Field Performances of Mediterranean Oaks in Replicate Common Gardens for Future Reforestation under Climate Change in Central and Southern Europe: First Results from a Four-Year Study
Abstract
:1. Introduction
- (1)
- Do the three Mediterranean species differ in survival and growth performance during the initial growth phase under different macroclimatic conditions?
- (2)
- Do Greek and Italian provenances of the Mediterranean species (consistently) show different behavior in different environments?
- (3)
- Do the local German oaks (Q. robur) outperform their Mediterranean relatives in the German plantations?
- (4)
- Is it possible to predict survival and performance in the field from easily accessible morphological and physiological seedling parameters?
2. Materials and Methods
2.1. Plant Material
2.2. Common Garden Experiments
2.3. Climate Data
2.4. Soil Analysis
2.5. Morphological Attributes
2.6. Relative Chlorophyll Content and Chlorophyll Fluorescence
2.7. Plant Fitness Parameter Scoring and Ranking
2.8. Statistical Analysis
3. Results
3.1. Soil Parameters
3.2. Climate Data and Extreme Climate Events
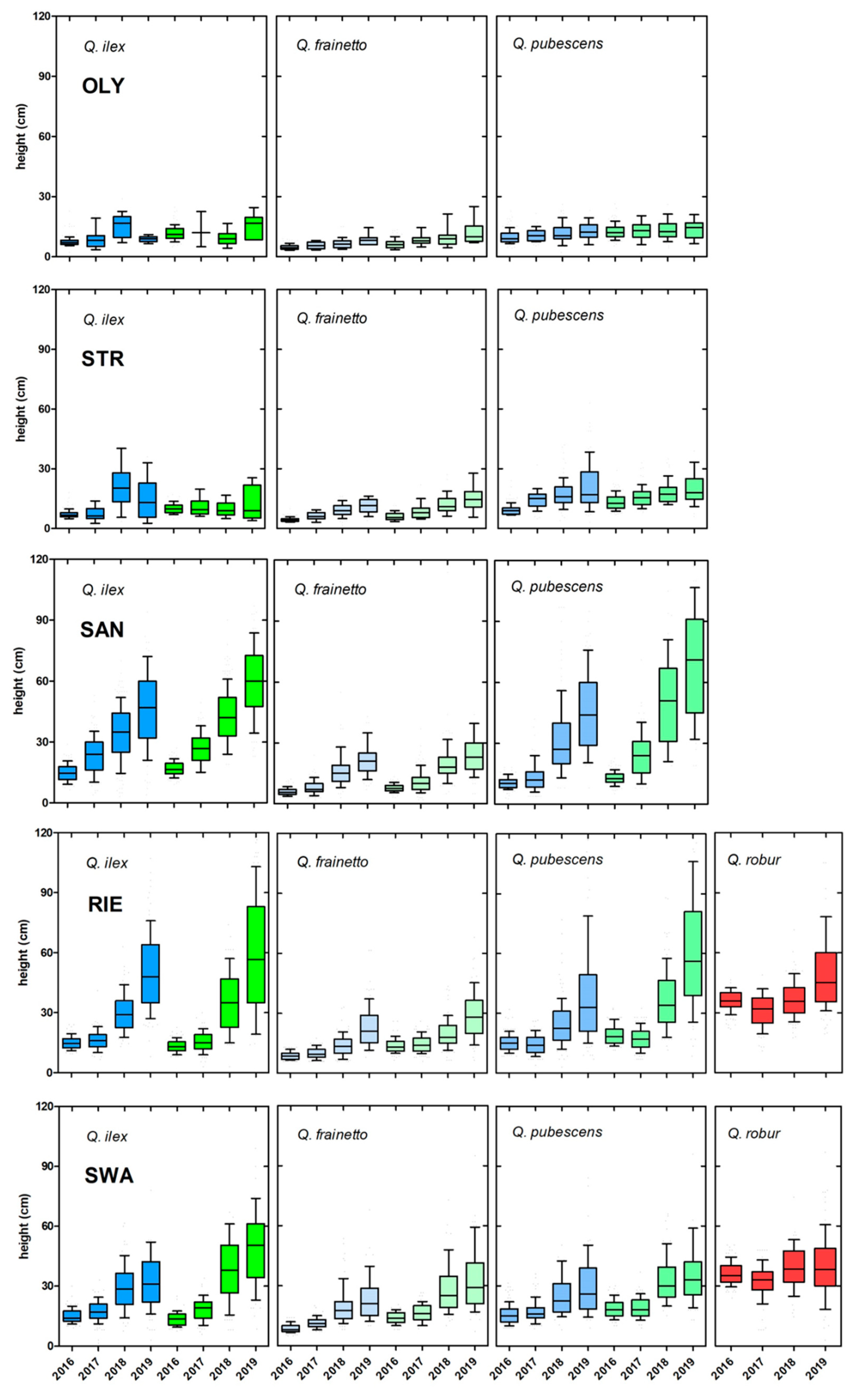
3.3. Seedling Survival and Growth
3.4. Relative Chlorophyll Content
3.5. Quality Scoring of Seedlings as Predictors for Survival and Growth and Population Fitness
4. Discussion and Conclusions
4.1. Seedling Survival and Establishment
4.2. Cumulative Growth Conditions at the Common Garden Sites as Reflected in Seedling Performance
4.3. Preliminary Conclusions for (Re)forestation Strategies
- (1)
- Of the Mediterranean species, Q. pubescens shows the best performance at all sites, followed by Q. ilex. Q. frainetto showed the lowest performance under all conditions, except for Greek Q. ilex at OLY. Since this finding was independent of provenance and site, it appears to be an inherent trait and we conclude that among the deciduous species, Q. frainetto is a “slow-growing” species (sensu Lambers and Poorter, [32]) as opposite to Q. pubescens (and Q. robur).
- (2)
- Whether Greek or Italian provenances perform better at a given site depends on site conditions. In general, the differences between the provenances were small.
- (3)
- In Germany, Q. robur did not outperform Q. pubescens. Both sites (RIE and SWA) were quite warm and suffered from heatwaves during the experimental period, which may have masked a potential advantage (under milder temperatures and higher precipitation) of the local species over the Mediterranean one.
- (4)
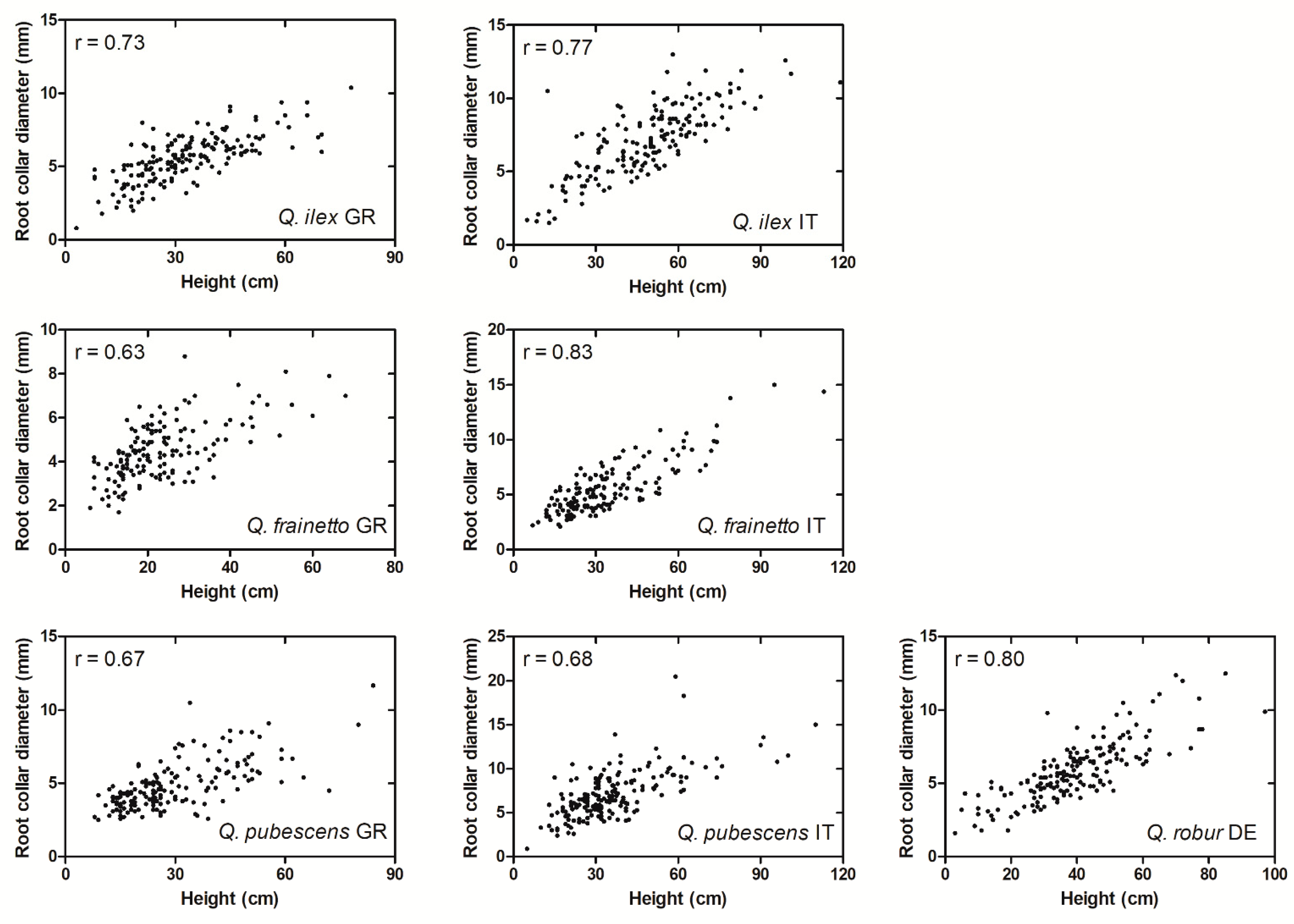
- The predictive value of the morphological and physiological measurements on the seedlings in 2016 is not considered of practical use, except for root collar diameter in certain combinations of provenance and growth site.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frank, D.; Reichstein, M.; Bahn, M.; Thonicke, K.; Frank, D.; Mahecha, M.D.; Smith, P.; van der Velde, M.; Vicca, S.; Babst, F.; et al. Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Chang. Biol. 2015, 21, 2861–2880. [Google Scholar] [CrossRef] [Green Version]
- Bantis, F.; Fruchtenicht, E.; Graap, J.; Stroll, S.; Reininger, N.; Schafer, L.; Pollastrini, M.; Holland, V.; Bussotti, F.; Radoglou, K.; et al. The JIP-test as a tool for forestry in times of climate change. Photosynthetica 2020, 58, 224–236. [Google Scholar] [CrossRef] [Green Version]
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.J.; Nabuurs, G.J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M.; Holland, V.; Brüggemann, W. Functional traits and adaptive capacity of European forests to climate change. Environ. Exp. Bot. 2015, 111, 91–113. [Google Scholar] [CrossRef]
- Koller, S.; Jedmowski, C.; Kamm, K.; Brüggemann, W. The South Hesse Oak Project (SHOP): Species- and site-specific efficiency of the photosynthetic apparatus of Mediterranean and Central European Oaks in Central Europe. Plant Biosyst. 2014, 148, 237–248. [Google Scholar] [CrossRef]
- Mátyás, C. Climatic adaptation of trees: Rediscovering provenance tests. Euphytica 1996, 92, 45–54. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Vitasse, Y.; Delzon, S.; Bresson, C.C. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res. 2009, 39, 1259–1269. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.R.; Dumroese, R.K. Preparing for climate change: Forestry and assisted migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Koralewski, T.E.; Wang, H.H.; Grant, W.E.; Byram, T.D. Plants on the move: Assisted migration of forest trees in the face of climate change. For. Ecol. Manang. 2015, 344, 30–37. [Google Scholar] [CrossRef]
- Zeng, X.; Fischer, G.A. Using multiple seedlots in restoration planting enhances genetic diversity compared to natural regeneration in fragmented tropical forests. For. Ecol. Manag. 2021, 482, 118819. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bladé, C.; Valdecantos, A.; Seva, J.P.; Fuentes, D.; Alloza, J.A.; Vilagrosa, A.; Bautista, S.; Cortina, J.; Vallejo, R. Pines and oaks in the restoration of Mediterranean landscapes of Spain: New perspectives for an old practice—A review. Plant Ecol. 2004, 171, 209–220. [Google Scholar] [CrossRef]
- Mattsson, A. Predicting field performance using seedling quality assessment. New For. 1996, 13, 223–248. [Google Scholar]
- Tsakaldimi, M.; Ganatsas, P.; Jacobs, D.F. Prediction of planted seedling survival of five Mediterranean species based on initial seedling morphology. New For. 2013, 44, 327–339. [Google Scholar] [CrossRef]
- Saha, S.; Kuehne, C.; Kohnle, U.; Brang, P.; Ehring, A.; Geisel, J.; Leder, B.; Muth, M.; Petersen, R.; Peter, J.; et al. Growth and quality of young oaks (Quercus robur and Quercus petraea) grown in cluster plantings in central Europe: A weighted meta-analysis. For. Ecol. Manang. 2012, 283, 106–118. [Google Scholar] [CrossRef]
- Saha, S.; Kuehne, C.; Bauhus, J. Lessons learned from oak cluster planting trials in central Europe. Can. J. For. Res. 2016, 47, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Skiadaresis, G.; Saha, S.; Bauhus, J. Oak group planting produces a higher number of future crop trees, with better spatial distribution than row planting. Forests 2016, 7, 289. [Google Scholar] [CrossRef] [Green Version]
- Köhn, M. Die mechanische Analyse des Bodens mittels Pipettmethode. J. Plant Nutr. Soil Sci. 1931, 21, 211–222. [Google Scholar] [CrossRef]
- Ad.Hoc-AG Boden. Bodenkundliche Kartieranleitung KA5 (Manual of Soil Mapping. In Bundesanstalt für Geowissenschaften und Rohstoffe, 5th ed.; Eckelmann, W. (Ed.) Schweizerbart: Frankfurt, Germany, 2005. [Google Scholar]
- Alfonso, S. Einfluss von Trockenstress auf die Photosynthese in der Gattung. Ph.D. Thesis, University of Frankfurt, Frankfurt, Germany, 2010. (In German). [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaption; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Radoglou, K.; Raftoyannis, Y. Effects of desiccation and freezing on vitality and field performance of broadleaved tree species. Ann. For. Sci. 2001, 58, 59–68. [Google Scholar] [CrossRef]
- Larcher, W. Zunahme des Frostabhärtungsvermögens von Quercus ilex im Laufe der Individualentwicklung. Planta 1969, 88, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Radoglou, K.; Raftoyannis, Y.; Halivopoulos, G. The effect of planting date and seedlings quality on field performance of Castanea sativa Mill and Quercus frainetto Ten. Forestry 2003, 76, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Siam, A.M.J.; Radoglou, K.; Noitsakis, B.; Smiris, P. Ecophysiology of seedlings of three deciduous oak trees during summer water deficit. Sudan J. Des. Res. 2009, 1, 71–87. [Google Scholar]
- Fotelli, M.N.; Radoglou, K.M.; Constantinidou, H.I.A. Water stress responses of seedlings of four Mediterranean oak species. Tree Physiol. 2000, 20, 1065–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayala, J.; Dianda, M.; Wilson, J.; Ouedraogo, S.J.; Sanon, K. Predicting field performance of five irrigated tree species using seedling quality assessment in Burkina Faso, West Africa. New For. 2009, 38, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, B.; Ziolkowska, A.; Bagard, M.; Keech, O.; Gardestrom, P. The impact of light intensity on shade-induced leaf senescence. Plant Cell Environ. 2012, 35, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Landi, M.; Massai, R.; Nali, C.; Guidi, L.; Lorenzini, G. Variations in physiological and biochemical traits of oak seedlings grown under drought and ozone stress. Physiol. Plant 2016, 157, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Rebetez, M.; Mayer, H.; Dupont, O.; Schindler, D.; Gartner, K.; Kropp, J.P.; Menzel, A. Heat and drought 2003 in Europe: A climate synthesis. Ann. For. Sci. 2006, 63, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Poorter, H. Inherent variation in growth rate between higher plants: A search for physiological causes and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar]
- David, T.S.; Pinto, C.A.; Nadezhdina, N.; David, J.S. Water and forests in the Mediterranean hot climate zone: A review based on a hydraulic interpretation of tree functioning. For. Syst. 2016, 25, eR02. [Google Scholar] [CrossRef] [Green Version]

| Species | Origin | Site | Survival | RPF | Ranking |
| German sites | |||||
| Q. pubescens | IT | RIE | 98.57 | 0.869 | 1 |
| Q. robur | DE | RIE | 88.10 | 0.808 | 2 |
| Q. pubescens | GR | RIE | 96.67 | 0.804 | 3 |
| Q. ilex | IT | RIE | 95.71 | 0.777 | 4 |
| Q. pubescens | GR | SWA | 78.57 | 0.763 | 5 |
| Q. ilex | GR | RIE | 94.76 | 0.740 | 6 |
| Q. pubescens | IT | SWA | 85.24 | 0.728 | 7 |
| Q. robur | DE | SWA | 81.90 | 0.690 | 8 |
| Q. ilex | IT | SWA | 80.48 | 0.682 | 9 |
| Q. frainetto | GR | RIE | 97.62 | 0.634 | 10 |
| Q. ilex | GR | SWA | 82.86 | 0.630 | 11 |
| Q. frainetto | IT | RIE | 95.71 | 0.623 | 12 |
| Q. frainetto | IT | SWA | 74.29 | 0.610 | 13 |
| Q. frainetto | GR | SWA | 72.38 | 0.609 | 14 |
| Species | Origin | Site | Survival | RPF | Ranking |
| Italian site | |||||
| Q. pubescens | IT | SAN | 81.90 | 0.944 | 1 |
| Q. pubescens | GR | SAN | 77.62 | 0.873 | 2 |
| Q. ilex | IT | SAN | 82.38 | 0.849 | 3 |
| Q. ilex | GR | SAN | 74.76 | 0.780 | 4 |
| Q. frainetto | GR | SAN | 60.95 | 0.540 | 5 |
| Q. frainetto | IT | SAN | 58.57 | 0.486 | 6 |
| Species | Origin | Site | Survival | RPF | Ranking |
| Greek site | |||||
| Q. pubescens | GR | OLY | 18.10 | 0.939 | 1 |
| Q. pubescens | IT | OLY | 11.43 | 0.820 | 2 |
| Q. ilex | IT | OLY | 2.86 | 0.509 | 3 |
| Q. frainetto | GR | OLY | 3.81 | 0.443 | 4 |
| Q. frainetto | IT | OLY | 4.29 | 0.429 | 5 |
| Q. ilex | GR | OLY | 1.90 | 0.314 | 6 |
| Site | SWA | RIE | SAN | OLY | STR |
|---|---|---|---|---|---|
| Granulometric analysis | |||||
| Sand (%) | 80.8 ± 0.8 | 11.6 ± 4.0 | 33.6 ± 6.5 | 55.2 ± 5.6 | 31.4 ± 4.2 |
| Silt (%) | 12.0 ± 0.3 | 64.1 ± 4.3 | 41.5 ± 3.7 | 26.3 ± 1.9 | 41.3 ± 3.5 |
| Clay (%) | 7.2 ± 0.5 | 24.2 ± 3.7 | 24.9 ± 2.9 | 18.5 ± 3.9 | 27.3 ± 4.4 |
| Soil type | Sl2 | Lu | Ls2-Ls3 | Ls4-Sl4 | Lt2 |
| pH (CaCl2) | 3.3 ± 0.3 | 7.2 ± 0.2 | 7.2 ± 0.1 | 5.4 ± 0.2 | 6.3 ± 0.6 |
| Organic matter (%) | 36.5 ± 19.5 | 3.1 ± 2.1 | 2.2 ± 0.3 | 4.7 ± 1.2 | 17.6 ± 1.6 |
| CaCO3 (%) | nd | 6.1 ± 4.6 | 56.7 ± 1.8 | 0 | 0.1 ± 0.3 |
| Field capacity (Vol-%) | 20.1 ± 4.6 | 31.1 ± 7.6 | 44.3 ± 1.1 | 45.8 ± 4.0 | 50.0 ± 3.8 |
| Plantation Site | Species and Provenance | |||||
|---|---|---|---|---|---|---|
| Q. Ilex | Q. Frainetto | Q. Pubescens | ||||
| Greece | Italy | Greece | Italy | Greece | Italy | |
| RIE | 95 | 96 | 98 | 96 | 97 | 99 |
| SWA | 83 | 80 | 72 | 74 | 79 | 85 |
| SAN | 75 | 82 | 61 | 59 | 78 | 82 |
| OLY | 2 | 3 | 4 | 5 | 18 | 11 |
| STR | 6 | 6 | 18 | 18 | 51 | 27 |
| Species and Provenance | Year | Plantation Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RIE | SWA | SAN | OLY | STR | |||||||
| Q. ilex GR | 2016 | 43.49 ± 0.29 | a | 43.24 ± 0.30 | a | 33.69 ± 0.55 | b | 26.10 ± 0.55 | c | 27.19 ± 0.51 | c |
| 2017 | 36.83 ± 0.47 | b | 35.99 ± 0.39 | bc | 39.74 ± 0.37 | a | 39.00 ± 1.68 | ab | 31.70 ± 1.82 | c | |
| 2018 | 36.72 ± 0.43 | a | 37.33 ± 0.35 | a | 38.60 ± 0.57 | a | 30.71 ± 1.03 | b | 30.64 ± 1.39 | b | |
| 2019 | 33.10 ± 0.33 | a | 33.84 ± 0.42 | a | 15.77 ± 5.49 | b | |||||
| Q. ilex IT | 2016 | 43.32 ± 0.33 | a | 42.88 ± 0.32 | a | 35.97 ± 0.65 | b | 33.56 ± 0.54 | c | 30.36 ± 0.52 | d |
| 2017 | 35.49 ± 0.44 | b | 36.30 ± 0.40 | b | 41.18 ± 0.41 | a | 34.73 ± 3.89 | ab | 34.24 ± 1.97 | b | |
| 2018 | 39.14 ± 0.49 | a | 38.59 ± 0.30 | a | 40.19 ± 0.52 | a | 30.97 ± 2.45 | b | 29.06 ± 1.65 | b | |
| 2019 | 33.95 ± 0.41 | a | 34.81 ± 0.38 | a | 22.13 ± 4.40 | b | |||||
| Q. frainetto GR | 2016 | 38.76 ± 0.31 | a | 38.34 ± 0.33 | a | 18.09 ± 0.29 | c | 24.72 ± 0.63 | b | 25.22 ± 0.63 | b |
| 2017 | 33.66 ± 0.47 | b | 37.10 ± 0.49 | a | 30.28 ± 0.56 | c | 30.98 ± 1.28 | bc | 30.05 ± 0.96 | c | |
| 2018 | 33.51 ± 0.38 | b | 37.08 ± 0.31 | a | 24.48 ± 0.89 | d | 23.75 ± 1.06 | d | 28.08 ± 0.80 | c | |
| 2019 | 27.51 ± 0.51 | b | 35.74 ± 0.34 | a | 19.93 ± 1.01 | c | |||||
| Q. frainetto IT | 2016 | 40.84 ± 0.27 | a | 41.42 ± 0.26 | a | 19.47 ± 0.49 | c | 23.52 ± 0.59 | b | 23.82 ± 0.60 | b |
| 2017 | 32.01 ± 0.42 | b | 36.61 ± 0.45 | a | 32.54 ± 0.48 | b | 30.88 ± 1.02 | b | 32.71 ± 1.06 | b | |
| 2018 | 30.85 ± 0.41 | b | 36.44 ± 0.32 | a | 24.48 ± 0.82 | c | 22.23 ± 0.88 | c | 31.06 ± 1.19 | b | |
| 2019 | 25.97 ± 0.48 | b | 34.72 ± 0.34 | a | 16.70 ± 1.47 | c | |||||
| Q. pubescens GR | 2016 | 41.85 ± 0.30 | a | 41.93 ± 0.30 | a | 29.29 ± 0.54 | c | 33.65 ± 0.55 | b | 35.25 ± 0.53 | b |
| 2017 | 37.05 ± 0.40 | b | 40.16 ± 0.36 | a | 40.45 ± 0.39 | a | 33.56 ± 0.60 | c | 34.54 ± 0.69 | c | |
| 2018 | 37.79 ± 0.32 | b | 40.18 ± 0.23 | a | 39.15 ± 0.49 | ab | 28.90 ± 0.76 | c | 31.64 ± 0.59 | c | |
| 2019 | 36.23 ± 0.28 | b | 38.48 ± 0.25 | a | 29.07 ± 1.32 | c | |||||
| Q. pubescens IT | 2016 | 40.57 ± 0.24 | a | 40.76 ± 0.26 | a | 27.25 ± 0.46 | b | 28.52 ± 0.51 | b | 27.37 ± 0.50 | b |
| 2017 | 36.08 ± 0.41 | b | 38.15 ± 0.39 | a | 39.20 ± 0.40 | a | 32.99 ± 0.88 | c | 32.19 ± 0.83 | c | |
| 2018 | 35.19 ± 0.32 | c | 39.66 ± 0.25 | a | 37.50 ± 0.47 | b | 23.59 ± 0.84 | e | 28.43 ± 0.68 | d | |
| 2019 | 34.77 ± 0.32 | b | 37.53 ± 0.36 | a | 19.83 ± 1.16 | c | |||||
| Q. robur DE | 2016 | 37.54 ± 0.41 | a | 37.89 ± 0.36 | a | ||||||
| 2017 | 27.73 ± 0.51 | b | 36.14 ± 0.57 | a | |||||||
| 2018 | 33.25 ± 0.46 | b | 36.10 ± 0.42 | a | |||||||
| 2019 | 32.39 ± 0.45 | b | 37.23 ± 0.44 | a | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bantis, F.; Graap, J.; Früchtenicht, E.; Bussotti, F.; Radoglou, K.; Brüggemann, W. Field Performances of Mediterranean Oaks in Replicate Common Gardens for Future Reforestation under Climate Change in Central and Southern Europe: First Results from a Four-Year Study. Forests 2021, 12, 678. https://doi.org/10.3390/f12060678
Bantis F, Graap J, Früchtenicht E, Bussotti F, Radoglou K, Brüggemann W. Field Performances of Mediterranean Oaks in Replicate Common Gardens for Future Reforestation under Climate Change in Central and Southern Europe: First Results from a Four-Year Study. Forests. 2021; 12(6):678. https://doi.org/10.3390/f12060678
Chicago/Turabian StyleBantis, Filippos, Julia Graap, Elena Früchtenicht, Filippo Bussotti, Kalliopi Radoglou, and Wolfgang Brüggemann. 2021. "Field Performances of Mediterranean Oaks in Replicate Common Gardens for Future Reforestation under Climate Change in Central and Southern Europe: First Results from a Four-Year Study" Forests 12, no. 6: 678. https://doi.org/10.3390/f12060678







