Photosynthetic Acclimation and Growth Responses to Elevated CO2 Associate with Leaf Nitrogen and Phosphorus Concentrations in Mulberry (Morus multicaulis Perr.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Plant Materials
2.3. Determination of Plant Growth
2.4. Determination of Photosynthetic Parameters
2.5. Plant N, P and K Measurements
2.6. Statistical Analysis
3. Results
3.1. Plant Growth Traits
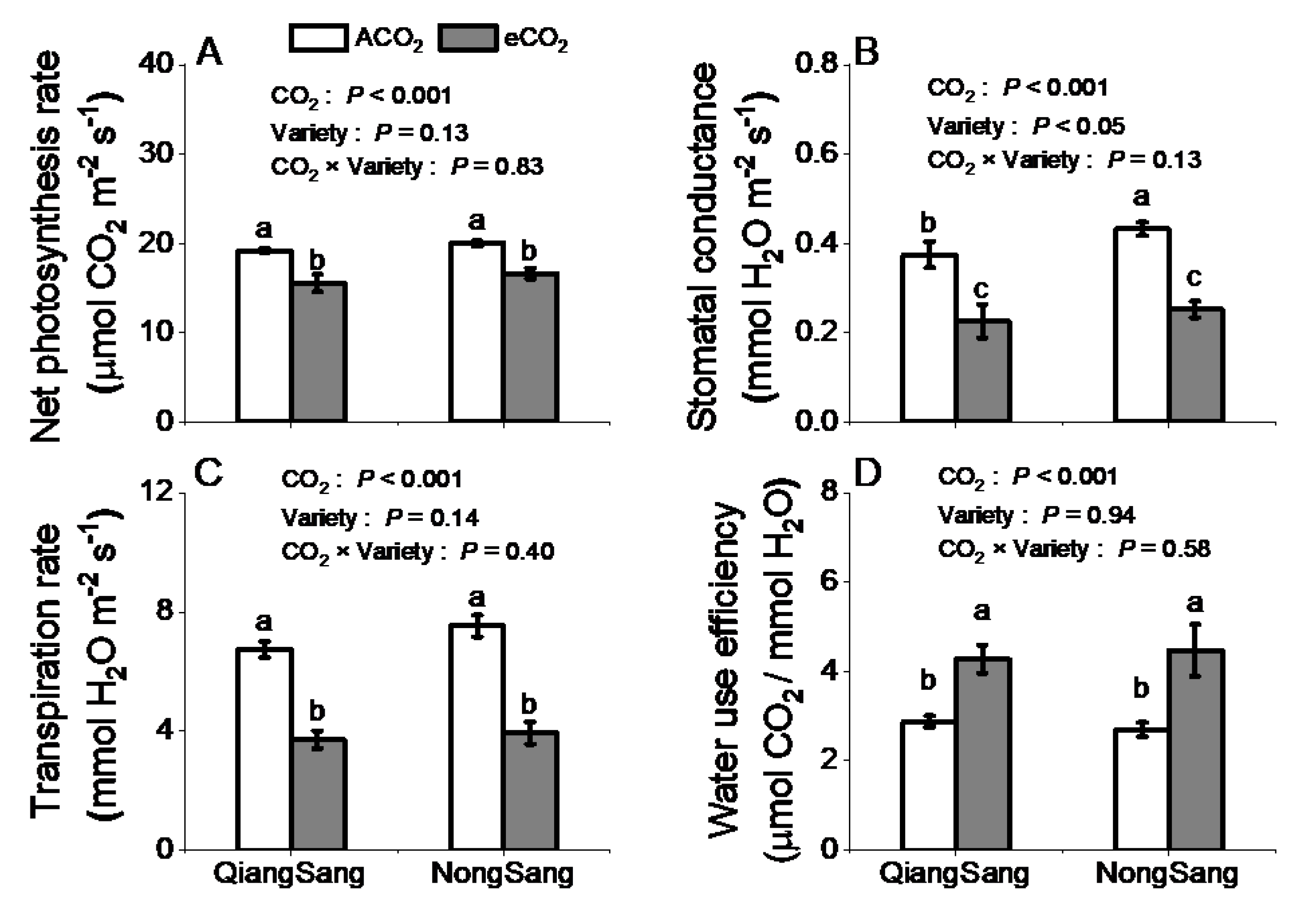
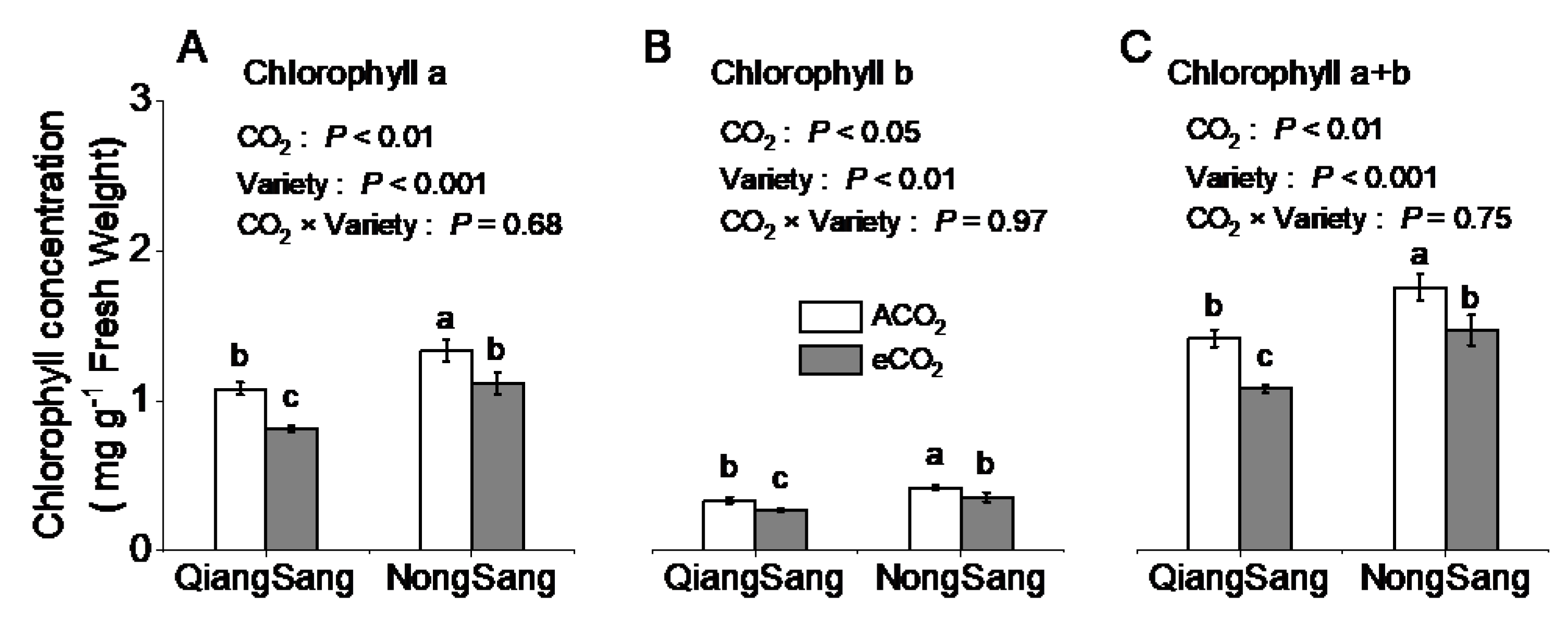
3.2. Leaf Photosynthetic Traits
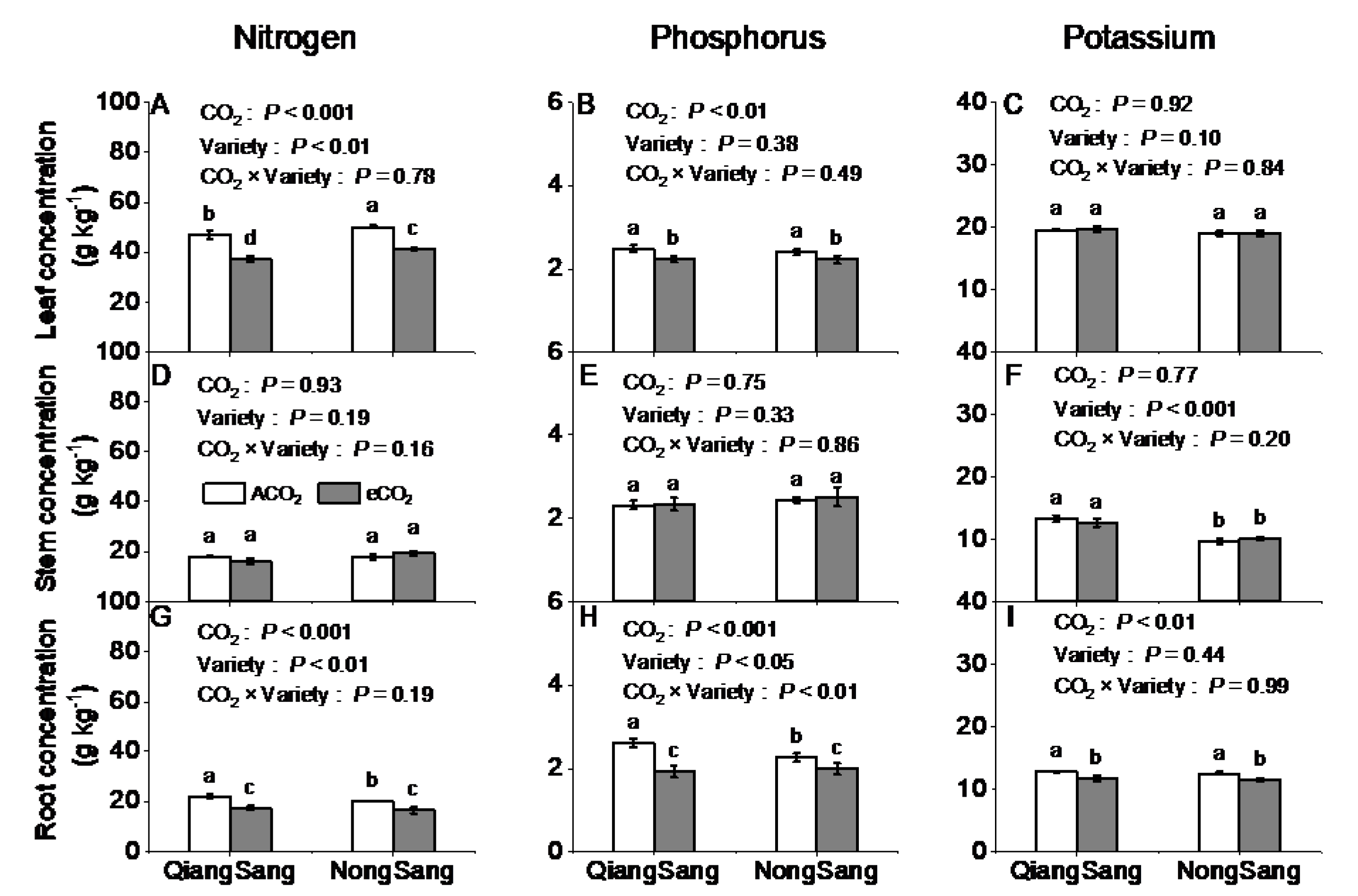
3.3. Plant Tissue N, P, and K Concentrations
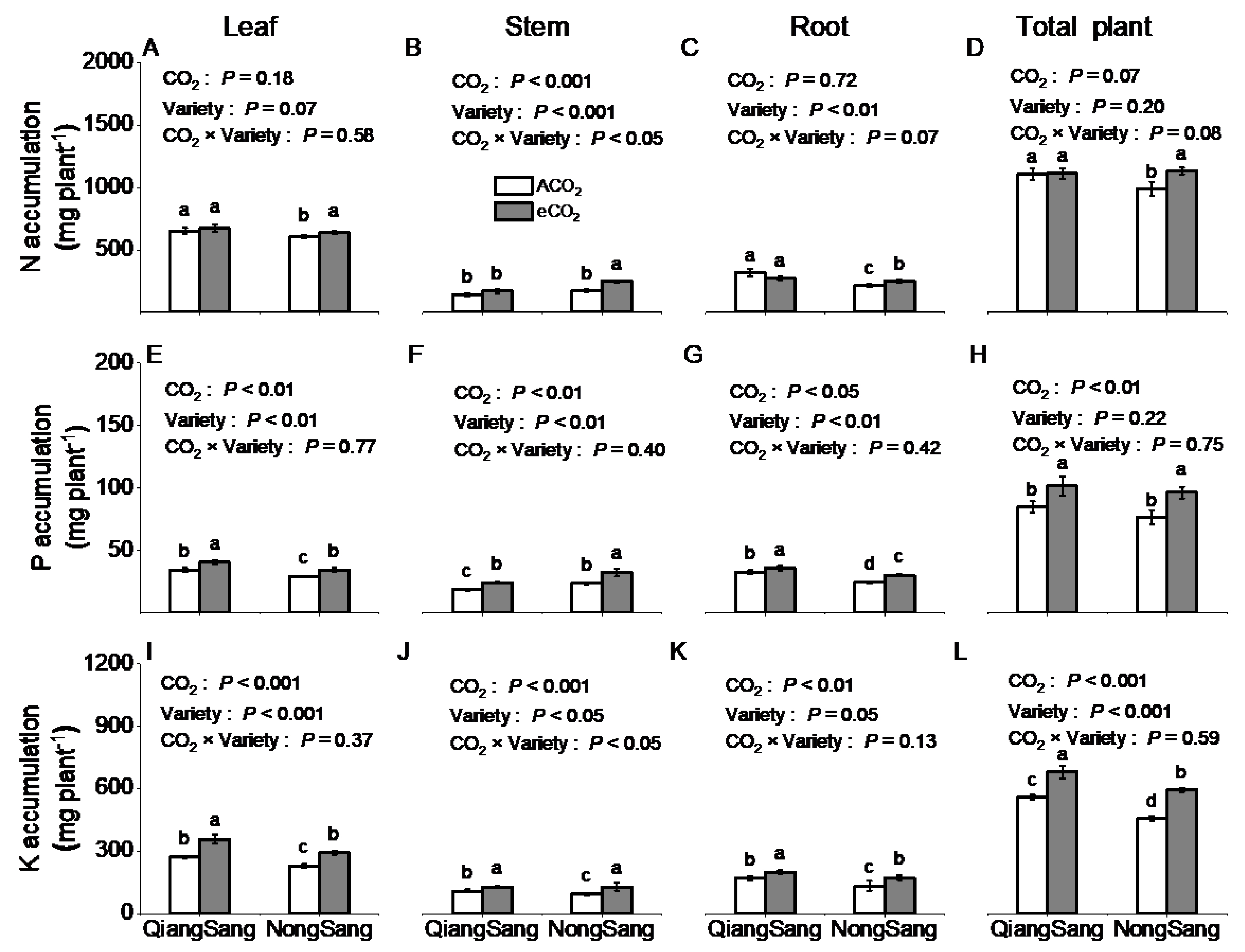
3.4. Plant N, P, and K Accumulations
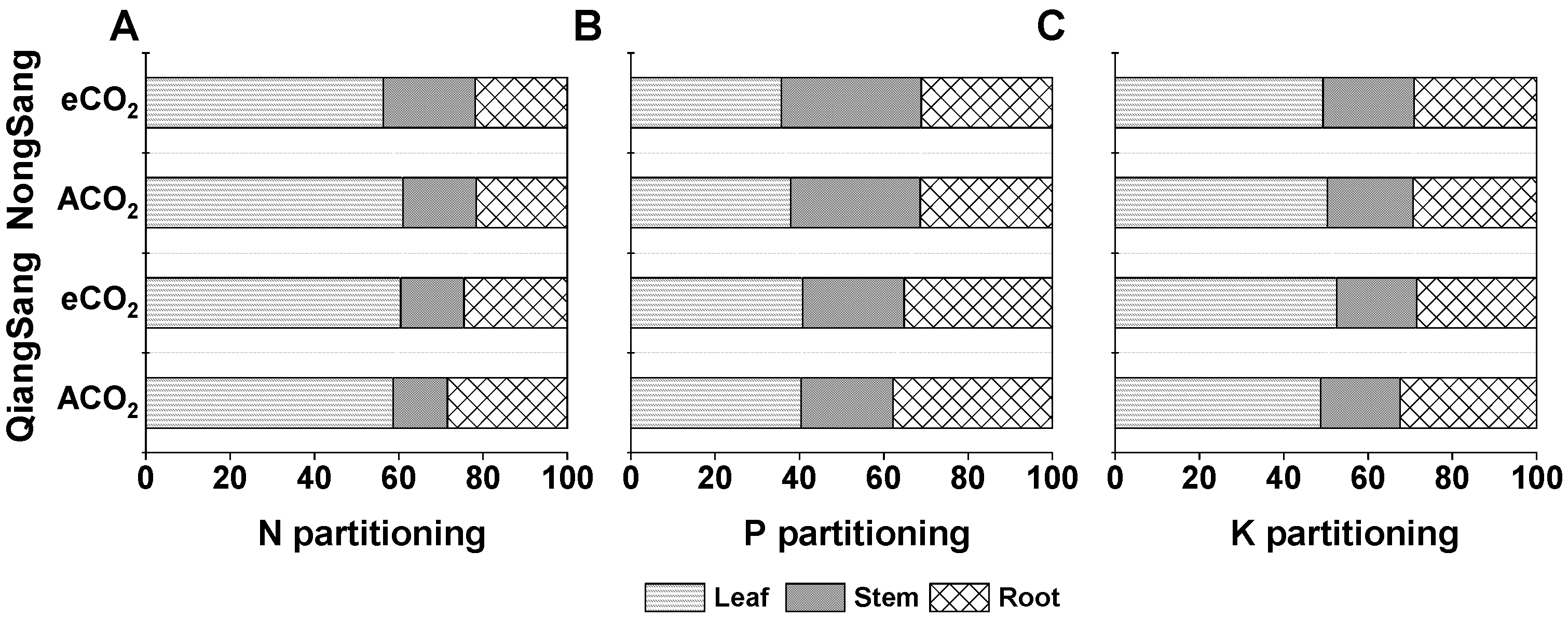
3.5. Plant N, P, and K Partitioning
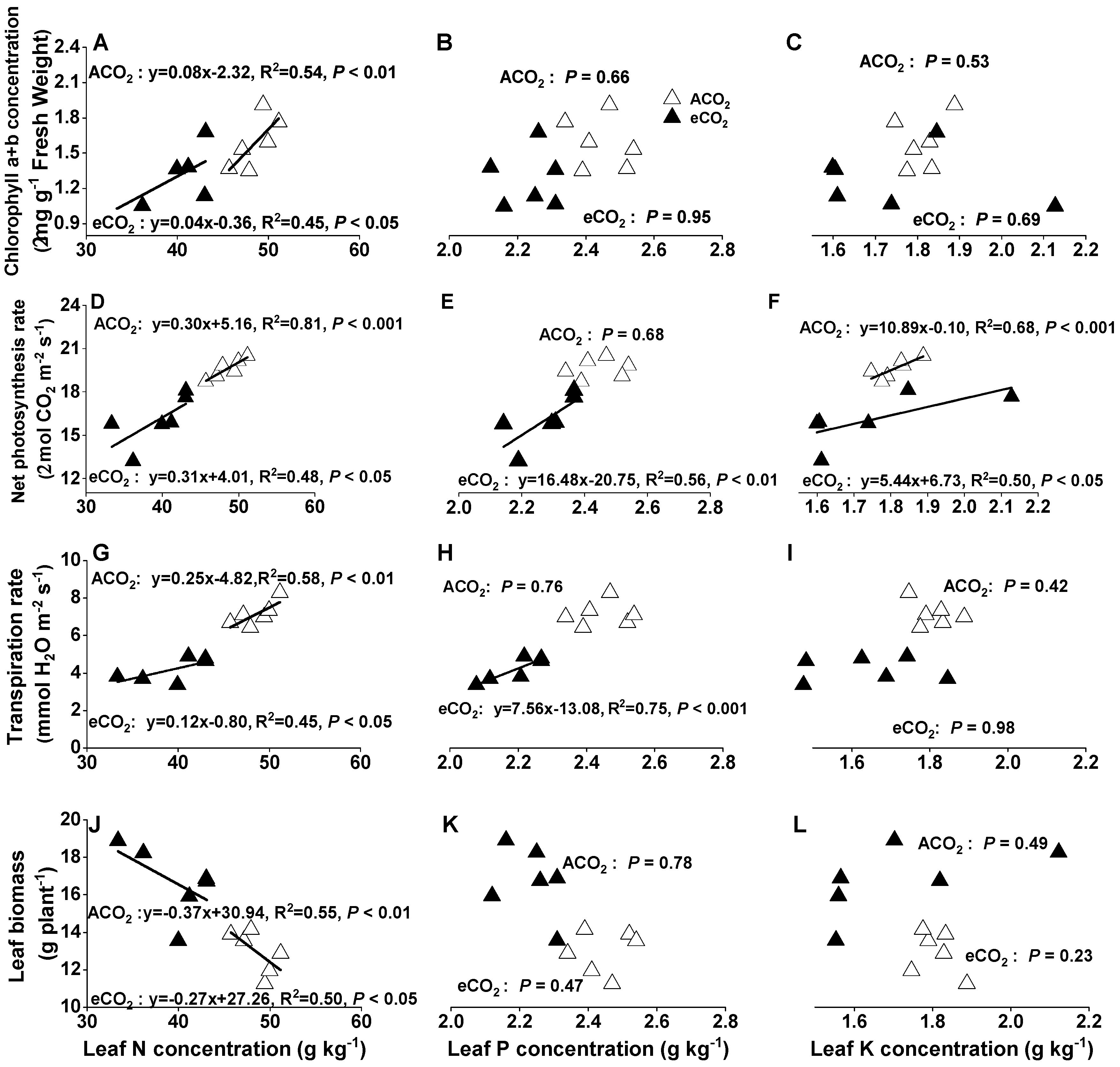
3.6. Relationships between Physiological Parameters and Tissue Nutrient Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Ofori-Amanfo, K.K.; Klem, K.; Vesela, B.; Holub, P.; Agyei, T.; Marek, M.V.; Grace, J.; Urban, O. Interactive effect of elevated CO2 and reduced summer precipitation on photosynthesis is species-specific: The case study with soil-planted norway spruce and sessile oak in a mountainous forest plot. Forests 2021, 12, 14–29. [Google Scholar]
- Salazar-Parra, C.; Aranjuelo, I.; Pascual, I.; Erice, G.; Sanz-Sáez, Á.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Araus, J.L.; Morales, F. Carbon balance, partitioning and photosynthetic acclimation in fruit-bearing grapevine (Vitis vinifera L. cv. Tempranillo) grown under simulated climate change (elevated CO2, elevated temperature and moderate drought) scenarios in temperature gradient greenhouses. J. Plant Physiol. 2015, 174, 97–109. [Google Scholar] [PubMed]
- Yuan, M.; Cai, C.; Wang, X.; Li, G.; Wu, G.; Wang, J.; Geng, W.; Liu, G.; Zhu, J.; Sun, Y. Warm air temperatures increase photosynthetic acclimation to elevated CO2 concentrations in rice under field conditions. Field Crop. Res. 2021, 262, 1–11. [Google Scholar] [CrossRef]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Morcuende, R. Improved responses to elevated CO2 in durum wheat at a low nitrate supply associated with the upregulation of photosynthetic genes and the activation of nitrate assimilation. Plant Sci. 2017, 260, 119–128. [Google Scholar] [CrossRef]
- Ainsworth, E.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Reich, P.B.; Naumburg, E.S.; Koch, G.W.; Kubiske, M.E.; Smith, S.D. Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob. Chang. Biol. 2004, 10, 2121–2138. [Google Scholar] [CrossRef]
- Nowak, R.S.; Ellsworth, D.S.; Smith, S.D. Functional responses of plants to elevated atmospheric CO2—Do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 2010, 162, 253–280. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Parvin, S.; Uddin, S.; Tausz-Posch, S.; Armstrong, R.; Tausz, M. Carbon sink strength of nodules but not other organs modulates photosynthesis of faba bean (Vicia faba) grown under elevated [CO2] and different water supply. New Phytol. 2020, 227, 132–145. [Google Scholar] [CrossRef]
- Dabu, X.; Li, S.; Cai, Z.; Ge, T.; Hai, M. The effect of potassium on photosynthetic acclimation in cucumber during CO2 enrichment. Photosynthetica 2019, 57, 640–645. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Lugassi, N.; Egbaria, A.; Granot, D.; Yermiyahu, U. The role of nitrogen in photosynthetic acclimation to elevated [CO2] in tomatoes. Plant Soil 2019, 434, 397–411. [Google Scholar] [CrossRef]
- Tcherkez, G.; Ben-Mariem, S.; Larraya, L.; Garcia-Mina, J.M.; Zamarreno, A.M.; Paradela, A.; Cui, J.; Badeck, F.W.; Meza, D.; Rizza, F.; et al. Elevated CO2 has concurrent effects on leaf and grain metabolism but minimal effects on yield in wheat. J. Exp. Bot. 2020, 71, 5990–6003. [Google Scholar] [CrossRef] [PubMed]
- Nasto, M.K.; Winter, K.; Turner, B.L.; Cleveland, C.C. Nutrient acquisition strategies augment growth in tropical N2-fixing trees in nutrient-poor soil and under elevated CO2. Ecology 2019, 100, e02646. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, G.; Liu, J.; Zhang, D.; Xu, Z.; Liu, S. Effects of elevated carbon dioxide and nitrogen addition on foliar stoichiometry of nitrogen and phosphorus of five tree species in subtropical model forest ecosystems. Environ. Pollut. 2012, 168, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.B.; Slot, M.; Dalling, J.W.; Winter, K.; Turner, B.L.; Zalamea, P.C.; Ostertag, R. Species-specific effects of phosphorus addition on tropical tree seedling response to elevated CO2. Funct. Ecol. 2019, 33, 1871–1881. [Google Scholar] [CrossRef]
- Sreeharsha, R.V.; Sekhar, K.M.; Reddy, A.R. Delayed flowering is associated with lack of photosynthetic acclimation in Pigeon pea (Cajanus cajan L.) grown under elevated CO2. Plant Sci. 2015, 231, 82–93. [Google Scholar] [CrossRef]
- Wujeska-Klause, A.; Crous, K.Y.; Ghannoum, O.; Ellsworth, D.S. Lower photorespiration in elevated CO2 reduces leaf N concentrations in mature Eucalyptus trees in the field. Glob. Chang. Biol. 2019, 25, 1–14. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Anderson, I.C.; Crous, K.Y.; Cooke, J.; Drake, J.E.; Gherlenda, A.N.; Gimeno, T.E.; Macdonald, C.A.; Medlyn, B.E.; Powell, J.R.; et al. Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nat. Clim. Chang. 2017, 7, 279–282. [Google Scholar] [CrossRef]
- Li, J.; Dang, Q.-L.; Man, R.; Marfo, J. Elevated CO2 alters N-growth relationship in spruce and causes unequal increases in N, P and K demands. For. Ecol. Manag. 2013, 298, 19–26. [Google Scholar] [CrossRef]
- Tissue, D.T.; Lewis, J.D. Photosynthetic responses of cottonwood seedlings grown in glacial through future atmospheric [CO2] vary with phosphorus supply. Tree Physiol. 2010, 30, 1361–1372. [Google Scholar] [CrossRef]
- Park, H.J.; Lim, S.S.; Yang, H.I.; Lee, K.S.; Kwak, J.H.; Park, S.I.; Kim, H.Y.; Lee, S.M.; Choi, W.J. Nitrogen effects on quantity, chemistry, and decomposability of Pinus densiflora and Quercus variabilis litters under elevated CO2 and warming. For. Ecol. Manag. 2020, 473, 1–14. [Google Scholar] [CrossRef]
- Zhuang, M.; Li, Y.; Guo, Z.; Li, Y.; Pan, W.; Chen, S. Elevated CO2 and O3 levels influence the uptake and leaf concentration of mineral N, P, K in Phyllostachys edulis (Carrière) J.Houz. and Oligostachyum lubricum (wen) King f. Forests 2018, 9, 195. [Google Scholar] [CrossRef]
- Shinano, T.; Yamamoto, T.; Tawaraya, K.; Tadokoro, M.; Koike, T.; Osaki, M. Effects of elevated atmospheric CO2 concentration on the nutrient uptake characteristics of Japanese larch (Larix kaempferi). Tree Physiol. 2007, 27, 97–104. [Google Scholar] [CrossRef]
- Özgen, M.; Serçe, S.; Kaya, C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic. 2009, 119, 275–279. [Google Scholar] [CrossRef]
- Lu, C.; Ji, F.D.; Zhu, F.R.; Zhao, A.C.; Luo, G.Q.; Su, C. Mulberry cultivation varieties in China. Chongqing Southwest Norm. Univ. 2017, 3–11. (In Chinese) [Google Scholar]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroën, K. Morus alba L. nature’s functional tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Papanastasis, V.P.; Yiakoulaki, M.D.; Decandia, M.; Dini-Papanastasi, O. Integrating woody species into livestock feeding in the Mediterranean areas of Europe. Anim. Feed Sci. Technol. 2008, 140, 1–17. [Google Scholar] [CrossRef]
- Lee, J.; Chae, K.; Ha, J.; Park, B.Y.; Lee, H.S.; Jeong, S.; Kim, M.Y.; Yoon, M. Regulation of obesity and lipid disorders by herbal extracts from Morus alba, Melissa officinalis, and Artemisia capillaris in high-fat diet-induced obese mice. J. Ethnopharmacol. 2008, 115, 263–270. [Google Scholar] [CrossRef]
- Singab, A.N.B.; El-Beshbishy, H.A.; Yonekawa, M.; Nomura, T.; Fukai, T. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef]
- Sivaci, A.; Sökmen, M. Seasonal changes in antioxidant activity, total phenolic and anthocyanin constituent of the stems of two Morus species (Morus alba L. and Morus nigra L.). Plant Growth Regul. 2004, 44, 251–256. [Google Scholar] [CrossRef]
- Sekhar, K.M.; Sreeharsha, R.V.; Mudalkar, S.; Reddy, A.R. Persistent stimulation of photosynthesis in short rotation coppice mulberry under elevated CO2 atmosphere. J. Photoch. Photobiol. B 2014, 137, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, K.M.; Sreeharsha, R.V.; Reddy, A.R. Differential responses in photosynthesis, growth and biomass yields in two mulberry genotypes grown under elevated CO2 atmosphere. J. Photoch. Photobiol. B 2015, 151, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Wang, J.; Wu, X.; Ma, S.; Xu, Z.; Zhou, T.; Xu, N.; Tang, X.; An, B. Increased CO2 concentrations increasing water use efficiency and improvement PSII function of mulberry seedling leaves under drought stress. J. Plant Interact. 2019, 14, 213–223. [Google Scholar] [CrossRef]
- Shi, S.; Qiu, Y.; Wen, M.; Xu, X.; Dong, X.; Xu, C.; He, X. Daytime, not nighttime, elevated atmospheric carbon dioxide exposure improves plant growth and leaf quality of mulberry (Morus alba L.) seedlings. Front. Plant Sci. 2021, 11, 609031. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Luo, X.; Dong, X.; Qiu, Y.; Xu, C.; He, X. Arbuscular mycorrhization enhances nitrogen, phosphorus and potassium accumulation in Vicia faba by modulating soil nutrient balance under elevated CO2. J. Fungi 2021, 7, 361–377. [Google Scholar] [CrossRef]
- Li, H.S. The Experiment Principle and Technique on Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000; pp. 78–102. (In Chinese) [Google Scholar]
- Yang, J.H.; Wang, C.L.; Dai, H.L. Soil Agrochemical Analysis and Environmental Monitoring Techniques; Chinese Dadi Press: Beijing, China, 2008; pp. 18–64. (In Chinese) [Google Scholar]
- Singh, A.K.; Rai, A.; Kushwaha, M.; Chauhan, P.S.; Pandey, V.; Singh, N. Tree growth rate regulate the influence of elevated CO2 on soil biochemical responses under tropical condition. J. Environ. Manag. 2019, 231, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, M.; Pokharel, S.S.; Li, C.; Parajulee, M.N.; Chen, F.; Fang, W. Effects of elevated CO2 on foliar soluble nutrients and functional components of tea, and population dynamics of tea aphid, Toxoptera aurantii. Plant Physiol. Biochem. 2019, 145, 84–94. [Google Scholar] [CrossRef]
- Dawes, M.A.; Hättenschwiler, S.; Bebi, P.; Hagedorn, F.; Handa, I.T.; Körner, C.; Rixen, C. Species-specific tree growth responses to 9 years of CO2 enrichment at the alpine treeline. J. Ecol. 2011, 99, 383–394. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, Q.; Yu, H.; Liu, S.; Dong, G.; Zhu, J. Effect of elevated CO2 on the growth and macronutrient (N, P and K) uptake of annual wormwood (Artemisia annua L.). Pedosphere 2016, 26, 235–242. [Google Scholar] [CrossRef]
- Kumar, S.; Chaitanya, B.S.K.; Ghatty, S.; Reddy, A.R. Growth, reproductive phenology and yield responses of a potential biofuel plant, Jatropha curcas grown under projected 2050 levels of elevated CO2. Physiol. Plant. 2014, 152, 501–519. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 2015, 6, 701. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Hobbie, S.E.; Lee, T.D.; Pastore, M.A. Unexpected reversal of C3 versus C4 grass response to elevated CO2 during a 20-year field experiment. Science 2018, 360, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Navas, M.L. Plant growth and competition at elevated CO2 on winners, losers and functional groups. New Phytol. 2003, 157, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhang, Y.G.; Ju, W.M.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssens, I.A.; Wu, M.S.; Berry, J.A.; et al. Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Z.X.; Wei, J.P.; Shen, C.; Yan, P.; Zhang, L.P.; Han, W.Y. Stimulation in primary and secondary metabolism by elevated carbon dioxide alters green tea quality in Camellia sinensis L. Sci. Rep. 2017, 7, 7937. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.M.; Souza, A.; Ament, M.R.; Gleadow, R.M.; Ort, D.R. High sink-strength prevents photosynthetic down-regulation in cassava grown at elevated CO2 concentration. J. Exp. Bot. 2021, 72, 542–560. [Google Scholar] [CrossRef]
- Slot, M.; Rifai, S.W.; Winter, K. Photosynthetic plasticity of a tropical tree species, Tabebuia rosea, in response to elevated temperature and [CO2]. Plant Cell Environ. 2021, 44. [Google Scholar] [CrossRef]
- Goicoechea, N.; Baslam, M.; Erice, G.; Irigoyen, J.J. Increased photosynthetic acclimation in alfalfa associated with arbuscular mycorrhizal fungi (AMF) and cultivated in greenhouse under elevated CO2. J. Plant Physiol. 2014, 171, 1774–1781. [Google Scholar] [CrossRef]
- Griffin, K.; Sims, D.; Seemann, J. Altered night-time CO2 concentration affects the growth, physiology and biochemistry of soybean. Plant Cell Environ. 1999, 22, 91–99. [Google Scholar] [CrossRef]
- Sakai, H.; Hasegawa, T.; Kobayashi, K. Enhancement of rice canopy carbon gain by elevated CO2 is sensitive to growth stage and leaf nitrogen concentration. New Phytologist. 2006, 170, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.P.; Xie, Y.; Ha, R.; Cao, B.; Song, L.H. Effects of elevated CO2 on photosynthetic accumulation, sucrose metabolism-related enzymes, and genes identification in Goji Berry (Lycium barbarum L.). Front. Plant Sci. 2021, 12, 643555. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Zaidi, P.H.; Pal, M.; Sengupta, U.K. Stage sensitivity of mungbean (Vigna radiata L. Wilczek) to an elevated level of carbon dioxide. J. Agron. Crop Sci. 2002, 188, 219–224. [Google Scholar] [CrossRef]
- Niinemets, Ü.J.D.; Tenhunen, N.R.; Canta, M.M.; Chavis, T.F.; Pereira, J.S.; Reynolds, J.F. Interactive effects of nitrogen and phosphorus on the acclimation potential of foliage photosynthetic properties of cork oak, Quercus suber, to elevated atmospheric CO2 concentrations. Glob. Chang. Biol. 2010, 5, 455–470. [Google Scholar] [CrossRef]
- Kontunen-Soppela, S.; Parviainen, J.; Ruhanen, H.; Brosche, M.; Keinänen, M.; Thakur, R.C.; Kolehmainen, M.; Kangasjärvi, J.; Oksanen, E.; Karnosky, D.F. Gene expression responses of paper birch (Betula papyrifera) to elevated CO2 and O3 during leaf maturation and senescence. Environ. Pollut. 2010, 158, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Kontunen-Soppela, S.; Riikonen, J.; Ruhanen, H.; Brosche, M.; Somervuo, P.; Peltonen, P.; Kangasjärvi, J.; Auvinen, P.; Paulin, L.; Keinänen, M.; et al. Differential gene expression in senescing leaves of two silver birch genotypes in response to elevated CO2 and tropospheric ozone. Plant Cell Environ. 2010, 33, 1016–1028. [Google Scholar] [CrossRef]
- Del Pozo, A.; Pérez, P.; Gutiérrez, D.; Alonso, A.; Morcuende, R.; Martínez-Carrasco, R. Gas exchange acclimation to elevated CO2 in upper-sunlit and lower-shaded canopy leaves in relation to nitrogen acquisition and partitioning in wheat grown in field chambers. Environ. Exp. Bot. 2007, 59, 371–380. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Rogers, A.; Ainsworth, E.A. Growth at elevated ozone or elevated carbon dioxide concentration alters antioxidant capacity and response to acute oxidative stress in soybean (Glycine max). J. Exp. Bot. 2011, 62, 2667–2678. [Google Scholar] [CrossRef]
- Agüera, E.; Haba, P.D. Leaf senescence in response to elevated atmospheric CO2 concentration and low nitrogen supply. Biol. Plant. 2018, 62, 1–8. [Google Scholar] [CrossRef]
- Geissler, N.; Hussin, S.; Koyro, H.W. Elevated atmospheric CO2 concentration ameliorates effects of NaCl salinity on photosynthesis and leaf structure of Aster tripolium L. J. Exp. Bot. 2009, 60, 137–151. [Google Scholar] [CrossRef]
- Watanabe, M.; Watanabe, Y.; Kitaoka, S.; Utsugi, H.; Kita, K.; Koike, T. Growth and photosynthetic traits of hybrid larch F1 (Larix gmelinii var. japonica × L. kaempferi) under elevated CO2 concentration with low nutrient availability. Tree Physiol. 2011, 31, 965–975. [Google Scholar]
- Li, L.; Manning, W.; Wang, X. Elevated CO2 increases root mass and leaf nitrogen resorption in Red Maple (Acer rubrum L.). Forests 2019, 10, 420. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ineson, P.; Scott, A. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob. Chang. Biol. 1998, 4, 43–54. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Asensio, J.S.R.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.D.; Tomaz, M.A.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C. Combined effects of elevated [CO2] and high temperature on leaf mineral balance in Coffea spp. plants. Clim. Chang. 2014, 126, 365–379. [Google Scholar] [CrossRef]
- Kanowski, J. Effects of elevated CO2 on the foliar chemistry of seedlings of two rainforest trees from north-east Australia implications for folivorous marsupials. Austral. Ecol. 2001, 26, 165–172. [Google Scholar] [CrossRef]
- Conti, T.R.; Geiger, D.R. Potassium nutrition and translocation in sugar beet. Plant Physiol. 1982, 70, 168–172. [Google Scholar] [CrossRef] [PubMed]

| Variable | CO2 (ppm) | Plant Height (cm) | Stem Diameter (mm) | Leaf Number (plant−1) | Leaf Biomass (g plant−1) | Stem Biomass (g plant−1) | Root Biomass (g plant−1) | Total Biomass (g plant−1) |
|---|---|---|---|---|---|---|---|---|
| QiangSang | 410/460 | 38.83 ± 0.83 c | 6.05 ± 0.09 b | 18.83 ± 0.44 b | 13.87 ± 0.17 c | 7.85 ± 0.29 c | 14.19 ± 0.61 a | 35.91 ± 1.02 b |
| 710/760 | 42.17 ± 1.69 b | 6.70 ± 0.17 a | 23.17 ± 1.09 a | 18.02 ± 0.59 a | 10.27 ± 0.60 b | 15.68 ± 0.68 a | 43.97 ± 1.61 a | |
| NongSang | 410/460 | 42.25 ± 0.88 b | 6.21 ± 0.34 b | 16.25 ± 0.66 c | 12.03 ± 0.47d | 9.59 ± 0.11 b | 10.64 ± 0.73 b | 32.26 ± 1.08 b |
| 710/760 | 49.33 ± 1.76 a | 6.94 ± 0.17 a | 18.58 ± 0.71 b | 15.09 ± 0.92 b | 12.75 ± 0.55 a | 15.11 ± 0.75 a | 42.95 ± 1.54 a | |
| ANOVA | ||||||||
| CO2 | ** | * | ** | *** | *** | ** | *** | |
| Variety | ** | ns | ** | ** | *** | * | ns | |
| CO2×variety | ns | ns | ns | ns | ns | ns | ns |
| Variable | N Partitioning | P Partitioning | K Partitioning | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | |
| CO2 | 0.07 | 0.001 | 0.129 | 0.441 | 0.002 | 0.251 | 0.208 | 0.312 | 0.119 |
| Variety | 0.244 | 0.001 | 0.003 | 0.011 | 0.001 | 0.002 | 0.401 | 0.026 | 0.326 |
| CO2×variety | 0.002 | 0.146 | 0.090 | 0.312 | 0.771 | 0.388 | 0.041 | 0.576 | 0.167 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Xu, X.; Dong, X.; Xu, C.; Qiu, Y.; He, X. Photosynthetic Acclimation and Growth Responses to Elevated CO2 Associate with Leaf Nitrogen and Phosphorus Concentrations in Mulberry (Morus multicaulis Perr.). Forests 2021, 12, 660. https://doi.org/10.3390/f12060660
Shi S, Xu X, Dong X, Xu C, Qiu Y, He X. Photosynthetic Acclimation and Growth Responses to Elevated CO2 Associate with Leaf Nitrogen and Phosphorus Concentrations in Mulberry (Morus multicaulis Perr.). Forests. 2021; 12(6):660. https://doi.org/10.3390/f12060660
Chicago/Turabian StyleShi, Songmei, Xiao Xu, Xingshui Dong, Chenyang Xu, Yuling Qiu, and Xinhua He. 2021. "Photosynthetic Acclimation and Growth Responses to Elevated CO2 Associate with Leaf Nitrogen and Phosphorus Concentrations in Mulberry (Morus multicaulis Perr.)" Forests 12, no. 6: 660. https://doi.org/10.3390/f12060660
APA StyleShi, S., Xu, X., Dong, X., Xu, C., Qiu, Y., & He, X. (2021). Photosynthetic Acclimation and Growth Responses to Elevated CO2 Associate with Leaf Nitrogen and Phosphorus Concentrations in Mulberry (Morus multicaulis Perr.). Forests, 12(6), 660. https://doi.org/10.3390/f12060660






