Pinus Pollen Emission Patterns in Different Bioclimatic Areas of the Iberian Peninsula
Abstract
:1. Introduction
2. Materials and Methods
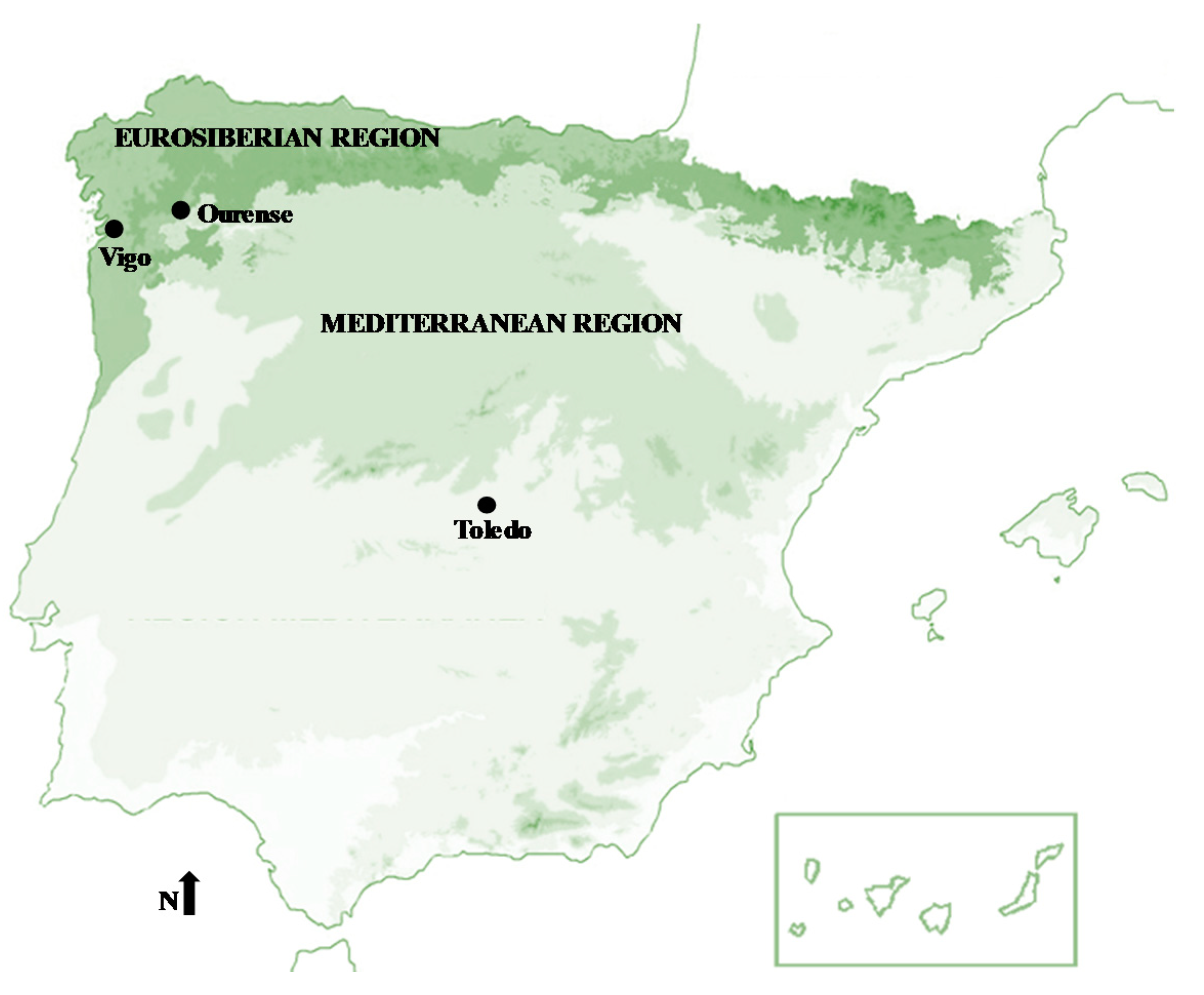
2.1. Characterization and Locations of Study Areas
2.2. Airborne Pollen Study
2.3. Meteorological Data
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, D.M.; Rundel, P.W. Ecology and biogeography of Pinus: An introduction. In Ecology and Biogeography of Pinus; Richardson, D.M., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 3–49. [Google Scholar]
- De Linares, C.; Delgado, R.; Aira, M.J.; Alcázar, P.; Alonso-Pérez, S.; Boi, M.; Cariñanos, P.; Cuevas, E.; Díaz de la Guardia, C.; Elvira-Rendueles, B.; et al. Changes in the Mediterranean pine forest: Pollination patterns and annual trends of airborne pollen. Aerobiologia 2017, 33, 375–391. [Google Scholar] [CrossRef]
- San-Miguel-Ayanz, J.; De Rigo, D.; Caudullo, G.; Houston Durrant, T.; Mauri, A. European Atlas of Forest Tree Species; European Commission: Luxembourg, 2016. [Google Scholar]
- Barberó, M.; Loisel, R.; Quézel, P.; Richardson, D.M.; Romane, F. Pines of the Mediterranean basin. In Ecology and Biogeography of Pinus; Richardson, D.M., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 153–170. [Google Scholar]
- DGCN; IFN3. Tercer Inventario Forestal Nacional, IFN3 (1997–2007) [Third National Forest Inventory, IFN3 (1997–2007)]; Ministerio de Medio Ambiente: Madrid, Spain, 2007; Available online: http://www.magrama.gob.es/es/biodiversidad/servicios/banco-datos-naturaleza/informacion-disponible/ifn3.aspx (accessed on 15 November 2020).
- Barrio-Anta, M.; Castedo-Dorado, F.; Cámara-Obregón, A.; López-Sánchez, C.A. Predicting current and future suitable habitat and productivity for Atlantic populations of maritime pine (Pinus pinaster Aiton) in Spain. Ann. For. Sci. 2020, 77, 41. [Google Scholar] [CrossRef]
- EEA. Climate Change, Impacts and Vulnerability in Europe 2016; An Indicator-Based Report, European Environment Agency Report N° 1; EEA: Copenhagen, Denmark, 2017. [Google Scholar]
- Monzón, J.; Moyer-Horner, L.; Palamar, M.B. Climate change and species range dynamics in protected areas. BioScience 2011, 61, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.E.; Shaw, R.G.; Etterson, J.R. Evolutionary responses to climate change. Ecology 2005, 86, 1704–1714. [Google Scholar] [CrossRef]
- Pereira, J.S.; Correia, A.V.; Correia, C.V.; Ferreira, M.T.; Onofre, N.; Freitas, H.; Godinho, F. Florestas e biodiversidade. In Alterações Climáticas em Portugal. Cenários, Impactos e Medidas de Adaptação (Projecto SIAM II); Santos, F.D., Miranda, P.M.A., Eds.; Gradiva: Lisbon, Portugal, 2006; pp. 301–344. [Google Scholar]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B.; et al. Attributing physical and biological impacts to anthropogenic climate change. Nature 2008, 453, 353–357. [Google Scholar] [CrossRef]
- Rodríguez-Rajo, F.J.; Jato, V.; Aira, M.J. Pollen content in the atmosphere of Lugo (NW Spain) with reference to meteorological factors (1999–2001). Aerobiologia 2003, 19, 213–225. [Google Scholar] [CrossRef]
- Docampo, S.; Recio, M.; Trigo, M.M.; Melgar, M.; Cabezudo, B. Risk of pollen allergy in Nerja (southern Spain): A pollen calendar. Aerobiologia 2007, 23, 189–199. [Google Scholar] [CrossRef]
- Schermer, É.; Bel-Venner, M.C.; Fouchet, D.; Siberchicot, A.; Boulanger, V.; Caignard, T.; Thibaudon, M.; Oliver, G.; Nicolas, M.; Gaillard, J.M.; et al. Pollen limitation as a main driver of fruiting dynamics in oak populations. Ecol. Lett. 2019, 22, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, L. Plant pollen production in selected tree species. Canterb. Bot. Soc. J. 1996, 31, 10–13. [Google Scholar]
- Tormo, R.; Muñóz, A.; Silva, I.; Gallardo, F. Pollen production in anemophilous trees. Grana 1996, 35, 38–46. [Google Scholar]
- Lo, F.; Bitz, C.M.; Battisti, D.S.; Hess, J.J. Pollen calendars and maps of allergenic pollen in North America. Aerobiologia 2019, 35, 613–633. [Google Scholar] [CrossRef] [Green Version]
- Lugonja, P.; Brdar, S.; Simović, I.; Mimić, G.; Palamarchuk, Y.; Sofiev, M.; Šikoparija, B. Integration of in situ and satellite data for top-down mapping of Ambrosia infection level. Remote Sens. Environ. 2019, 235, 111455. [Google Scholar] [CrossRef]
- Rojo, J.; Orlandi, F.; Ben Dhiab, A.; Lara, B.; Picornell, A.; Oteros, J.; Msallem, M.; Fornaciari, M.; Pérez-Badia, R. Estimation of Chilling and Heat Accumulation Periods Based on the Timing of Olive Pollination. Forests 2020, 11, 835. [Google Scholar] [CrossRef]
- Fernández-González, M.; Rodríguez-Rajo, F.J.; Jato, V.; Escuredo, O.; Aira, J.M. Estimation of yield ‘‘Loureira’’ variety with an aerobiological and phenological model. Grana 2011, 50, 63–72. [Google Scholar] [CrossRef]
- Tormo-Molina, R.; Gonzalo-Garijo, M.A.; Silva-Palacios, I.; Muñoz-Rodríguez, A.F. General trends in airborne pollen production and pollination periods at a Mediterranean site (Badajoz, Southwest Spain). J. Investig. Allergol. Clin. Immunol. 2010, 20, 567–574. [Google Scholar] [PubMed]
- Orlandi, F.; Rojo, J.; Picornell, A.; Oteros, J.; Pérez-Badia, R.; Fornaciari, M. Impact of Climate Change on Olive Crop Production in Italy. Atmosphere 2020, 11, 595. [Google Scholar] [CrossRef]
- Cariñanos, P.; Galán, C.; Alcázar, P.; Domínguez, E. Airborne pollen records and status of the anemophilous flora in arid areas of the Iberian Peninsula. J. Arid. Environ. 2010, 74, 1102–1105. [Google Scholar] [CrossRef]
- Womack, A.M.; Bohannan, B.J.M.; Green, J.L. Biodiversity and biogeography of the atmosphere. Philos. Trans. R. Soc. B 2010, 365, 3645–3653. [Google Scholar] [CrossRef] [Green Version]
- Ziello, C.; Sparks, T.H.; Estella, N.; Belmonte, J.; Bergmann, K.; Bucher, E.; Brighetti, M.; Damialis, A.; Detandt, M.; Galán, C.; et al. Changes to airborne pollen counts across Europe. PLoS ONE 2012, 7, e34076. [Google Scholar] [CrossRef]
- Galán, C.; Alcázar, P.; Oteros, J.; García-Mozo, H.; Aira, M.J.; Belmonte, J.; Diaz de la Guardia, C.; Fernández-González, D.; Gutierrez-Bustillo, M.; Moreno-Grau, S.; et al. Airborne pollen trends in the Iberian Peninsula. Sci. Total Environ. 2016, 550, 53–59. [Google Scholar] [CrossRef]
- Ziska, L.H.; Makra, L.; Harry, S.K.; Bruffaerts, N.; Hendrickx, M.; Coates, F.; Saarto, A.; Thibaudon, M.; Oliver, G.; Damialis, A.; et al. Temperature-related changes in airborne allergenic pollen abundance and seasonality across the northern hemisphere: A retrospective data analysis. Lancet Planet. Health 2019, 3, e124–e131. [Google Scholar] [CrossRef] [Green Version]
- Rojo, J.; Picornell, A.; Oteros, J.; Werchan, M.; Werchan, B.; Bergmann, K.-C.; Smith, M.; Weichenmeier, I.; Schmidt-Weber, C.B.; Buters, J. Consequences of climate change on airborne pollen in Bavaria, Central Europe. Reg. Environ. Chang. 2021, 20, 9. [Google Scholar] [CrossRef]
- AEMET Agencia Estatal de Meteorología. Guía Resumida del Clima en España 1981–2010; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2017. [Google Scholar]
- Hirst, J.M. An automatic volumetric spore-trap. Ann. Appl. Biol. 1952, 36, 257–265. [Google Scholar] [CrossRef]
- Galán, C.; Cariñanos, P.; Alcázar, P.; Domínguez, E. Spanish Aerobiology Network: Management and Quality Manual; University of Córdoba: Córdoba, Spain, 2007; pp. 1–61. [Google Scholar]
- Galán, C.; Ariatti, A.; Bonini, M.; Clot, B.; Crouzy, B.; Dahl, A.; Fernandez-González, D.; Frenguelli, G.; Gehrig, R.; Isard, S.; et al. Recommended terminology for aerobiological studies. Aerobiologia 2017, 33, 293–295. [Google Scholar] [CrossRef]
- Rojo, J.; Picornell, A.; Oteros, J. AeRobiology: The computational tool for biological data in the air. Methods Ecol. Evol. 2019, 10, 1371–1376. [Google Scholar] [CrossRef] [Green Version]
- Cunha, M.; Abreu, I.; Pinto, P.; Castro, R. Airborne pollen samples for early-season estimates of wine production in a Mediterranean climate of Northern Portugal. Am. J. Enol. Vitic. 2003, 54, 189–194. [Google Scholar]
- Ribeiro, H.; Cunha, M.; Abreu, I. Definition of the main pollen season using a logistic model. Ann. Agric. Environ. Med. 2007, 14, 159–167. [Google Scholar]
- Anderegg, W.R.; Kane, J.M.; Anderegg, L.D. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Li, D.; Wu, S.; Liu, L.; Zhang, Y.; Li, S. Vulnerability of the global terrestrial eco-systems to climate change. Glob. Chang. Biol. 2018, 24, 4095–4106. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Chang. 2014, 4, 806. [Google Scholar] [CrossRef] [Green Version]
- Thom, D.; Seidl, R. Natural disturbance impacts on ecosystem services and bio-diversity in temperate and boreal forests. Biol. Rev. 2016, 91, 760–781. [Google Scholar] [CrossRef]
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; et al. Global change pressures on soils from land use and management. Glob. Chang. Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef]
- Kume, A.; Fujimoto, M.; Mizoune, N.; Honoki, H.; Nakajima, H.; Ishida, M. Impact of reduced ozone concentration on the mountain forests of Mt. Tateyama, Japan. Environ. Pollut. 2020, 267, 115407. [Google Scholar] [CrossRef] [PubMed]
- Skjøth, C.A.; Baker, P.; Sadyś, M.; Adams-Groom, B. Pollen from alder (Alnus sp.), birch (Betula sp.) and oak (Quercus sp.) in the UK originate from small woodlands. Urban Clim. 2015, 14, 414–428. [Google Scholar] [CrossRef]
- Ghasemifard, H.; Ghada, W.; Estrella, N.; Lüpke, M.; Oteros, J.; Damialis, A.; Traidl-Hoffmann, C.; Buters, J.; Menzel, A. High post-season Alnus pollen loads successfully identified as long-range transport of an alpine species. Atmos. Environ. 2020, 231, 117453. [Google Scholar] [CrossRef]
- Gernandt, D.S.; López, G.G.; García, S.O.; Liston, A. Phylogeny and classification of Pinus. Taxon 2005, 54, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Montero, G. Pinus Pine (“Pinus pinea L.”) in Andalusia: Ecology, Distribution and Forestry; Junta de Andalucia: Seville, Spain, 2004. [Google Scholar]
- Sheffer, E. A review of the development of Mediterranean pine–oak ecosystems after land abandonment and afforestation: Are they novel ecosystems? Ann. For. Sci. 2012, 69, 429–443. [Google Scholar] [CrossRef] [Green Version]
- Tapias, R.; Climent, J.; Pardos, J.A.; Gil, L. Life histories of Mediterranean pines. Plant Ecol. 2004, 171, 53–68. [Google Scholar] [CrossRef]
- Frenguelli, G.; Tedeschini, E.; Veronesi, F.; Bricchi, E. Airborne pine (Pinus spp.) pollen in the atmosphere of Perugia (Central Italy): Behaviour of pollination in the two last decades. Aerobiologia 2002, 18, 223–228. [Google Scholar] [CrossRef]
- Castroviejo, S. Flora Iberica. Nord. J. Bot. 2003, 22, 686. [Google Scholar] [CrossRef]
- Thuiller, W.; Vayreda, J.; Pino, J.; Sabate, S.; Lavorel, S.; Garcia, C. Large-scale environmental correlates of forest tree distributions in Catalonia (NE Spain). Glob. Ecol. Biogeogr. 2003, 12, 313–325. [Google Scholar] [CrossRef] [Green Version]
- Rojo, J.; Pérez-Badia, R. Effects of topography and crown-exposure on olive tree phenology. Trees 2014, 28, 449–459. [Google Scholar] [CrossRef]
- Fu, Y.H.; Zhao, H.; Piao, S.; Peaucelle, M.; Peng, S.; Zhou, G.; Ciais, P.; Huang, M.; Menzel, A.; Peñuelas, J.; et al. Declining global warming effects on the phenology of spring leaf unfolding. Nature 2015, 526, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, F.; García-Mozo, H.; Galán, C.; Romano, B.; Díaz de la Guardia, C.; Ruiz, L. Olive flowering trends in a large Mediterranean area (Italy and Spain). Int. J. Biometeorol. 2010, 54, 151–163. [Google Scholar] [CrossRef]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araujo, M.B. Multiple Dimensions of Climate Change and Their Implications for Biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makra, L.; Matyasovszky, I.; Deák, A.J. Trends in the characteristics of allergenic pollen circulation in central Europe based on the example of Szeged, Hungary. Atmos. Environ. 2011, 45, 6010–6018. [Google Scholar] [CrossRef]
- Damialis, A.; Halley, J.M.; Gioulekas, D.; Vokou, D. Long-term trends in atmospheric pollen levels in the city of Thessaloniki, Greece. Atmos. Environ. 2007, 41, 7011–7021. [Google Scholar] [CrossRef]
- Valcarcel, J.P.; Pilona, F.P. The contribution of registers photographs for wildlife studies: The case of the iberobalear expansion of the invasive species Leptoglossus Occidentalis Heidemann, 1910 (Hemiptera, Coreidae). Arq. Entomol. 2010, 4, 42–52. [Google Scholar]
- Graham, M.D.; Vinebrooke, R.D.; Turner, M. Coupling of boreal forests and lakes: Effects of conifer pollen on littoral communities. Limnol. Oceanogr. 2006, 51, 1524–1529. [Google Scholar] [CrossRef]
- Masclaux, H.; Perga, M.E.; Kagami, M.; Desvilettes, C.; Bourdier, G.; Bec, A. How pollen organic matter enters fresh water food webs. Limnol. Oceanogr. 2013, 58, 1185–1195. [Google Scholar] [CrossRef]
- Filipiak, M. Pollen Stoichiometry May Influence Detrital Terrestrial and Aquatic Food Webs. Front. Ecol. Evol. 2016, 4, 138. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, L. Weight loss and release of mineral nitrogen from decomposing pollen. Soil Biol. Biochem. 1999, 31, 353–361. [Google Scholar] [CrossRef]
- Cho, Y.J.; Sung Kim, I.; Kim, P.; Ju Lee, E. Deposition of airborne pine pollen in a temperate pine forest. Grana 2003, 42, 178–182. [Google Scholar] [CrossRef]
- Webster, E.A.; Tilston, E.L.; Chudek, J.A.; Hopkins, D.W. Decomposition in soil and chemical characteristics of pollen. Eur. J. Soil Sci. 2008, 59, 551–558. [Google Scholar] [CrossRef]
- Rösel, S.; Rychła, A.; Wurzbacher, C.; Grossart, H. Effects of pollen leaching and microbial degradation on organic carbon and nutrient availability in lake water. Aquat. Sci. 2012, 74, 87–99. [Google Scholar] [CrossRef]
- Lee, E.J.; Booth, T. Macronutrient input from pollen in two regenerating pine stands in southeast Korea. Ecol. Res. 2003, 18, 423–430. [Google Scholar] [CrossRef]
- Lee, E.J.; Kenkel, N.; Booth, T. Atmospheric deposition of macronutrients by pollen in the boreal forest. Ecoscience 1996, 3, 304–309. [Google Scholar] [CrossRef]
- Maggs, J. Litter fall and retranslocation of nutrients in a refertilized and prescribed burned Pinus elliottii plantation. For. Ecol. Manag. 1985, 12, 253–268. [Google Scholar] [CrossRef]
- Bullejos, F.J.; Carrillo, P.; Gorokhova, E.; Medina-Sánchez, J.M.; Balseiro, E.G.; Villar-Argaiz, M. Shifts in food quality for herbivorous consumer growth: Multiple golden means in the life history. Ecology 2014, 95, 1272–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jato, V.; Rodríguez-Rajo, F.J.; Méndez, J.; Aira, M.J. Phenological behaviour of Quercus in Ourense (NW Spain) and its relationship with the atmospheric pollen season. Int. J. Biometeorol. 2002, 46, 176–184. [Google Scholar]
- Recio, M.; Picornell, A.; Trigo, M.M.; Gharbia, D.; García-Sánchez, J.; Cabezudo, B. Intensity and temporality of airborne Quercus pollen in the southwest Mediterranean area: Correlation with meteorological and phenoclimatic variables, trends and possible adaptation to climate change. Agric. For. Meteorol. 2018, 250, 308–318. [Google Scholar] [CrossRef]
- Fernández-González, M.; González-Fernández, E.; Ribeiro, H.; Abreu, I.; Rodríguez-Rajo, F.J. Pollen Production of Quercus in the North-Western Iberian Peninsula and Airborne Pollen Concentration Trends during the Last 27 Years. Forests 2020, 11, 702. [Google Scholar] [CrossRef]
- Xunta Galicia. Primera Revisión del Plan Forestal de Galicia: Documento de Diagnóstico del Monte y el Sector Forestal Gallego; Xunta de Galicia: Santiago, Spain, 2018; Available online: https://distritoforestal.es/images/DIAGNOSTICO_PFG_CAST_1.pdf (accessed on 21 October 2020).
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Snyder, P.K. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mozo, H.; Oteros, J.; Galán, C. Impact of land cover changes and climate on the main airborne pollen types in Southern Spain. Sci. Total Environ. 2016, 548–549, 221–228. [Google Scholar] [CrossRef]
- Ruiz-Valenzuela, L.; Aguilera, F. Trends in airborne pollen and pollen-season-related features of anemophilous species in Jaen (South Spain): A 23-year perspective. Atmos. Environ. 2018, 80, 234–243. [Google Scholar] [CrossRef]
- Bishan, C.; Bing, L.; Chixin, C.; Junxia, S.; Shulin, Z.; Cailang, L.; Siqiao, Y.; Chuanxiu, L. Relationship between airborne pollen assemblages and major meteorological parameters in Zhanjiang, South China. PLoS ONE 2020, 15, e0240160. [Google Scholar] [CrossRef]
- Kluska, K.; Piotrowicz, K.; Kasprzyka, I. The impact of rainfall on the diurnal patterns of atmospheric pollen concentrations. Agric. For. Meteorol. 2020, 291, 108042. [Google Scholar] [CrossRef]
- Pérez, C.F.; Gassmann, M.I.; Covi, M. An evaluation of the airborne pollen–precipitation relationship with the superposed epoch method. Aerobiologia 2009, 25, 313–320. [Google Scholar] [CrossRef]
- Pecero-Casimiro, R.; Maya-Manzano, J.M.; Fernández-Rodríguez, S.; Tormo-Molina, R.; Silva-Palacios, I.; Monroy-Colín, A.; Gonzalo-Garijo, A. Pollen calendars and regional gradients as information tools in the Extremadura pollen monitoring network (SW Spain). Aerobiologia 2020, 36, 731–748. [Google Scholar] [CrossRef]





| MPS—Vigo | ||||||
|---|---|---|---|---|---|---|
| Year | Start (MPS) | End (MPS) | Length (MPS) | API | Pollen Peak | Peak Day |
| 1995 | 28-Feb. | 23-Apr. | 55 | 5616 | 542 | 25-Mar. |
| 1996 | 3-Mar. | 30-Apr. | 59 | 3588 | 294 | 22-Mar. |
| 1997 | 21-Feb. | 4-Apr. | 43 | 5993 | 606 | 10-Mar. |
| 1998 | 11-Feb. | 27-Mar. | 45 | 5297 | 1105 | 3-Mar. |
| 1999 | 28-Feb. | 17-Apr. | 49 | 4080 | 489 | 7-Apr. |
| 2000 | 3-Mar. | 24-Mar. | 22 | 7704 | 1131 | 11-Mar. |
| 2001 | 2-Jan. | 19-May | 138 | 1529 | 408 | 5-Mar. |
| 2002 | 3-Mar. | 15-Apr. | 44 | 6894 | 1222 | 26-Mar. |
| 2003 | 4-Mar. | 8-Apr. | 36 | 5598 | 989 | 17-Mar. |
| 2004 | 10-Feb. | 1-May | 82 | 4749 | 351 | 3-Mar. |
| 2005 | 14-Mar. | 17-Apr. | 35 | 3343 | 1129 | 31-Mar. |
| 2006 | 13-Mar. | 8-May | 57 | 4962 | 863 | 3-Apr. |
| 2007 | 26-Feb. | 7-May | 71 | 5488 | 374 | 17-Apr. |
| 2008 | 5-Feb. | 24-Apr. | 80 | 2158 | 185 | 19-Mar. |
| 2009 | 27-Feb. | 12-Apr. | 45 | 4004 | 347 | 17-Mar. |
| 2010 | 15-Mar. | 6-May | 53 | 2951 | 321 | 11-Apr. |
| 2011 | 1-Mar. | 27-Apr. | 58 | 4469 | 576 | 1-Apr. |
| 2012 | 5-Mar. | 8-Apr. | 35 | 4338 | 575 | 23-Mar. |
| 2013 | 27-Jan. | 12-Jun. | 137 | 2365 | 167 | 18-Apr. |
| 2014 | 4-Mar. | 6-May | 64 | 3042 | 323 | 9-Apr. |
| 2015 | 19-Mar. | 21-Apr. | 34 | 8780 | 1027 | 8-Apr. |
| 2016 | 12-Feb. | 18-May | 97 | 2617 | 220 | 21-Mar. |
| 2017 | 25-Feb. | 22-Apr. | 57 | 5734 | 844 | 25-Mar. |
| 2018 | 27-Mar. | 21-May | 56 | 3657 | 522 | 25-Apr. |
| 2019 | 27-Feb. | 15-Apr. | 48 | 1538 | 147 | 26-Mar. |
| Mean | 25-Feb. | 24-Apr. | 60 | 4420 | 590 | 26-Mar. |
| Max | 27-Mar. | 12-Jun. | 138 | 8780 | 1222 | 26-Mar. |
| Min | 2-Jan. | 24-Mar. | 22 | 1529 | 147 | 26-Mar. |
| SD | 17.519 | 18.353 | 28.666 | 1840.404 | 345.096 | 14.325 |
| RSD% | 31.019 | 15.893 | 47.777 | 41.641 | 58.463 | 16.712 |
| MPS—Ourense | ||||||
|---|---|---|---|---|---|---|
| Year | Start (MPS) | End (MPS) | Length (MPS) | API | Pollen Peak | Peak Day |
| 1995 | 6-Mar. | 22-Apr. | 48 | 3696 | 627 | 25-Mar. |
| 1996 | 22-Mar. | 9-May | 49 | 3505 | 442 | 9-Apr. |
| 1997 | 4-Mar. | 7-Apr. | 35 | 2422 | 287 | 18-Mar. |
| 1998 | 22-Feb. | 21-Apr. | 59 | 3114 | 292 | 22-Mar. |
| 1999 | 20-Mar. | 21-Apr. | 33 | 1694 | 323 | 6-Apr. |
| 2000 | 3-Mar. | 19-Apr. | 48 | 2600 | 233 | 27-Mar. |
| 2001 | 23-Feb. | 3-May | 70 | 1778 | 290 | 1-Apr. |
| 2002 | 11-Mar. | 24-Apr. | 45 | 6262 | 1003 | 25-Mar. |
| 2003 | 19-Mar. | 13-Apr. | 26 | 3369 | 818 | 1-Apr. |
| 2004 | 2-Mar. | 3-May | 63 | 3294 | 314 | 4-Apr. |
| 2005 | 24-Mar. | 22-Apr. | 30 | 2851 | 656 | 7-Apr. |
| 2006 | 31-Mar. | 7-May | 38 | 5615 | 728 | 10-Apr. |
| 2007 | 11-Mar. | 8-May | 59 | 5814 | 287 | 16-Apr. |
| 2008 | 13-Mar. | 26-Apr. | 45 | 3690 | 552 | 6-Apr. |
| 2009 | 5-Mar. | 22-Apr. | 49 | 5986 | 464 | 24-Mar. |
| 2010 | 2-Apr. | 8-May | 37 | 5532 | 644 | 12-Apr. |
| 2011 | 18-Mar. | 28-Apr. | 42 | 5494 | 649 | 11-Apr. |
| 2012 | 12-Mar. | 15-Apr. | 35 | 5507 | 565 | 30-Mar. |
| 2013 | 25-Mar. | 14-May | 51 | 4552 | 285 | 17-Apr. |
| 2014 | 15-Mar. | 3-May | 50 | 7990 | 899 | 10-Apr. |
| 2015 | 11-Mar. | 5-May | 56 | 6783 | 548 | 3-Apr. |
| 2016 | 23-Feb. | 31-May | 99 | 5250 | 278 | 28-Feb. |
| 2017 | 9-Mar. | 6-May | 59 | 5576 | 295 | 8-Apr. |
| 2018 | 7-Apr. | 17-May | 41 | 6095 | 604 | 27-Apr. |
| 2019 | 24-Feb. | 10-May | 76 | 1660 | 178 | 31-Mar. |
| Mean | 13-Mar. | 30-Apr. | 50 | 4405 | 490 | 3-Apr. |
| Max | 7-Apr. | 31-May | 99 | 7990 | 1003 | 25-Mar. |
| Min | 22-Feb. | 7-Apr. | 26 | 1660 | 178 | 31-Mar. |
| SD | 12.123 | 12.066 | 16.017 | 1833.928 | 226.165 | 11.394 |
| RSD% | 16.913 | 10.021 | 32.214 | 40.992 | 46.117 | 12.241 |
| MPS—Toledo | ||||||
|---|---|---|---|---|---|---|
| Year | Start (MPS) | End (MPS) | Length (MPS) | API | Pollen Peak | Peak Day |
| 2003 | 24-Feb. | 10-Jun. | 107 | 781 | 43 | 22-Mar. |
| 2004 | 22-Feb. | 15-Jul. | 145 | 1092 | 98 | 31-Mar. |
| 2005 | 15-Mar. | 6-Jul. | 114 | 1491 | 89 | 5-Jun. |
| 2006 | 8-Mar. | 14-Jun. | 99 | 788 | 81 | 4-Apr. |
| 2007 | 4-Mar. | 1-Jul. | 120 | 1633 | 127 | 30-Mar. |
| 2008 | 18-Feb. | 15-Jun. | 119 | 1755 | 235 | 30-Mar. |
| 2009 | 3-Mar. | 30-Jun. | 120 | 1629 | 117 | 27-May |
| 2010 | 20-Mar. | 11-Jul. | 114 | 1332 | 81 | 4-Jun. |
| 2011 | 5-Mar. | 20-Jun. | 108 | 1039 | 152 | 3-Apr. |
| 2012 | 5-Mar. | 21-Jun. | 109 | 1332 | 70 | 10-Apr. |
| 2013 | 5-Mar. | 3-Jul. | 121 | 1273 | 103 | 11-Apr. |
| 2014 | 27-Feb. | 23-Jun. | 117 | 2695 | 503 | 1-Apr. |
| 2015 | 11-Apr. | 23-Jun. | 74 | 2654 | 339 | 20-May |
| 2016 | 23-Feb. | 25-Jul. | 154 | 1775 | 148 | 3-Apr. |
| 2017 | 21-Feb. | 2-Jun. | 102 | 1615 | 155 | 26-Mar. |
| 2018 | 22-Mar. | 25-Jul. | 126 | 2390 | 178 | 17-Jun. |
| 2019 | 8-Mar. | 7-Jul. | 122 | 2228 | 131 | 28-May |
| Mean | 6-Mar. | 29-Jun. | 116 | 1618 | 156 | 23-Apr. |
| Max | 11-Apr. | 25-Jul. | 154 | 2695 | 503 | 1-Apr. |
| Min | 18-Feb. | 2-Jun. | 74 | 781 | 43 | 22-Mar. |
| SD | 12.967 | 15.120 | 17.569 | 588.611 | 113.404 | 30.658 |
| RSD% | 19.896 | 8.394 | 15.154 | 36.385 | 72.750 | 27.089 |
| Area | MaxT | MinT | AvgT | Rainfall | Humidity | |
|---|---|---|---|---|---|---|
| MPS Start date | Eurosiberian (Vigo) | 0.406 ** | 0.437 ** | 0.489 ** | −0.721 *** | −0.383 * |
| Transition (Orense) | −0.467 ** | −0.340 * | ||||
| Mediterranean (Toledo) | 0.787 *** | −0.430 * | 0.731 *** | 0.827 *** | ||
| MPS End date | Eurosiberian (Vigo) | 0.561 *** | 0.427 ** | |||
| Transition (Orense) | 0.592 *** | |||||
| Mediterranean (Toledo) | 0.472 * | |||||
| MPS Length | Eurosiberian (Vigo) | −0.375 * | 0.801 *** | 0.509 *** | ||
| Transition (Orense) | −0.337 * | 0.454 ** | 0.414 ** | |||
| Mediterranean (Toledo) | 0.581 ** | |||||
| API | Eurosiberian (Vigo) | −0.532 *** | −0.340 * | |||
| Transition (Orense) | −0.369 * | −0.451 ** | ||||
| Mediterranean (Toledo) | 0.444 * | −0.473 * | 0.469 * | |||
| Peak date | Eurosiberian (Vigo) | 0.616 ** | 0.458 ** | |||
| Transition (Orense) | 0.364 * | |||||
| Mediterranean (Toledo) | 0.757 ** | −0.546 ** | 0.718 ** | 0.778 ** |
| Eurosiberian Vigo (1995–2019) | Transition Ourense (1995–2019) | Mediterranean Toledo (2003–2019) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slope | R2 | p | Slope | R2 | p | Slope | R2 | p | ||
| MPS | Start MPS | 0.493 | 0.043 | 0.331 | 0.265 | 0.026 | 0.462 | 0.645 | 0.063 | 0.329 |
| End MPS | 0.994 | 0.158 | 0.049 | 0.961 | 0.346 | 0.002 | 0.649 | 0.048 | 0.410 | |
| Length MPS | 0.506 | 0.017 | 0.536 | 0.702 | 0.104 | 0.116 | −0.007 | 0.000 | 0.994 | |
| Pollen integral | −68.523 | 0.075 | 0.185 | 126.130 | 0.256 | 0.009 | 79.717 | 0.468 | 0.002 | |
| Pollen peak | −13.718 | 0.085 | 0.156 | 0.270 | 0.000 | 0.967 | 8.843 | 0.155 | 0.118 | |
| Peak date | 0.994 | 0.255 | 0.009 | 0.391 | 0.063 | 0.228 | 1.750 | 0.082 | 0.265 | |
| Year | Max T | 0.022 | 0.009 | 0.650 | 0.003 | 0.000 | 0.958 | 0.173 | 0.176 | 0.093 |
| Min T | 0.169 | 0.491 | 0.000 | 0.037 | 0.039 | 0.343 | 0.106 | 0.119 | 0.175 | |
| Average T | 0.078 | 0.169 | 0.041 | −0.013 | 0.009 | 0.647 | 0.139 | 0.156 | 0.116 | |
| Rainfall | −0.741 | 0.000 | 0.931 | 1.632 | 0.018 | 0.520 | −2.922 | 0.043 | 0.422 | |
| Rel Hum | 0.067 | 0.012 | 0.608 | −0.173 | 0.102 | 0.118 | −0.464 | 0.185 | 0.085 | |
| Pre-peak Period | Max T | 0.016 | 0.004 | 0.766 | −0.040 | 0.012 | 0.594 | 0.133 | 0.044 | 0.419 |
| Min T | 0.151 | 0.322 | 0.003 | −0.040 | 0.024 | 0.456 | 0.095 | 0.038 | 0.454 | |
| Average T | 0.067 | 0.085 | 0.157 | −0.075 | 0.129 | 0.077 | 0.114 | 0.044 | 0.148 | |
| Rainfall | 1.646 | 0.006 | 0.715 | −0.588 | 0.005 | 0.734 | 1582 | 0.033 | 0.486 | |
| Rel Hum | 0.010 | 0.000 | 0.950 | −0.227 | 0.073 | 0.192 | −0.121 | 0.006 | 0.761 | |
| Post-peak Period | Max T | 0.042 | 0.023 | 0.469 | 0.016 | 0.002 | 0.831 | 0.307 | 0.152 | 0.121 |
| Min T | 0.202 | 0.493 | 0.702 | 0.009 | 0.172 | 0.039 | 0.194 | 0.094 | 0.231 | |
| Average T | 0.103 | 0.219 | 0.018 | 0.027 | 0.0153 | 0.556 | 0.250 | 0.125 | 0.163 | |
| Rainfall | −2.388 | 0.012 | 0.602 | 2.220 | 0.050 | 0.281 | −4504 | 0.139 | 0.140 | |
| Rel Hum | 0.132 | 0.032 | 0.393 | −0.113 | 0.037 | 0.356 | −0.914 | 0.265 | 0.034 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-González, M.; Lara, B.; González-Fernández, E.; Rojo, J.; Pérez-Badia, R.; Rodríguez-Rajo, F.J. Pinus Pollen Emission Patterns in Different Bioclimatic Areas of the Iberian Peninsula. Forests 2021, 12, 688. https://doi.org/10.3390/f12060688
Fernández-González M, Lara B, González-Fernández E, Rojo J, Pérez-Badia R, Rodríguez-Rajo FJ. Pinus Pollen Emission Patterns in Different Bioclimatic Areas of the Iberian Peninsula. Forests. 2021; 12(6):688. https://doi.org/10.3390/f12060688
Chicago/Turabian StyleFernández-González, María, Beatriz Lara, Estefanía González-Fernández, Jesús Rojo, Rosa Pérez-Badia, and Fco. Javier Rodríguez-Rajo. 2021. "Pinus Pollen Emission Patterns in Different Bioclimatic Areas of the Iberian Peninsula" Forests 12, no. 6: 688. https://doi.org/10.3390/f12060688






