
- Shen, C.; Wang, L.; Li, M. The altitudinal variability and temporal instability of the climate—Tree-ring growth relationships for Changbai larch (Larix olgensis Henry) in the Changbai Mountains area, Jilin, Northeastern China. Trees 2015, 30, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Puhua, H. Betula platyphylla SUK., 1911. In Enzyklopädieder Holzgewächse-Handbuch und Atlas der Dendrologie; Schütt, P., Weisgerber, H., Schuck, H., Lang, U.M., Roloff, A., Eds.; Schütt, P., Translator; Wiley-VCH: Weinheim, Germany, 2013; p. 6. (In German) [Google Scholar]
- Gradel, A.; Haensch, C.; Ganbaatar, B.; Dovdondemberel, B.; Nadaldorj, O.; Günther, B. Response of white birch (Betula platyphylla Sukaczev) to temperature and precipitation in the mountain forest steppe and taiga of Northern Mongolia. Dendrochronologia 2017, 41, 24–33. [Google Scholar] [CrossRef]
- Zyryanova, O.A.; Terazawa, M.; Koike, T.; Zyryanov, V.I. White birch trees as resource species of Russia: Their distribution, ecophysiological features, multiple utilizations. Eurasian J. For. Res. 2010, 13, 25–40. [Google Scholar]
- Doležal, J.; Ishii, H.; Kyncl, T.; Takahashi, K.; Vetrova, V.P.; Homma, K.; Sumida, A.; Hara, T. Climatic factors affecting radial growth of Betula ermanii and Betula platypylla in Kamchatka. Can. J. For. Res. 2010, 40, 273–285. [Google Scholar] [CrossRef]
- Koichi, T.; Hiroto, A.; Koh, Y. Effects of climate on the radial growth of tree species in the upper and lower distribution limits of an altitudinal ecotone on Mount Norikura, Central Japan. Ecol. Res. 2003, 18, 549–558. [Google Scholar]
- Otoda, T.; Sakamoto, K.; Hirobe, M.; Undarmaa, J.; Yoshikawa, K. Influences of anthropogenic disturbances on the dynamics of white birch (Betula platyphylla) forests at the southern boundary of the Mongolian forest-steppe. J. For. Res. 2013, 18, 82–92. [Google Scholar] [CrossRef]
- Zhu, H.F.; Fang, X.Q.; Shao, X.M.; Yin, Z.Y. Tree ring-based February–April temperature reconstruction for Changbai Mountain in Northeast China and its implication for East Asian winter monsoon. Clim. Past 2009, 5, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 44, 69–75. [Google Scholar]
- Yamaguchi, D.K.; Cook, E.R.; Kairiukstis, L.A. Methods of Dendrochronology: Applications in the Environmental Sciences; Springer: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Wigley, T.M.L.; Briffa, K.R.; Jones, P.D. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Climatol. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Harris, I.R.; Jones, P.; Osborn, T.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Liu, Q. Larix olgensis growth-climate response between lower and upper elevation limits: An intensive study along the Eastern slope of the Changbai Mountains, Northeastern China. J. For. Res. 2018, 31, 231–244. [Google Scholar] [CrossRef]
- Shao, X.; Wu, X. Reconstruction of climate change on Changbai Mountain, Northeast China using tree-ring data. Quat. Sci. 1997, 1, 76–85. (In Chinese) [Google Scholar]
- Li, G.Q.; Bai, F.; Sang, W.G. Different responses of radial growth to climate warming in Pinus koraiensis and Piceajezoensis var. komarovii at their upper elevational limits in Changbai Mountain, China. Chin. J. Plant Ecol. 2011, 35, 500–511. (In Chinese) [Google Scholar] [CrossRef]
- Dang, H.; Jiang, M.; Zhang, Q.; Zhang, Y. Growth responses of subalpine fir (Abiesfargesii) to climate variability in the Qinling Mountain, China. For. Ecol. Manag. 2007, 240, 143–150. [Google Scholar] [CrossRef]
- Jump, A.S.; Hunt, J.M.; Peñuelas, J. Rapid climate change-related growth decline at the southern range edge of Fagus sylvatica. Glob. Chang. Biol. 2006, 12, 2163–2174. [Google Scholar] [CrossRef] [Green Version]



| Parameters | E550 | E760 | E995 | E1210 | E1425 |
|---|---|---|---|---|---|
| Elevation | 550 | 760 | 995 | 1210 | 1425 |
| Latitude | 42°41′ | 42°24′ | 42°15′ | 42°09′ | 42°03′ |
| Longitude | 128°01′ | 128°06′ | 128°09′ | 128°12′ | 128°16′ |
| No. of trees/cores | 22/43 | 28/53 | 28/55 | 33/62 | 31/59 |
| Period | 1943–2016 | 1947–2016 | 1939–2016 | 1926–2016 | 1921–2016 |
| Mean sensitivity (MS) | 0.277 | 0.29 | 0.295 | 0.304 | 0.309 |
| Standard deviation (SD) | 0.18 | 0.21 | 0.22 | 0.14 | 0.19 |
| Skewness | 1.27 | 1.157 | 0.345 | 1.005 | 1.021 |
| Kurtosis | 4.36 | 4.168 | 2.322 | 3.693 | 4.231 |
| Signal-to-noise ratio (SNR) | 8.5 | 11.6 | 11.7 | 9.3 | 8.7 |
| Mean ring width (mm) | 1.58 ± 0.06 | 1.16 ± 0.05 | 1.42 ± 0.07 | 1.03 ± 0.03 | 1.13 ± 0.06 |
| Mean inter-series correlation (RBAR) | 0.566 | 0.559 | 0.613 | 0.545 | 0.587 |
| Auto correlation | 0.582 | 0.713 | 0.708 | 0.784 | 0.703 |
| Expressed population signal (EPS) | 0.92 | 0.92 | 0.95 | 0.93 | 0.96 |
| First year where EPS > 0.85 | 1946 | 1951 | 1945 | 1929 | 1927 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Cao, Y.; Han, S.; Zhang, J.; Hao, L. Spatial Variation and Temporal Instability in the Growth/Climate Relationship of White Birch (Betula platyphylla Suk) in the Changbai Mountain, China. Forests 2021, 12, 589. https://doi.org/10.3390/f12050589
Jiang Y, Cao Y, Han S, Zhang J, Hao L. Spatial Variation and Temporal Instability in the Growth/Climate Relationship of White Birch (Betula platyphylla Suk) in the Changbai Mountain, China. Forests. 2021; 12(5):589. https://doi.org/10.3390/f12050589
Chicago/Turabian StyleJiang, Yangao, Yuting Cao, Shijie Han, Junhui Zhang, and Lin Hao. 2021. "Spatial Variation and Temporal Instability in the Growth/Climate Relationship of White Birch (Betula platyphylla Suk) in the Changbai Mountain, China" Forests 12, no. 5: 589. https://doi.org/10.3390/f12050589





