Calcium Biogeochemical Cycle in a Typical Karst Forest: Evidence from Calcium Isotope Compositions
Abstract
:1. Introduction
2. Materials and Methods
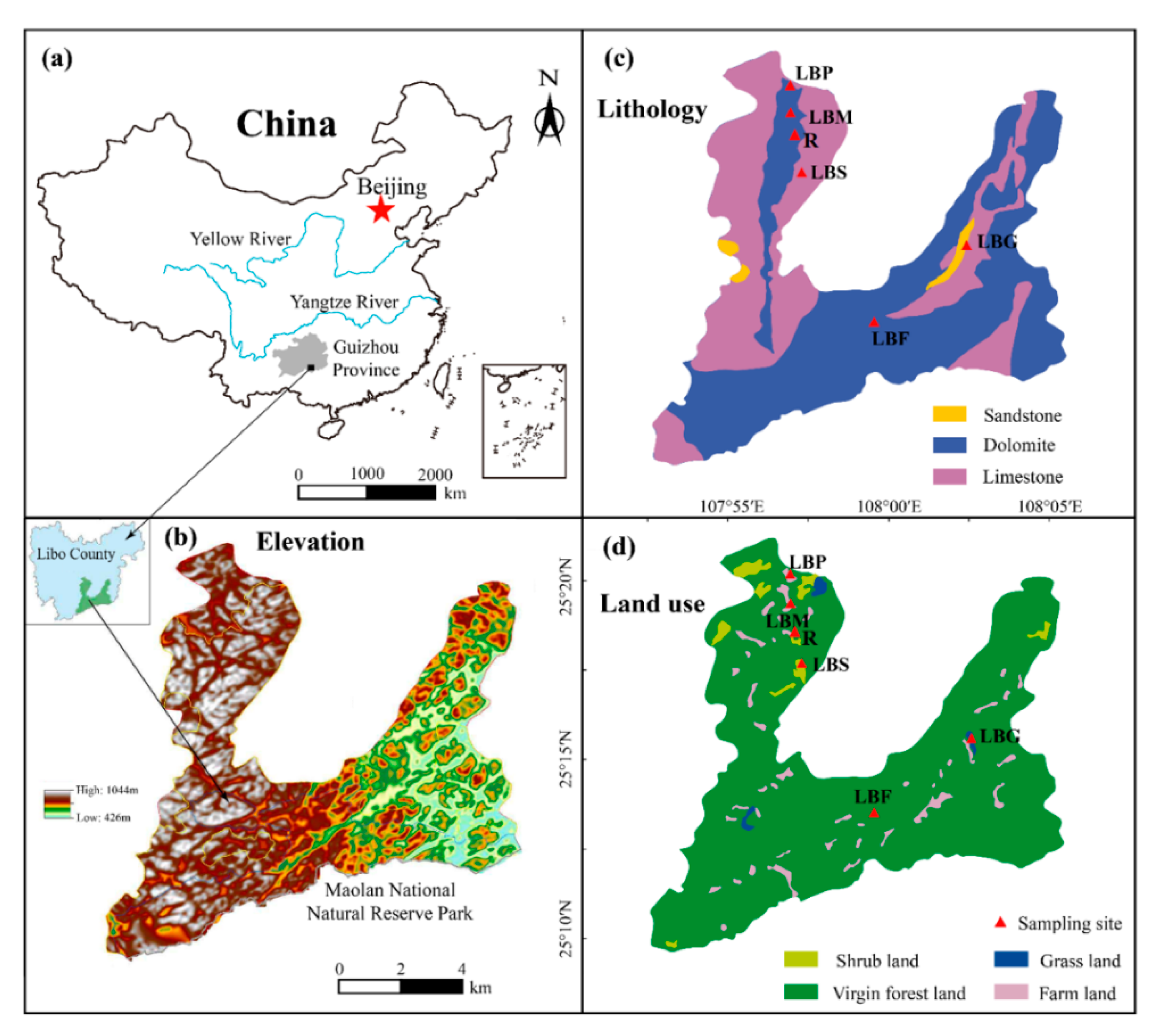
2.1. Geological Setting
2.2. Sampling Procedure
2.3. Chemical Analysis
2.4. Calcium Isotope Analysis
3. Results
3.1. δ44/40Ca Ratios of Rainwater
3.2. δ44/40Ca Ratios of Groundwater
3.3. δ44/40Ca Ratios of Bedrock and Soils
3.4. δ44/40Ca Ratios of Vegetation
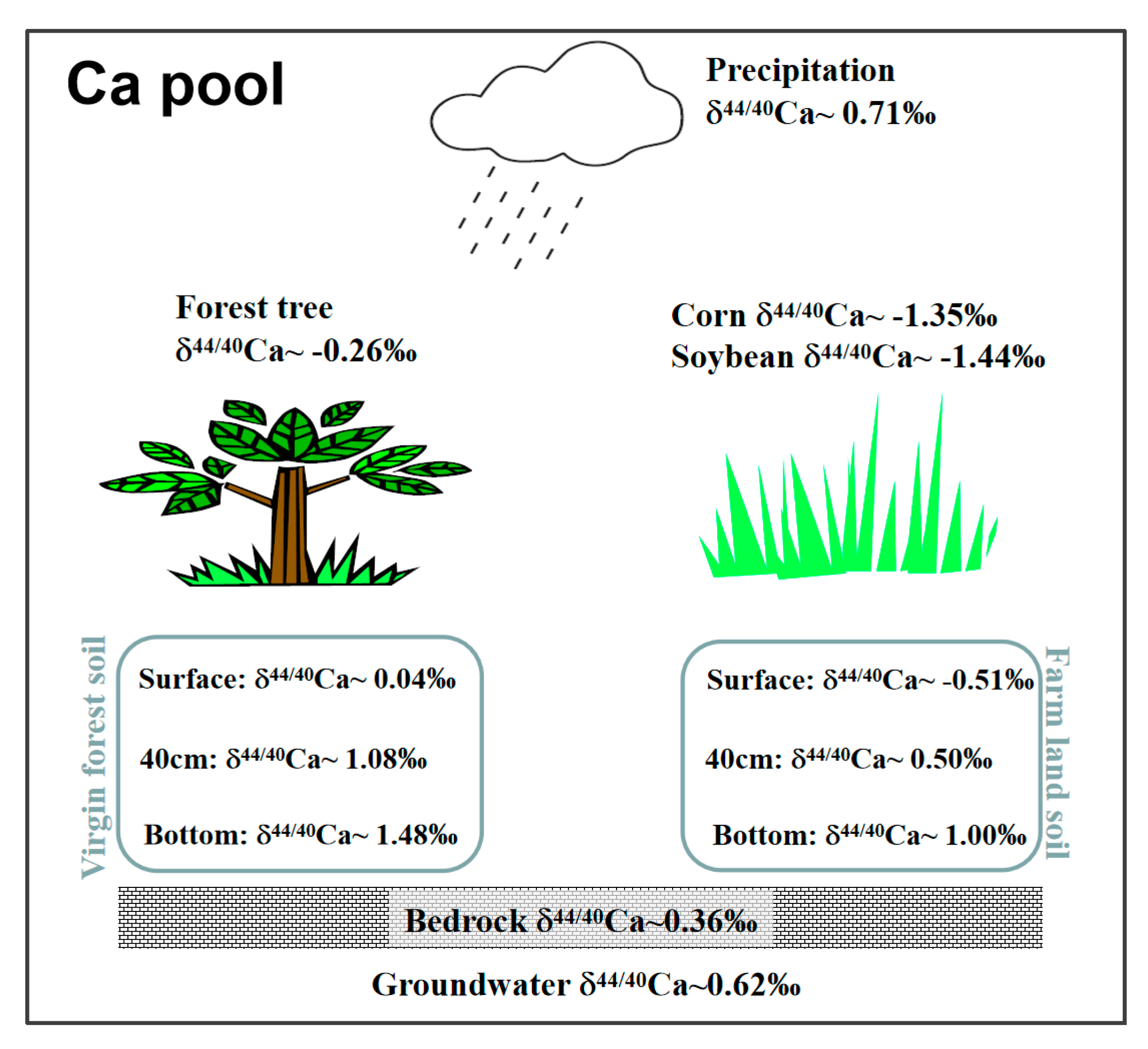
4. Discussion
4.1. Atmospheric Inputs of Calcium
4.2. Plant and Soil Calcium
4.3. Local-Scale Ca Cycling Effects in Maolan Karst Forest Ecosystem
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Momoshima, N.; Bondietti, E.A. Cation binding in wood: Applications to understanding historical changes in divalent cation availability to red spruce. Can. J. For. Res. 1990, 20, 1840–1849. [Google Scholar] [CrossRef]
- Likens, G.E.; Driscoll, C.T.; Buso, D.C. Long-term effects of acid rain: Response and recovery of a forest ecosystem. Science 1996, 272, 244. [Google Scholar] [CrossRef]
- Hindshaw, R.S.; Bourdon, B.; Pogge von Strandmann, P.A.E.; Vigier, N.; Burton, K.W. The stable calcium isotopic composition of rivers draining basaltic catchments in Iceland. Earth Planet. Sci. Lett. 2013, 374, 173–184. [Google Scholar] [CrossRef]
- Hindshaw, R.S.; Reynolds, B.C.; Wiederhold, J.G.; Kiczka, M.; Kretzschmar, R.; Bourdon, B. Calcium isotope fractionation in alpine plants. Biogeochemistry 2013, 112, 373–388. [Google Scholar] [CrossRef] [Green Version]
- Huntington, T.G. Assessment of calcium status in Maine forests: Review and future projection. Can. J. For. Res. 2005, 35, 1109–1121. [Google Scholar] [CrossRef]
- Lawrence, A.D.; Bu, J.; Gokulakrishnan, P. The interactions between SO2, NOx, HCl and Ca in a bench-scale fluidized combustor. J. Inst. Energy 1999, 72, 34–40. [Google Scholar]
- Blum, J.D.; Klaue, A.; Nezat, C.A.; Driscoll, C.T.; Johnson, C.E.; Siccama, T.G.; Eagar, C.; Fahey, T.J.; Likens, G.E. Mycorrhizal weathering of apatite as an important calcium source in base-poor forest ecosystems. Nature 2002, 417, 729–731. [Google Scholar] [CrossRef] [Green Version]
- Bullen, T.D.; Bailey, S.W. Identifying calcium sources at an acid deposition-impacted spruce forest: A strontium isotope, alkaline earth element multi-tracer approach. Biogeochemistry 2005, 74, 63–99. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Hedin, L.O.; Derry, L.A. Decoupling of unpolluted temperate forests from rock nutrient sources revealed by natural 87Sr/86Sr and 84Sr tracer addition. Proc. Natl. Acad. Sci. USA 2002, 99, 9639. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K.; Blum, J.D.; Friedland, A.J. Determination of soil exchangeable-cation loss and weathering rates using Sr isotopes. Nature 1993, 362, 438–441. [Google Scholar] [CrossRef]
- Åberg, G.; Jacks, G.; Hamilton, P.J. Weathering rates and 87Sr/86Sr ratios: An isotopic approach. J. Hydrol. 1989, 109, 65–78. [Google Scholar] [CrossRef]
- Bailey, S.W.; Hornbeck, J.W.; Driscoll, C.T.; Gaudette, H.E. Calcium inputs and transport in a base-poor forest ecosystem as interpreted by Sr isotopes. Water Resour. Res. 1996, 32, 707–719. [Google Scholar] [CrossRef]
- Elias, R.W.; Hirao, Y.; Patterson, C.C. The circumvention of the natural biopurification of calcium along nutrient pathways by atmospheric inputs of industrial lead. Geochim. Cosmochim. Acta 1982, 46, 2561–2580. [Google Scholar] [CrossRef]
- Poszwa, A.; Dambrine, E.; Pollier, B.; Atteia, O. A comparison between Ca and Sr cycling in forest ecosystems. Plant Soil 2000, 225, 299–310. [Google Scholar] [CrossRef]
- Åberg, G.; Jacks, G.; Wickman, T.; Hamilton, P.J. Strontium isotopes in trees as an indicator for calcium availability. Catena 1990, 17, 1–11. [Google Scholar] [CrossRef]
- Schmitt, A.-D.; Chabaux, F.; Stille, P. The calcium riverine and hydrothermal isotopic fluxes and the oceanic calcium mass balance. Earth Planet. Sci. Lett. 2003, 213, 503–518. [Google Scholar] [CrossRef]
- Schmitt, A.-D.; Stille, P.; Vennemann, T. Variations of the 44Ca/40Ca ratio in seawater during the past 24 million years: Evidence from δ44Ca and δ18O values of Miocene phosphates. Geochim. Cosmochim. Acta 2003, 67, 2607–2614. [Google Scholar] [CrossRef]
- Capo, R.C.; Stewart, B.W.; Chadwick, O.A. Strontium isotopes as tracers of ecosystem processes: Theory and methods. Geoderma 1998, 82, 197–225. [Google Scholar] [CrossRef]
- Pearce, C.R.; Parkinson, I.J.; Gaillardet, J.; Charlier, B.L.A.; Mokadem, F.; Burton, K.W. Reassessing the stable (δ88/86Sr) and radiogenic (87Sr/86Sr) strontium isotopic composition of marine inputs. Geochim. Cosmochim. Acta 2015, 157, 125–146. [Google Scholar] [CrossRef]
- Pearce, C.R.; Parkinson, I.J.; Gaillardet, J.; Chetelat, B.; Burton, K.W. Characterising the stable (δ88/86Sr) and radiogenic (87Sr/86Sr) isotopic composition of strontium in rainwater. Chem. Geol. 2015, 409, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, B.A.; Chadwick, O.A.; Vitousek, P.M.; Wooden, J.L. Ca cycling and isotopic fluxes in forested ecosystems in Hawaii. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef] [Green Version]
- Fantle, M.S.; DePaolo, D.J. Ca isotopes in carbonate sediment and pore fluid from ODP Site 807A: The Ca2+(aq)–calcite equilibrium fractionation factor and calcite recrystallization rates in Pleistocene sediments. Geochim. Cosmochim. Acta 2007, 71, 2524–2546. [Google Scholar] [CrossRef]
- Farkaš, J.; Buhl, D.; Blenkinsop, J.; Veizer, J. Evolution of the oceanic calcium cycle during the late Mesozoic: Evidence from δ44/40Ca of marine skeletal carbonates. Earth Planet. Sci. Lett. 2007, 253, 96–111. [Google Scholar] [CrossRef]
- Nägler, T.F.; Eisenhauer, A.; Müller, A.; Hemleben, C.; Kramers, J. The δ44Ca-temperature calibration on fossil and cultured Globigerinoides sacculifer: New tool for reconstruction of past sea surface temperatures. Geochem. Geophys. Geosyst. 2000, 1. [Google Scholar] [CrossRef] [Green Version]
- Skulan, J.; DePaolo, D.J.; Owens, T.L. Biological control of calcium isotopic abundances in the global calcium cycle. Geochim. Cosmochim. Acta 1997, 61, 2505–2510. [Google Scholar] [CrossRef]
- Zhu, P.; Macdougall, J.D. Calcium isotopes in the marine environment and the oceanic calcium cycle. Geochim. Cosmochim. Acta 1998, 62, 1691–1698. [Google Scholar] [CrossRef]
- Cenki-Tok, B.; Chabaux, F.; Lemarchand, D.; Schmitt, A.D.; Pierret, M.C.; Viville, D.; Bagard, M.L.; Stille, P. The impact of water–rock interaction and vegetation on calcium isotope fractionation in soil- and stream waters of a small, forested catchment (the Strengbach case). Geochim. Cosmochim. Acta 2009, 73, 2215–2228. [Google Scholar] [CrossRef]
- Cobert, F.; Schmitt, A.-D.; Bourgeade, P.; Labolle, F.; Badot, P.-M.; Chabaux, F.; Stille, P. Experimental identification of Ca isotopic fractionations in higher plants. Geochim. Cosmochim. Acta 2011, 75, 5467–5482. [Google Scholar] [CrossRef]
- Farkaš, J.; Déjeant, A.; Novák, M.; Jacobsen, S.B. Calcium isotope constraints on the uptake and sources of Ca2+ in a base-poor forest: A new concept of combining stable (δ44/42Ca) and radiogenic (εCa) signals. Geochim. Cosmochim. Acta 2011, 75, 7031–7046. [Google Scholar] [CrossRef]
- Han, G.; Song, Z.; Tang, Y.; Wu, Q.; Wang, Z. Ca and Sr isotope compositions of rainwater from Guiyang city, Southwest China: Implication for the sources of atmospheric aerosols and their seasonal variations. Atmos. Environ. 2019, 214, 116854. [Google Scholar] [CrossRef]
- Page, B.D.; Bullen, T.D.; Mitchell, M.J. Influences of calcium availability and tree species on Ca isotope fractionation in soil and vegetation. Biogeochemistry 2008, 88, 1–13. [Google Scholar] [CrossRef]
- Schmitt, A.-D.; Stille, P. The source of calcium in wet atmospheric deposits: Ca-Sr isotope evidence. Geochim. Cosmochim. Acta 2005, 69, 3463–3468. [Google Scholar] [CrossRef]
- Turchyn, A.V.; DePaolo, D.J. Calcium isotope evidence for suppression of carbonate dissolution in carbonate-bearing organic-rich sediments. Geochim. Cosmochim. Acta 2011, 75, 7081–7098. [Google Scholar] [CrossRef]
- Wiegand, B.A.; Schwendenmann, L. Determination of Sr and Ca sources in small tropical catchments (La Selva, Costa Rica)—A comparison of Sr and Ca isotopes. J. Hydrol. 2013, 488, 110–117. [Google Scholar] [CrossRef]
- van der Heijden, G.; Dambrine, E.; Pollier, B.; Zeller, B.; Ranger, J.; Legout, A. Mg and Ca uptake by roots in relation to depth and allocation to aboveground tissues: Results from an isotopic labeling study in a beech forest on base-poor soil. Biogeochemistry 2015, 122, 375–393. [Google Scholar] [CrossRef]
- Hindshaw, R.S.; Reynolds, B.C.; Wiederhold, J.G.; Kretzschmar, R.; Bourdon, B. Calcium isotopes in a proglacial weathering environment: Damma glacier, Switzerland. Geochim. Cosmochim. Acta 2011, 75, 106–118. [Google Scholar] [CrossRef]
- Holmden, C.; Bélanger, N. Calcium isotope fractionation in a boreal forest ecosystem. Geochim. Cosmochim. Acta 2006, 70, A261. [Google Scholar] [CrossRef]
- Holmden, C.; Bélanger, N. Ca isotope cycling in a forested ecosystem. Geochim. Cosmochim. Acta 2010, 74, 995–1015. [Google Scholar] [CrossRef] [Green Version]
- Fantle, M.S.; Tipper, E.T. Calcium isotopes in the global biogeochemical Ca cycle: Implications for development of a Ca isotope proxy. Earth Sci. Rev. 2014, 129, 148–177. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Wu, Q.; Tang, Y. Effects of agricultural alkaline substances on reducing the rainwater acidification: Insight from chemical compositions and calcium isotopes in a karst forests area. Agric. Ecosyst. Environ. 2020, 290, 106782. [Google Scholar] [CrossRef]
- Likens, G.E.; Driscoll, C.T.; Buso, D.C.; Siccama, T.G.; Johnson, C.E.; Lovett, G.M.; Fahey, T.J.; Reiners, W.A.; Ryan, D.F.; Martin, C.W.; et al. The biogeochemistry of calcium at Hubbard Brook. Biogeochemistry 1998, 41, 89–173. [Google Scholar] [CrossRef]
- Dijkstra, F.A. Calcium mineralization in the forest floor and surface soil beneath different tree species in the northeastern US. For. Ecol. Manag. 2003, 175, 185–194. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Van Breemen, N.; Jongmans, A.G.; Davies, G.R.; Likens, G.E. Calcium weathering in forested soils and the effect of different tree species. Biogeochemistry 2003, 62, 253–275. [Google Scholar] [CrossRef]
- Fujinuma, R.; Bockheim, J.; Balster, N. Base-cation cycling by individual tree species in old-growth forests of upper michigan, USA. Biogeochemistry 2005, 74, 357–376. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G. Rainwater chemistry reveals air pollution in a karst forest: Temporal variations, source apportionment, and implications for the forest. Atmosphere 2020, 11, 1315. [Google Scholar] [CrossRef]
- Han, G.; Tang, Y.; Wu, Q. Hydrogeochemistry and dissolved inorganic carbon isotopic composition on karst groundwater in Maolan, southwest China. Environ. Earth Sci. 2010, 60, 893–899. [Google Scholar] [CrossRef]
- Han, G.; Li, F.; Tang, Y. Variations in soil organic carbon contents and isotopic compositions under different land uses in a typical karst area in Southwest China. Geochem. J. 2015, 49, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Tang, Y.; Wu, Q.; Tan, Q. Chemical and strontium isotope characterization of rainwater in karst virgin forest, Southwest China. Atmos. Environ. 2010, 44, 174–181. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G.; Yang, K. Assessment and sources of heavy metals in suspended particulate matter in a tropical catchment, northeast Thailand. J. Clean. Prod. 2020, 265, 121898. [Google Scholar] [CrossRef]
- Li, X.; Han, G. One-step chromatographic purification of K, Ca, and Sr from geological samples for high precision stable and radiogenic isotope analysis by MC-ICP-MS. J. Anal. At. Spectrom. 2021, 36, 676–684. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Wang, Y.; Tsang, D.C.W.; Yang, X.; Beiyuan, J.; Yin, M.; Xiao, T.; Jiang, Y.; Lin, W.; et al. Emerging risks of toxic metal(loid)s in soil-vegetables influenced by steel-making activities and isotopic source apportionment. Environ. Int. 2021, 146, 106207. [Google Scholar] [CrossRef] [PubMed]
- Heuser, A.; Eisenhauer, A.; Gussone, N.; Bock, B.; Hansen, B.T.; Nägler, T.F. Measurement of calcium isotopes (δ44Ca) using a multicollector TIMS technique. Int. J. Mass Spectrom. 2002, 220, 385–397. [Google Scholar] [CrossRef]
- De La Rocha, C.L.; DePaolo, D.J. Isotopic evidence for variations in the marine calcium cycle over the Cenozoic. Science 2000, 289, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Han, G. Preliminary copper isotope study on particulate matter in Zhujiang River, southwest China: Application for source identification. Ecotoxicol. Environ. Saf. 2020, 198, 110663. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Zhang, Q. Effects of agricultural abandonment on soil aggregation, soil organic carbon storage and stabilization: Results from observation in a small karst catchment, Southwest China. Agric. Ecosyst. Environ. 2020, 288, 106719. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Li, X. Comparative analysis of soil nutrients under different land-use types in the Mun River basin of Northeast Thailand. J. Soils Sediments 2021. [Google Scholar] [CrossRef]
- Schmitt, A.-D.; Cobert, F.; Bourgeade, P.; Ertlen, D.; Labolle, F.; Gangloff, S.; Badot, P.-M.; Chabaux, F.; Stille, P. Calcium isotope fractionation during plant growth under a limited nutrient supply. Geochim. Cosmochim. Acta 2013, 110, 70–83. [Google Scholar] [CrossRef]
- Liu, J.; Han, G. Tracing riverine particulate black carbon sources in Xijiang River basin: Insight from stable isotopic composition and Bayesian mixing model. Water Res. 2021, 194, 116932. [Google Scholar] [CrossRef]
- Liu, J.; Han, G. Major ions and δ34SSO4 in Jiulongjiang River water: Investigating the relationships between natural chemical weathering and human perturbations. Sci. Total. Environ. 2020, 724, 138208. [Google Scholar] [CrossRef]
- Hu, Z.-L.; Pan, G.-X.; Li, L.-Q.; Du, Y.-X.; Wang, X.-Z. Changes in pools and heterogeneity of soil organic carbon, nitrogen and phosphorus under different vegetation types in Karst mountainous area of central Guizhou Province, China. Acta Ecol. Sin. 2009, 29, 4187–4195, (In Chinese with English abstract). [Google Scholar]
- Schmitt, A.-D.; Gangloff, S.; Labolle, F.; Chabaux, F.; Stille, P. Calcium biogeochemical cycle at the beech tree-soil solution interface from the Strengbach CZO (NE France): Insights from stable Ca and radiogenic Sr isotopes. Geochim. Cosmochim. Acta 2017, 213, 91–109. [Google Scholar] [CrossRef]



| Sample Number | Date (Year-Month-Day) | Ca2+ | Mg2+ | δ44/40Ca | 2SD | n |
|---|---|---|---|---|---|---|
| μmol/L | μmol/L | ‰ | ‰ | |||
| Rainwater | ||||||
| LB-32 | 2008-6-08 | 14.5 | 3.2 | 0.61 | 0.29 | 3 |
| LB-41 | 2008-7-24 | 21.6 | 2.7 | 0.55 | 0.24 | 3 |
| LB-44 | 2008-8-18 | 24.0 | 2.9 | 0.65 | 0.23 | 2 |
| LB-47 | 2008-9-24 | 12.9 | 0.9 | 1.01 | 0.15 | 3 |
| Groundwater | ||||||
| 2 | 2007-7-23 | 1163 | 648 | 0.40 | 0.14 | 2 |
| 3 | 2007-7-23 | 1309 | 501 | 0.52 | 0.04 | 3 |
| 7 | 2007-7-23 | 1312 | 373 | 0.60 | - | 1 |
| 11 | 2007-7-23 | 1452 | 114 | 0.63 | - | 1 |
| 13 | 2007-7-23 | 1728 | 289 | 0.72 | - | 1 |
| 16 | 2007-7-24 | 1759 | 126 | 0.36 | 0.13 | 2 |
| 20 | 2007-7-24 | 2538 | 394 | 0.43 | 0.01 | 2 |
| 24 | 2007-7-24 | 1334 | 1353 | 1.08 | 0.21 | 2 |
| 27 | 2007-7-27 | 1394 | 609 | 0.83 | - | 1 |
| Bedrock | mmol/kg | mmol/kg | ||||
| Dolomite | 6066 | 5872 | 0.36 | 0.09 | 3 | |
| Soil | depth (cm) | |||||
| Grassland | 0 | 20 | 137 | 0.28 | 0.10 | 2 |
| 40 | 56 | 135 | 0.75 | 0.14 | 3 | |
| 110 | 2275 | 2501 | 0.91 | 0.18 | 3 | |
| Shrubland | 0 | 34 | 43 | 0.29 | 0.15 | 3 |
| 40 | 159 | 166 | 0.30 | 0.24 | 3 | |
| 90 | 23 | 74 | 0.94 | 0.15 | 2 | |
| Farmland | 0 | 106 | 464 | −0.51 | 0.25 | 3 |
| 40 | 13 | 16 | 0.50 | 0.22 | 3 | |
| 110 | 21 | 25 | 1.00 | 0.04 | 2 | |
| Burnt grassland | 0 | 31 | 97 | −0.38 | 0.19 | 2 |
| 40 | 159 | 25 | 0.34 | 0.03 | 3 | |
| 70 | 16 | 25 | 1.00 | 0.15 | 2 | |
| Virgin forests | 0 | 60 | 124 | 0.04 | 0.20 | 3 |
| 40 | 11 | 36 | 1.08 | 0.21 | 2 | |
| 120 | 21 | 155 | 1.48 | 0.23 | 3 | |
| Forest tree | ||||||
| Nandian domestica | 231 | 101 | −0.26 | 0.18 | 2 | |
| Folium platycaryae | 602 | 194 | −0.22 | 0.08 | 2 | |
| Handliodendron bodinier | 672 | 234 | −0.26 | - | 1 | |
| Crop | ||||||
| Soybean | 47 | 233 | −1.44 | 0.14 | 3 | |
| Corn | 51 | 249 | −1.35 | 0.20 | 2 |
| Shrubland | Virgin Forests | Grassland | Burnt Grassland | Farmland | |
|---|---|---|---|---|---|
| Mg/Ca | 1.27 | 2.08 | 2.45 | 3.15 | 4.37 |
| δ44/40Ca | 0.29 | 0.04 | 0.28 | −0.38 | −0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Eisenhauer, A.; Zeng, J.; Liu, M. Calcium Biogeochemical Cycle in a Typical Karst Forest: Evidence from Calcium Isotope Compositions. Forests 2021, 12, 666. https://doi.org/10.3390/f12060666
Han G, Eisenhauer A, Zeng J, Liu M. Calcium Biogeochemical Cycle in a Typical Karst Forest: Evidence from Calcium Isotope Compositions. Forests. 2021; 12(6):666. https://doi.org/10.3390/f12060666
Chicago/Turabian StyleHan, Guilin, Anton Eisenhauer, Jie Zeng, and Man Liu. 2021. "Calcium Biogeochemical Cycle in a Typical Karst Forest: Evidence from Calcium Isotope Compositions" Forests 12, no. 6: 666. https://doi.org/10.3390/f12060666
APA StyleHan, G., Eisenhauer, A., Zeng, J., & Liu, M. (2021). Calcium Biogeochemical Cycle in a Typical Karst Forest: Evidence from Calcium Isotope Compositions. Forests, 12(6), 666. https://doi.org/10.3390/f12060666








