Past and Future of Temperate Forests State under Climate Change Effects in the Romanian Southern Carpathians
Abstract
:1. Introduction
2. Materials and Methods
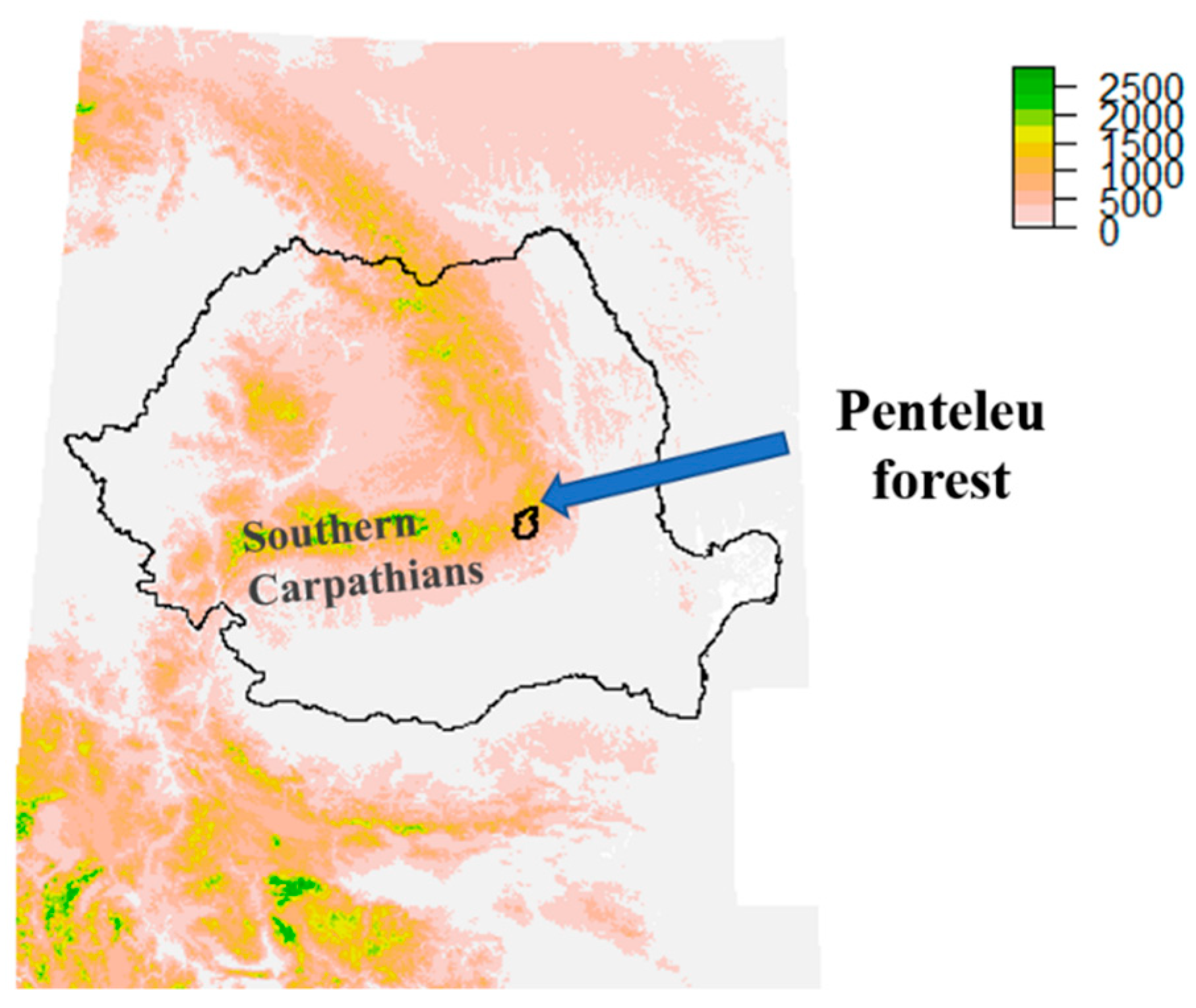
2.1. Study Area
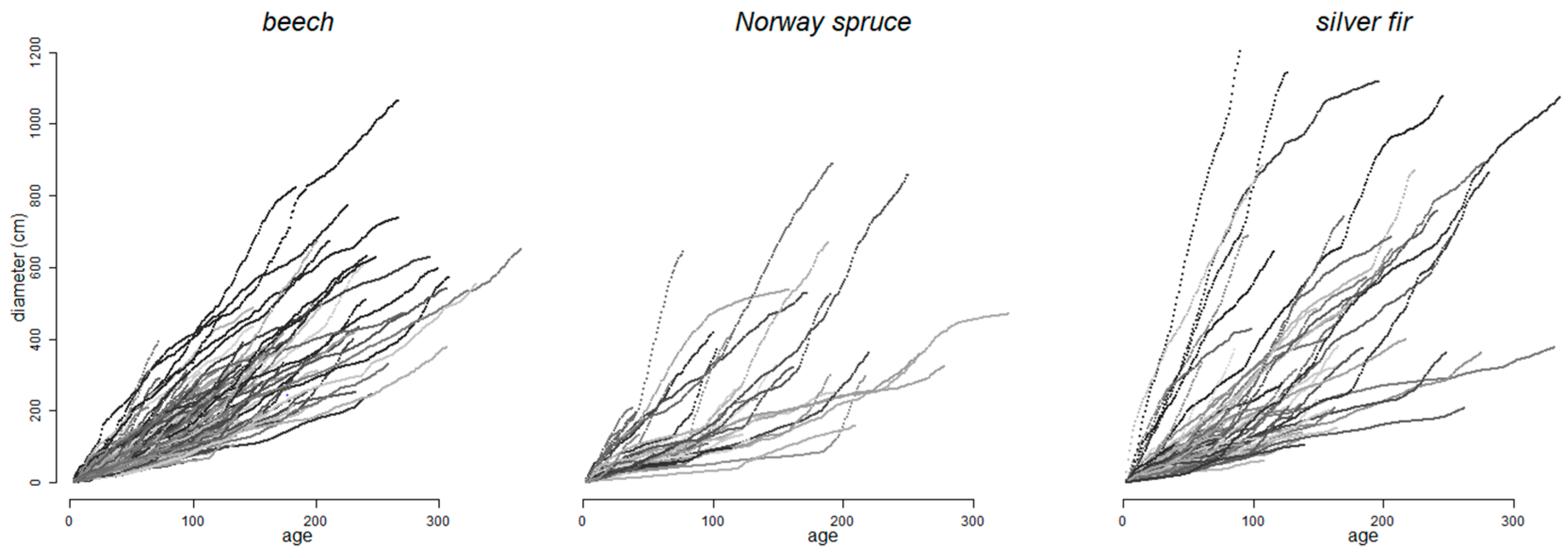
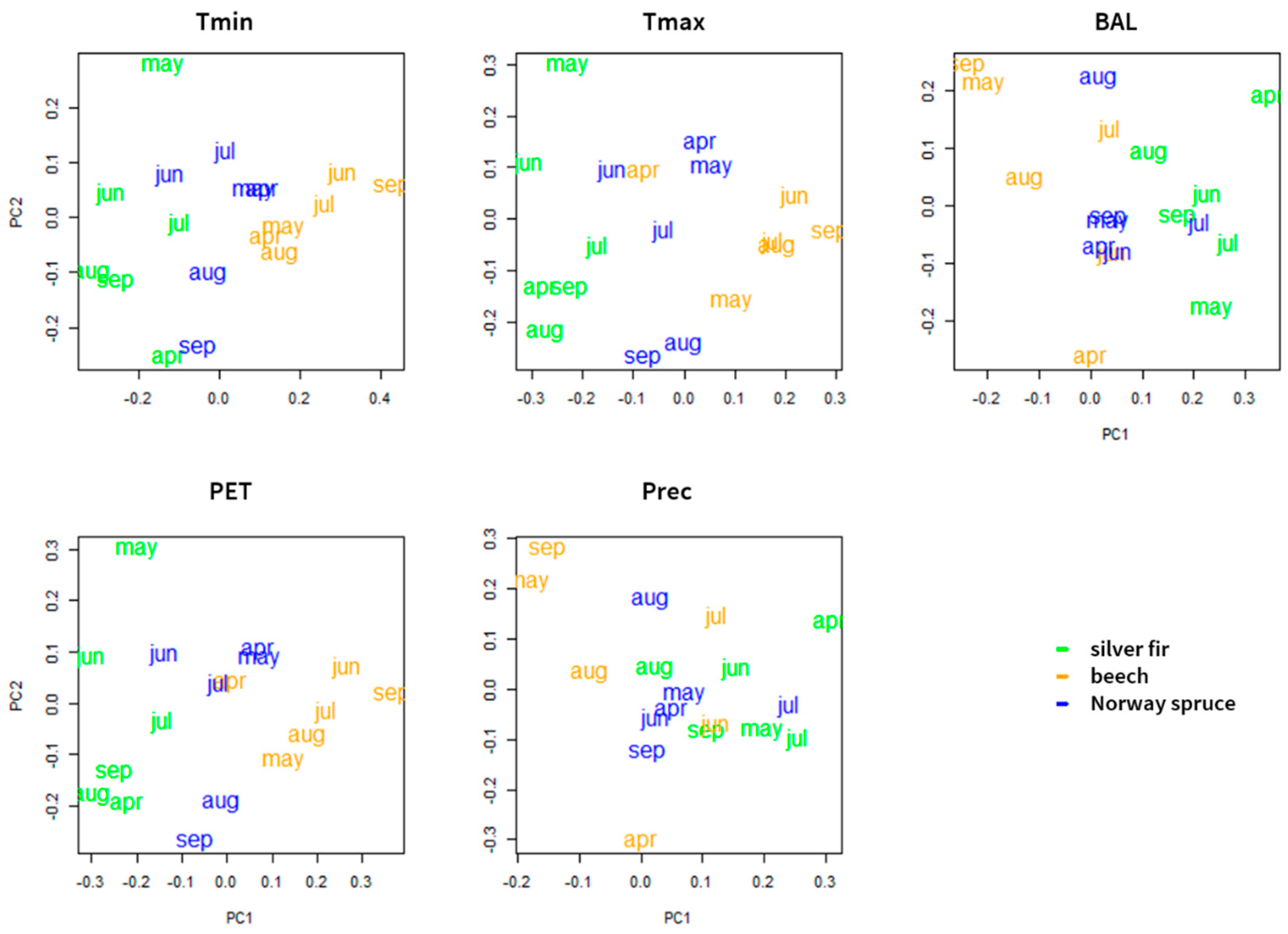
2.2. Dendrochronological Series and Climate Signal
2.3. Climate Change Projections
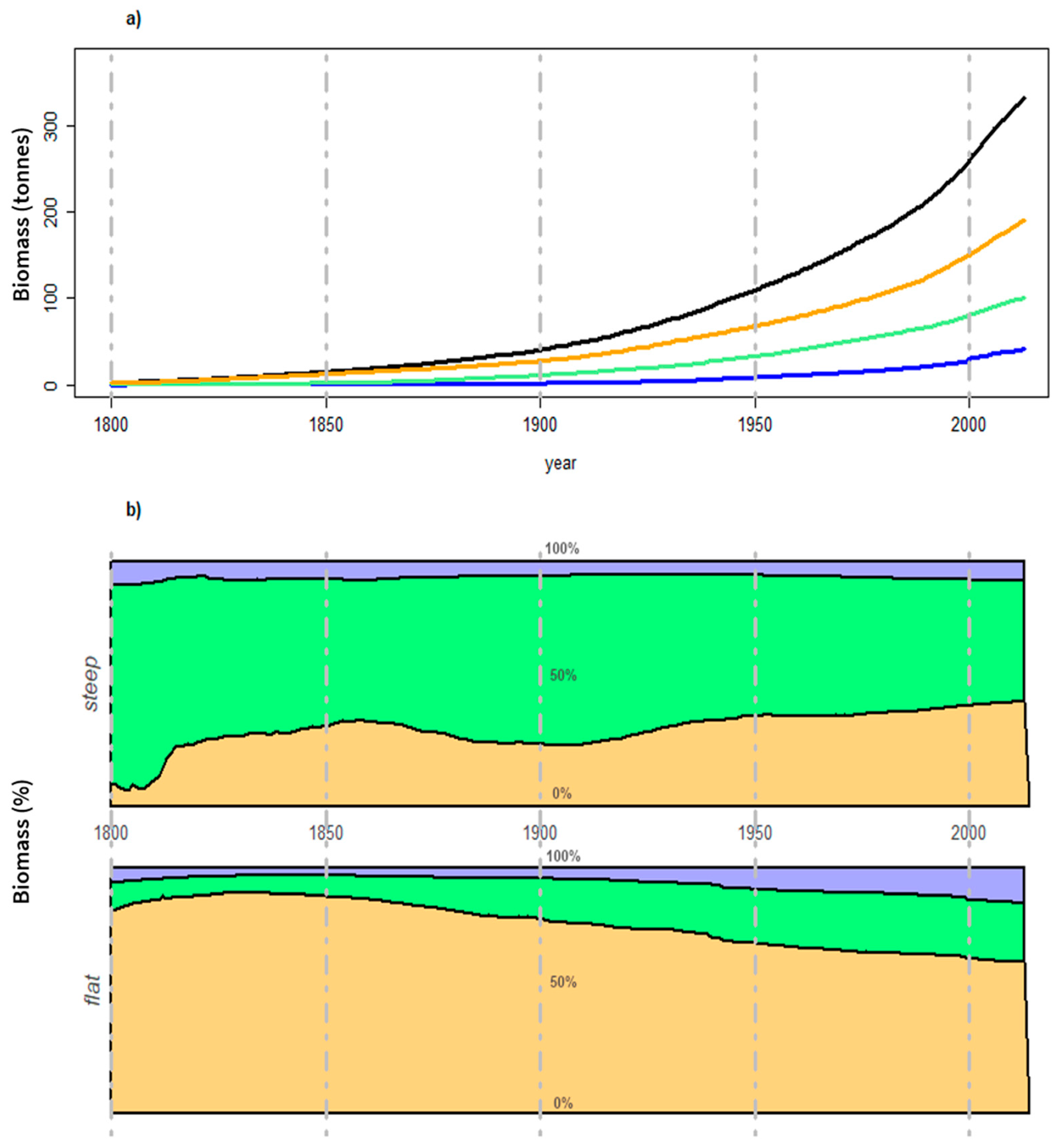
3. Results
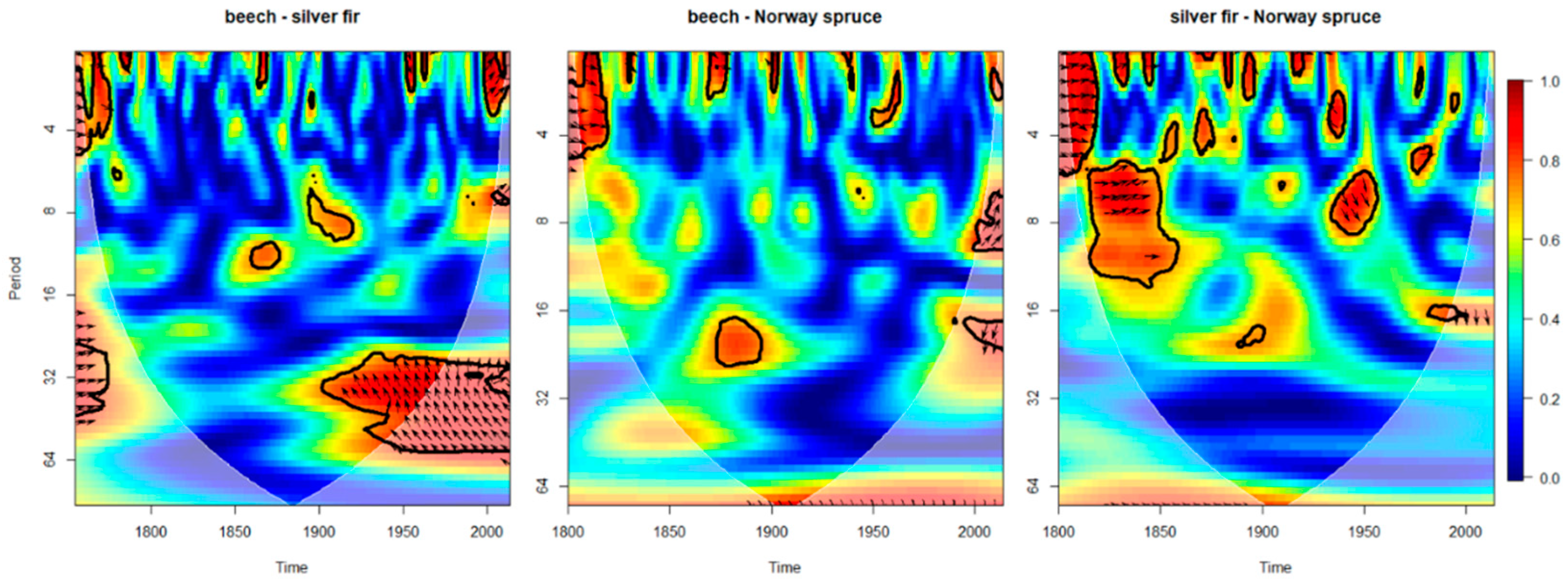
3.1. Forest Sensitivity to Past Climate
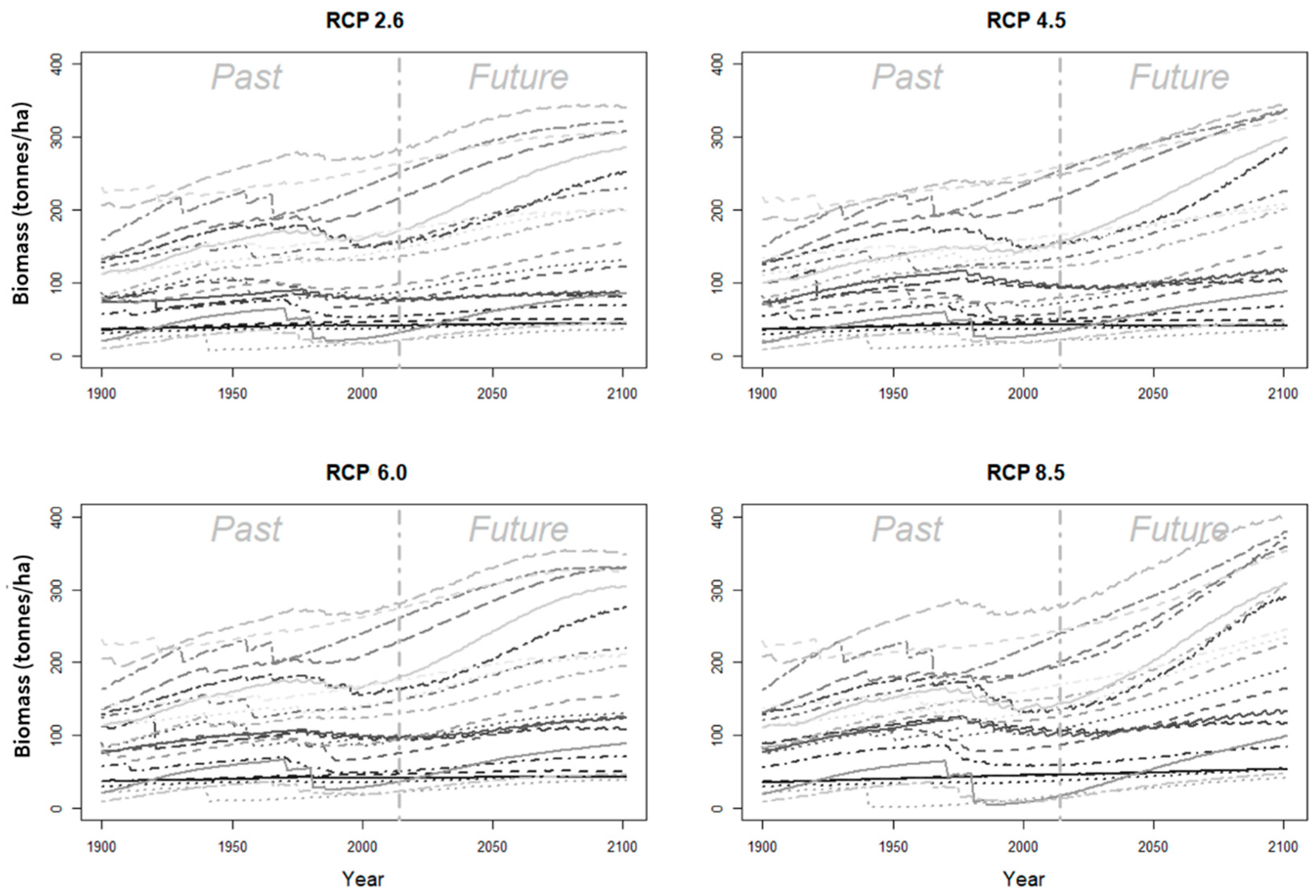
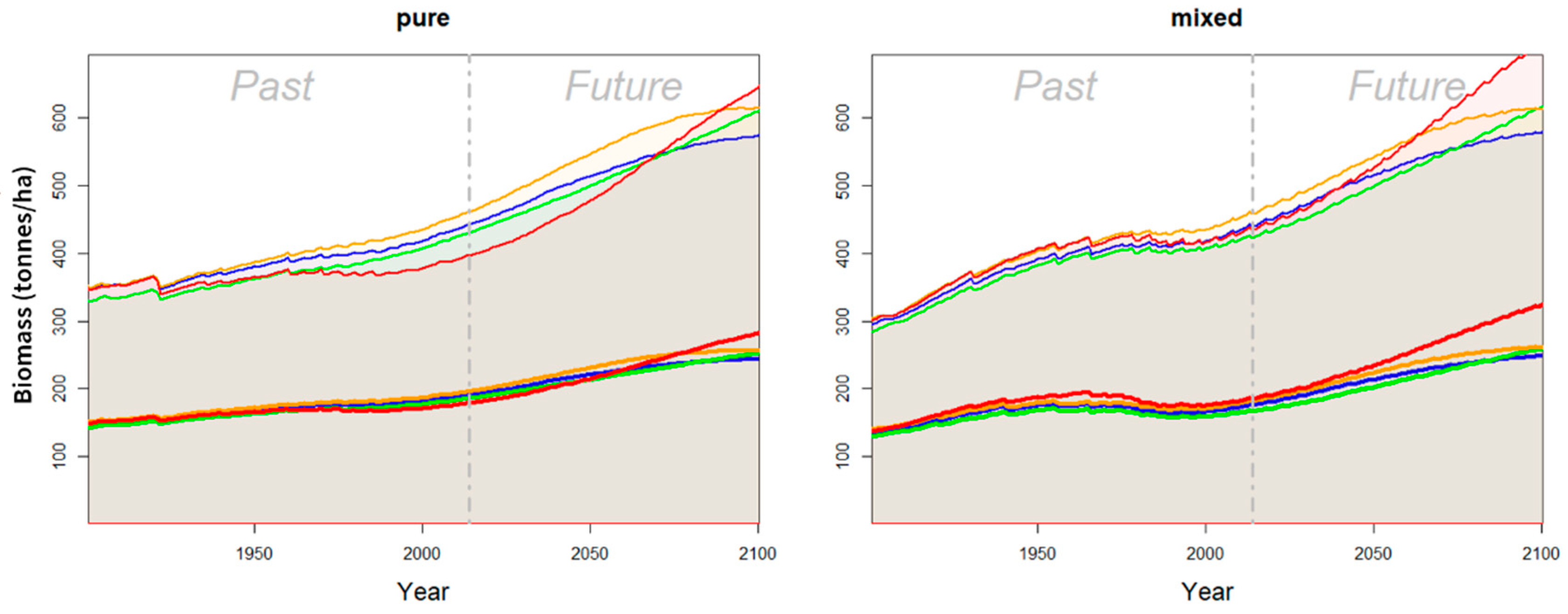
3.2. Aboveground Living Biomass under Climate Change Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dymond, C.C.; Beukema, S.; Nitschke, C.R.; Coates, K.D.; Scheller, R.M. Carbon Sequestration in Managed Temperate Coniferous Forests under Climate Change. Biogeosciences 2016, 13, 1933–1947. [Google Scholar] [CrossRef] [Green Version]
- Nabuurs, G.-J.; Lindner, M.; Verkerk, P.J.; Gunia, K.; Deda, P.; Michalak, R.; Grassi, G. First Signs of Carbon Sink Saturation in European Forest Biomass. Nat. Clim. Chang. 2013, 3, 792–796. [Google Scholar] [CrossRef]
- van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F.; et al. The Representative Concentration Pathways: An Overview. Clim. Chang. 2011, 109, 5–31. [Google Scholar] [CrossRef]
- Micu, D.M.; Dumitrescu, A.; Cheval, S.; Birsan, M.-V. Climate of the Romanian Carpathians; Springer Atmospheric Sciences; Springer International Publishing: Cham, Switzerlands, 2015; ISBN 978-3-319-02885-9. [Google Scholar]
- Huntley, B.; Baxter, R. Vegetation Ecology and Global Change. In Vegetation Ecology; van der Maarel, E., Franklin, J., Eds.; John Wiley & Sons, Ltd.: Oxford, UK, 2013; pp. 509–530. ISBN 978-1-118-45259-2. [Google Scholar]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A.; et al. Pervasive Shifts in Forest Dynamics in a Changing World. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest Disturbances under Climate Change. Nat. Clim. Chang. 2017, 7, 395. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H. The Course of Tree Growth. Theory and Reality. For. Ecol. Manag. 2020, 478, 118508. [Google Scholar] [CrossRef]
- Chivulescu, S.; Leca, S.; Silaghi, D.; Cristea, V. Structural Biodiversity and Dead Wood in Virgin Forests from Eastern Carpathians. J. Agric. For. 2018, 64. [Google Scholar] [CrossRef] [Green Version]
- Grantham, H.S.; Duncan, A.; Evans, T.D.; Jones, K.R.; Beyer, H.L.; Schuster, R.; Walston, J.; Ray, J.C.; Robinson, J.G.; Callow, M.; et al. Anthropogenic Modification of Forests Means Only 40% of Remaining Forests Have High Ecosystem Integrity. Nat. Commun. 2020, 11, 5978. [Google Scholar] [CrossRef]
- Vrška, T.; Adam, D.; Hort, L.; Kolář, T.; Janík, D. European Beech (Fagus Sylvatica L.) and Silver Fir (Abies Alba Mill.) Rotation in the Carpathians—A Developmental Cycle or a Linear Trend Induced by Man? For. Ecol. Manag. 2009, 258, 347–356. [Google Scholar] [CrossRef]
- Şofletea, N.; Curtu, L. Dendrologie; Pentru Viaţă: Braşov, Romania, 2001; Volume II, ISBN 978-973-99456-1-5. [Google Scholar]
- Bosela, M.; Lukac, M.; Castagneri, D.; Sedmák, R.; Biber, P.; Carrer, M.; Konôpka, B.; Nola, P.; Nagel, T.A.; Popa, I.; et al. Contrasting Effects of Environmental Change on the Radial Growth of Co-Occurring Beech and Fir Trees across Europe. Sci. Total Environ. 2018, 615, 1460–1469. [Google Scholar] [CrossRef]
- Primicia, I.; Camarero, J.J.; Janda, P.; Čada, V.; Morrissey, R.C.; Trotsiuk, V.; Bače, R.; Teodosiu, M.; Svoboda, M. Age, Competition, Disturbance and Elevation Effects on Tree and Stand Growth Response of Primary Picea Abies Forest to Climate. For. Ecol. Manag. 2015, 354, 77–86. [Google Scholar] [CrossRef]
- Griess, V.C.; Acevedo, R.; Härtl, F.; Staupendahl, K.; Knoke, T. Does Mixing Tree Species Enhance Stand Resistance against Natural Hazards? A Case Study for Spruce. For. Ecol. Manag. 2012, 267, 284–296. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of European Tree Species to Drought Stress in Mixed versus Pure Forests: Evidence of Stress Release by Inter-Specific Facilitation: Drought Stress Release by Inter-Specific Facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Magh, R.-K.; Bonn, B.; Grote, R.; Burzlaff, T.; Pfautsch, S.; Rennenberg, H. Drought Superimposes the Positive Effect of Silver Fir on Water Relations of European Beech in Mature Forest Stands. Forests 2019, 10, 897. [Google Scholar] [CrossRef] [Green Version]
- MacPherson, D.M.; Lieffers, V.J.; Blenis, P.V. Productivity of Aspen Stands with and without a Spruce Understory in Alberta’s Boreal Mixedwood Forests. For. Chron. 2001, 77, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Man, R.; Lieffers, V.J. Are Mixtures of Aspen and White Spruce More Productive than Single Species Stands? For. Chron. 1999, 75, 505–513. [Google Scholar] [CrossRef]
- Laiho, O.; Lahde, E.; Pukkala, T. Uneven- vs Even-Aged Management in Finnish Boreal Forests. Forestry 2011, 84, 547–556. [Google Scholar] [CrossRef]
- Barsoum, N.; Coote, L.; Eycott, A.E.; Fuller, L.; Kiewitt, A.; Davies, R.G. Diversity, Functional Structure and Functional Redundancy of Woodland Plant Communities: How Do Mixed Tree Species Plantations Compare with Monocultures? For. Ecol. Manag. 2016, 382, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Petritan, I.C.; Commarmot, B.; Hobi, M.L.; Petritan, A.M.; Bigler, C.; Abrudan, I.V.; Rigling, A. Structural Patterns of Beech and Silver Fir Suggest Stability and Resilience of the Virgin Forest Sinca in the Southern Carpathians, Romania. For. Ecol. Manag. 2015, 356, 184–195. [Google Scholar] [CrossRef]
- Chivulescu, S.; Ciceu, A.; Leca, S.; Apostol, B.; Popescu, O.; Badea, O. Development Phases and Structural Characteristics of the Penteleu-Viforta Virgin Forest in the Curvature Carpathians. IForest Biogeosci. For. 2020, 13, 389–395. [Google Scholar] [CrossRef]
- Mirtl, M. Introducing the Next Generation of Ecosystem Research in Europe: LTER-Europe’s Multi-Functional and Multi-Scale Approach. In Long-Term Ecological Research; Müller, F., Baessler, C., Schubert, H., Klotz, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 75–93. ISBN 978-90-481-8781-2. [Google Scholar]
- Parviainen, J. Virgin and Natural Forests in the Temperate Zone of Europe. For. Snow Landsc. Res. 2005, 79, 9–18. [Google Scholar]
- Dănescu, A.; Albrecht, A.T.; Bauhus, J. Structural Diversity Promotes Productivity of Mixed, Uneven-Aged Forests in Southwestern Germany. Oecologia 2016, 182, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Vacek, S.; Vacek, Z.; Podrázský, V.; Bílek, L.; Bulušek, D.; Štefančík, I.; Remeš, J.; Štícha, V.; Ambrož, R. Structural Diversity of Autochthonous Beech Forests in Broumovské Stěny National Nature Reserve, Czech Republic. Austrian J. For. Sci. 2014, 131, 191–214. [Google Scholar]
- Duveneck, M.J.; Scheller, R.M. Measuring and Managing Resistance and Resilience under Climate Change in Northern Great Lake Forests (USA). Landsc. Ecol. 2016, 31, 669–686. [Google Scholar] [CrossRef]
- Curovic, M.; Spalevic, V.; Sestras, P.; Motta, R.; Dan, C.; Garbarino, M.; Vitali, M.; Urbinati, C. Structural and Ecological Characteristics of Mixed Broadleaved Old-Growth Forest (Biogradska Gora-Montenegro). Turk. J. Agric. For. 2020, 44, 428–438. [Google Scholar] [CrossRef]
- Bouriaud, L.; Bouriaud, O.; Elkin, C.; Temperli, C.; Reyer, C.; Duduman, G.; Barnoaiea, I.; Nichiforel, L.; Zimmermann, N.; Bugmann, H. Age-Class Disequilibrium as an Opportunity for Adaptive Forest Management in the Carpathian Mountains, Romania. Reg. Environ. Chang. 2015, 15, 1557–1568. [Google Scholar] [CrossRef]
- Pretzsch, H.; Hilmers, T.; Uhl, E.; Bielak, K.; Bosela, M.; del Rio, M.; Dobor, L.; Forrester, D.I.; Nagel, T.A.; Pach, M.; et al. European Beech Stem Diameter Grows Better in Mixed than in Mono-Specific Stands at the Edge of Its Distribution in Mountain Forests. Eur. J. For. Res. 2020. [Google Scholar] [CrossRef]
- Piovesan, G.; Biondi, F.; Filippo, A.D.; Alessandrini, A.; Maugeri, M. Drought-Driven Growth Reduction in Old Beech (Fagus Sylvatica L.) Forests of the Central Apennines, Italy. Glob. Chang. Biol. 2008, 14, 1265–1281. [Google Scholar] [CrossRef]
- Schwarz, J.A.; Bauhus, J. Benefits of Mixtures on Growth Performance of Silver Fir (Abies Alba) and European Beech (Fagus Sylvatica) Increase with Tree Size Without Reducing Drought Tolerance. Front. For. Glob. Chang. 2019, 2, 79. [Google Scholar] [CrossRef]
- Scheller, R.M.; Domingo, J.B.; Sturtevant, B.R.; Williams, J.S.; Rudy, A.; Gustafson, E.J.; Mladenoff, D.J. Design, Development, and Application of LANDIS-II, a Spatial Landscape Simulation Model with Flexible Temporal and Spatial Resolution. Ecol. Model. 2007, 201, 409–419. [Google Scholar] [CrossRef]
- Aber, J.D.; Ollinger, S.V.; Federer, C.A.; Reich, P.B.; Goulden, M.L.; Sicklighter, D.W.; Melillo, J.M.; Lathrop, R.G. Predicting the Effects of Climate Change on Water Yield and Forest Production in the Northeastern United States. Clim. Res. 1995, 5, 207–222. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Sturtevant, B.R.; de Bruijn, A.M.G.; Lichti, N.; Jacobs, D.F.; Kashian, D.M.; Miranda, B.R.; Townsend, P.A. Forecasting Effects of Tree Species Reintroduction Strategies on Carbon Stocks in a Future without Historical Analog. Glob. Chang. Biol. 2018, 24, 5500–5517. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.M. Pattern Recognition and Machine Learning; Information Science and Statistics; Springer: New York, NY, USA, 2006; ISBN 978-0-387-31073-2. [Google Scholar]
- Scrucca, L. On Some Extensions to GA Package: Hybrid Optimisation, Parallelisation and Islands Evolution. R J. 2017, 9, 187–206. [Google Scholar] [CrossRef] [Green Version]
- Scrucca, L. GA: A Package for Genetic Algorithms in R. J. Stat. Softw. 2013, 53, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK; New York, NY, USA, 1976; ISBN 978-0-12-268450-0. [Google Scholar]
- Briffa, K.R.; Jones, P.D.; Wigley, T.M.; Pilcher, J.R.; Baillie, M.G.L. Climate Reconstruction from Tree Rings: Part 1, Basic Methodology and Preliminary Results for England. J. Climatol. 1983, 3, 233–242. [Google Scholar] [CrossRef]
- Cook, E.R.; Johnson, A.H.; Blasing, T.J. Forest Decline: Modeling the Effect of Climate in Tree Rings12. Tree Physiol. 1987, 3, 27–40. [Google Scholar] [CrossRef]
- Pauling, A.; Luterbacher, J.; Casty, C.; Wanner, H. Five Hundred Years of Gridded High-Resolution Precipitation Reconstructions over Europe and the Connection to Large-Scale Circulation. Clim. Dyn. 2006, 26, 387–405. [Google Scholar] [CrossRef] [Green Version]
- Popa, I. Fundamente Metodologice Şi Aplicaţii de Dendrocronologie; Editura Tehnicǎ Silvicǎ, 2004. [Google Scholar]
- Hasenauer, H. Dimensional Relationships of Open-Grown Trees in Austria. For. Ecol. Manag. 1997, 96, 197–206. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Chaskovskyy, O.; Knorn, J.; Radeloff, V.C.; Kruhlov, I.; Keeton, W.S.; Hostert, P. Forest Cover Change and Illegal Logging in the Ukrainian Carpathians in the Transition Period from 1988 to 2007. Remote Sens. Environ. 2009, 113, 1194–1207. [Google Scholar] [CrossRef]
- Sidor, C.G.; Popa, I.; Vlad, R.; Roibu, C.C. Rețeaua Națională de Serii Dendrocronologice Pentru Pinul Silvestru (Pinus Sylvestris) Din România-PIDECRO; Editura Silvica: Voluntari, Romania, 2020; ISBN 978-606-8020-73-0. [Google Scholar]
- ICAS. Amenajamentul. O.S. Penteleu; Regia Naţională a pădurilor Romsilva; Institutul de Cercetări şi amenajări Silvice: Bucureşti, Romania, 2012. [Google Scholar]
- INCDS. Amenajamentul. O.S. Penteleu; Regia Naţională a pădurilor Romsilva; Institutul de Cercetări şi amenajări Silvice: Bucureşti, Romania, 2021. [Google Scholar]
- Cristea, V.C. Dendrometrics and Auxological Identification and Characterization of Forests with Natural and Semi-Natural Structure from Penteleu Massif, Transilvania. Ph.D. Thesis, University of Brașov, Brașov, Romania, 2019. [Google Scholar]
- Van Oldenborgh, G.J.; Drijfhout, S.; van Ulden, A.; Haarsma, R.; Sterl, A.; Severijns, C.; Hazeleger, W.; Dijkstra, H. Western Europe Is Warming Much Faster than Expected. Clim. Past 2009, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Luterbacher, J. European Seasonal and Annual Temperature Variability, Trends, and Extremes since 1500. Science 2004, 303, 1499–1503. [Google Scholar] [CrossRef] [Green Version]
- Zorita, E.; von Storch, H. The Analog Method as a Simple Statistical Downscaling Technique: Comparison with More Complicated Methods. J. Clim. 1999, 12, 2474–2489. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Giurgiu, V.; Decei, I.; Drăghiciu, D. Metode Şi Tabele Dendrometrice [Methods and Dendrometric Tables]; Ceres Publishing House, Bucharest: Bucharest, Romania, 2004; ISBN 973-40-0639-8. [Google Scholar]
- Larson, L.A. Online User Manual for Cybis CooRecorder and CDendro Programs. Available online: http://www.cybis.se/forfun/dendro/ (accessed on 1 July 2020).
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 3, 69–78. [Google Scholar]
- Cook, B.D.; Krusic, P.J. ARSTAN v. 4.1d: A Tree-Ring Standardization Program Based on Detrending and Autoregressive Time Series Modeling, with Interactive Graphics; Tree-Ring Laboratory, Lamont-Doherty Earth Observatory of Columbia University: Palisades, NY, USA, 2005. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bunn, A.G. A Dendrochronology Program Library in R (DplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R Package for the Numerical Calibration of Proxy-Climate Relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Whitcher, B. Waveslim: Basic Wavelet Routines for One-, Two-, and Three-Dimensional Signal Processing; R Package Version 1.8.2. Available online: https://cran.r-project.org/web/packages/waveslim/ (accessed on 1 January 2021).
- Gouhier, T.C.; Grinsted, A.; Simko, V. R Package Biwavelet: Conduct Univariate and Bivariate Wavelet Analyses. Available online: https://cran.r-project.org/web/packages/biwavelet/ (accessed on 1 January 2021).
- Duveneck, M.J.; Scheller, R.M.; White, M.A. Effects of Alternative Forest Management on Biomass and Species Diversity in the Face of Climate Change in the Northern Great Lakes Region (USA). Can. J. For. Res. 2014, 44, 700–710. [Google Scholar] [CrossRef]
- Scheller, R.M.; Mladenoff, D.J. A Forest Growth and Biomass Module for a Landscape Simulation Model, LANDIS: Design, Validation, and Application. Model. Disturb. Succession For. Landsc. Using LANDIS 2004, 180, 211–229. [Google Scholar] [CrossRef]
- Gustafson, E.J.; De Bruijn, A.M.G.; Pangle, R.E.; Limousin, J.-M.; McDowell, N.G.; Pockman, W.T.; Sturtevant, B.R.; Muss, J.D.; Kubiske, M.E. Integrating Ecophysiology and Forest Landscape Models to Improve Projections of Drought Effects under Climate Change. Glob. Chang. Biol. 2015, 21, 843–856. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Shifley, S.R.; Mladenoff, D.J.; Nimerfro, K.K.; He, H.S. Spatial Simulation of Forest Succession and Timber Harvesting Using LANDIS. Can. J. For. Res. 2000, 30, 32–43. [Google Scholar] [CrossRef]
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY–A Global Database of Plant Traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Vittoz, P.; Engler, R. Seed Dispersal Distances: A Typology Based on Dispersal Modes and Plant Traits. Bot. Helv. 2007, 117, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Giurgiu, V.; Draghiciu, D. Modele Matematico-Auxologice Si Tabele de Productie Pentru Arborete [Mathematic-Auxologic Models and Yield Tables for Forest Stands]; Ceres Publishing House: Bucharest, Romania, 2004; ISBN 973-40-0637-1. [Google Scholar]
- Tanase, M.A.; Villard, L.; Pitar, D.; Apostol, B.; Petrila, M.; Chivulescu, S.; Leca, S.; Borlaf-Mena, I.; Pascu, I.-S.; Dobre, A.-C.; et al. Synthetic Aperture Radar Sensitivity to Forest Changes: A Simulations-Based Study for the Romanian Forests. Sci. Total Environ. 2019, 689, 1104–1114. [Google Scholar] [CrossRef]
- Schmitt, L.M. Theory of Genetic Algorithms. Theor. Comput. Sci. 2001, 259, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Bivand, R.; Rundel, C. Rgeos: Interface to Geometry Engine-Open Source (‘GEOS’). Available online: https://cran.r-project.org/web/packages/rgeos/ (accessed on 1 January 2021).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bivand, R.; Keitt, T.; Rowlingson, B. Rgdal: Bindings for the “Geospatial” Data Abstraction Library. Available online: https://cran.r-project.org/web/packages/rgdal/ (accessed on 1 January 2021).
- Bivand, R.; Lewin-Koh, N. Maptools: Tools for Reading and Handling Spatial Objects. Available online: https://cran.r-project.org/web/packages/maptools/ (accessed on 1 January 2021).
- White, T.; van der Ende, J.; Nichols, T.E. Beyond Bonferroni Revisited: Concerns over Inflated False Positive Research Findings in the Fields of Conservation Genetics, Biology, and Medicine. Conserv. Genet. 2019, 20, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. Available online: https://cran.r-project.org/web/packages/nlme/ (accessed on 1 January 2021).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, H.; Dieler, J. The Dependency of the Size-Growth Relationship of Norway Spruce (Picea Abies [L.] Karst.) and European Beech (Fagus Sylvatica [L.]) in Forest Stands on Long-Term Site Conditions, Drought Events, and Ozone Stress. Trees 2011, 25, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Bose, A.K.; Weiskittel, A.; Wagner, R.G. Occurrence, Pattern of Change, and Factors Associated with American Beech-Dominance in Stands of the Northeastern USA Forest. For. Ecol. Manag. 2017, 392, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Čater, M.; Levanič, T. Beech and Silver Fir’s Response along the Balkan’s Latitudinal Gradient. Sci. Rep. 2019, 9, 16269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costea, C. Codrul Grădinărit; Editura Agro-silvica: Bucharest, Romania, 1962. [Google Scholar]
- Čater, M.; Kobler, A. Light Response of Fagus Sylvatica L. and Abies Alba Mill. in Different Categories of Forest Edge–Vertical Abundance in Two Silvicultural Systems. For. Ecol. Manag. 2017, 391, 417–426. [Google Scholar] [CrossRef]
- Töchterle, P.; Yang, F.; Rehschuh, S.; Rehschuh, R.; Ruehr, N.K.; Rennenberg, H.; Dannenmann, M. Hydraulic Water Redistribution by Silver Fir (Abies Alba Mill.) Occurring under Severe Soil Drought. Forests 2020, 11, 162. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Duro, J.; Manzoni, L.; Arias, I.; Casal, M.; Cruz, O.; Pesqueira Xose, M.; Munoz, A.; Alvarez, R.; Mariot, L.; Bandini, S.; et al. Hidden Costs of Modelling Post-Fire Plant Community Assembly Using Cellular Automata; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- García-Duro, J.; Cruz, O.; Casal, M.; Reyes, O. Fire as Driver of the Expansion of Paraserianthes Lophantha (Willd.) I. C. Nielsen in SW Europe. Biol. Invasions 2019, 21, 1427–1438. [Google Scholar] [CrossRef]
- García-Duro, J.; Álvarez, R.; Basanta, M.; Casal, M. Aplicación de redes bayesianas ás relacións entre especies vexetais despois de incendio forestal e a súa sensibilidade ó tamaño das unidades de mostraxe. In BIOapps2016. Encontro Galaico-Portugués de Biometría, con Aplicación ás Ciencias da Saúde, á Ecoloxía e ás Ciencias do Medio Ambiente; Ginzo, M.J., Pértega, S., Santiago Pérez, M.I., Eds.; SGAPEIO & SPE: Santiago de Compostela, Spain, 2016. [Google Scholar]
- Pausas, J.G.; Fernández-Muñoz, S. Fire Regime Changes in the Western Mediterranean Basin: From Fuel-Limited to Drought-Driven Fire Regime. Clim. Chang. 2012, 110, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Hubau, W.; Lewis, S.L.; Phillips, O.L.; Affum-Baffoe, K.; Beeckman, H.; Cuní-Sanchez, A.; Daniels, A.K.; Ewango, C.E.N.; Fauset, S.; Mukinzi, J.M.; et al. Asynchronous Carbon Sink Saturation in African and Amazonian Tropical Forests. Nature 2020, 579, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chivulescu, S.; García-Duro, J.; Pitar, D.; Leca, Ș.; Badea, O. Past and Future of Temperate Forests State under Climate Change Effects in the Romanian Southern Carpathians. Forests 2021, 12, 885. https://doi.org/10.3390/f12070885
Chivulescu S, García-Duro J, Pitar D, Leca Ș, Badea O. Past and Future of Temperate Forests State under Climate Change Effects in the Romanian Southern Carpathians. Forests. 2021; 12(7):885. https://doi.org/10.3390/f12070885
Chicago/Turabian StyleChivulescu, Serban, Juan García-Duro, Diana Pitar, Ștefan Leca, and Ovidiu Badea. 2021. "Past and Future of Temperate Forests State under Climate Change Effects in the Romanian Southern Carpathians" Forests 12, no. 7: 885. https://doi.org/10.3390/f12070885
APA StyleChivulescu, S., García-Duro, J., Pitar, D., Leca, Ș., & Badea, O. (2021). Past and Future of Temperate Forests State under Climate Change Effects in the Romanian Southern Carpathians. Forests, 12(7), 885. https://doi.org/10.3390/f12070885








