Seasonal Shifts in Cold Tolerance and the Composition of the Gut Microbiome of Dendroctonus valens LeConte Occur Concurrently
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bark Beetle Collection and Dissection
2.2. DNA Extraction, PCR Amplification, and Sequencing of Partial 16S rRNA and ITS Genes
2.3. Processing and Statistical Analysis of Sequencing Data
2.4. Determination of the Supercooling Point
2.5. Antioxidant Enzyme Activity Assays
3. Results
3.1. Richness and Diversity Analysis
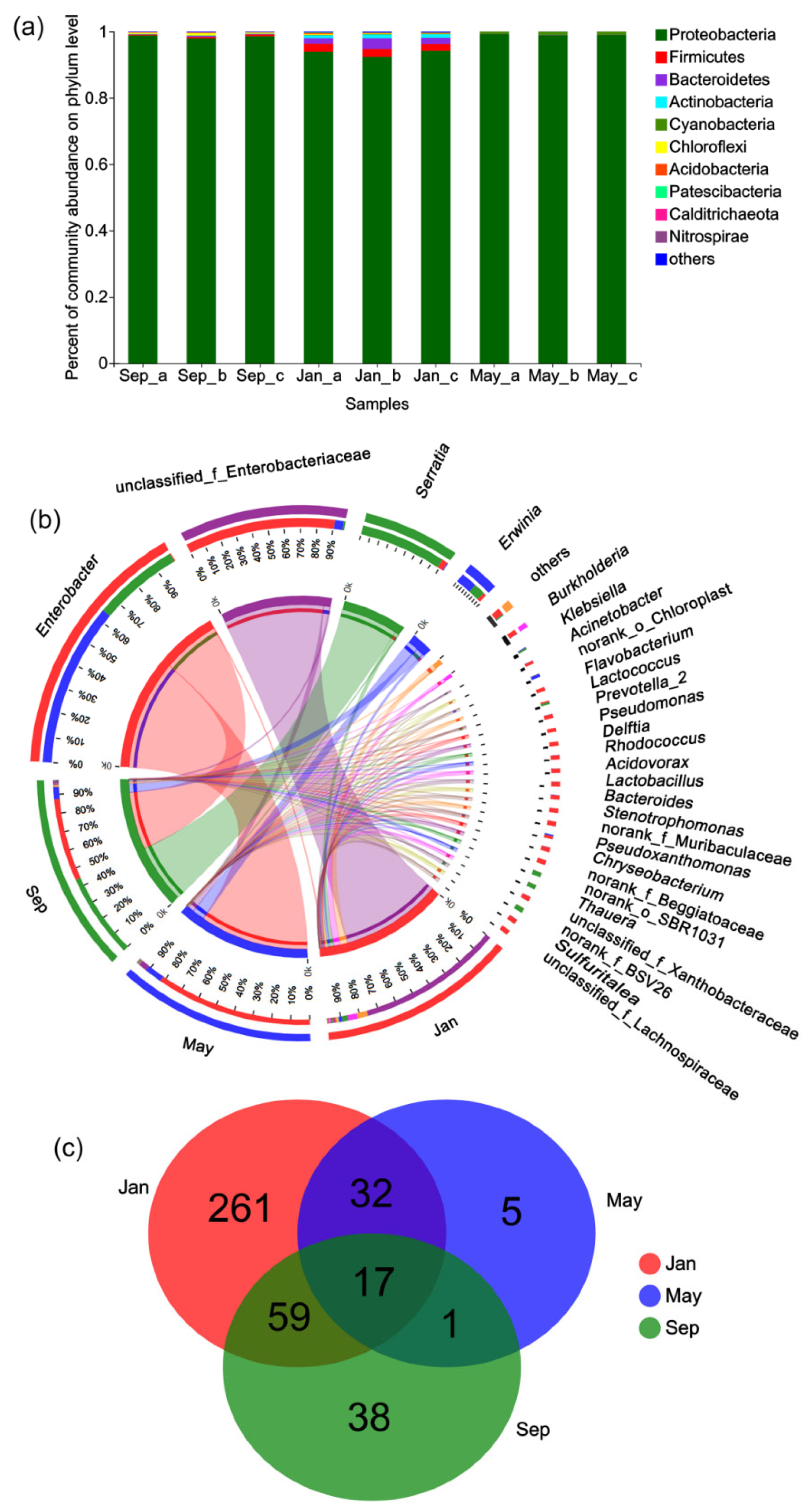
3.2. Taxonomic Profiling of the Bacterial Community
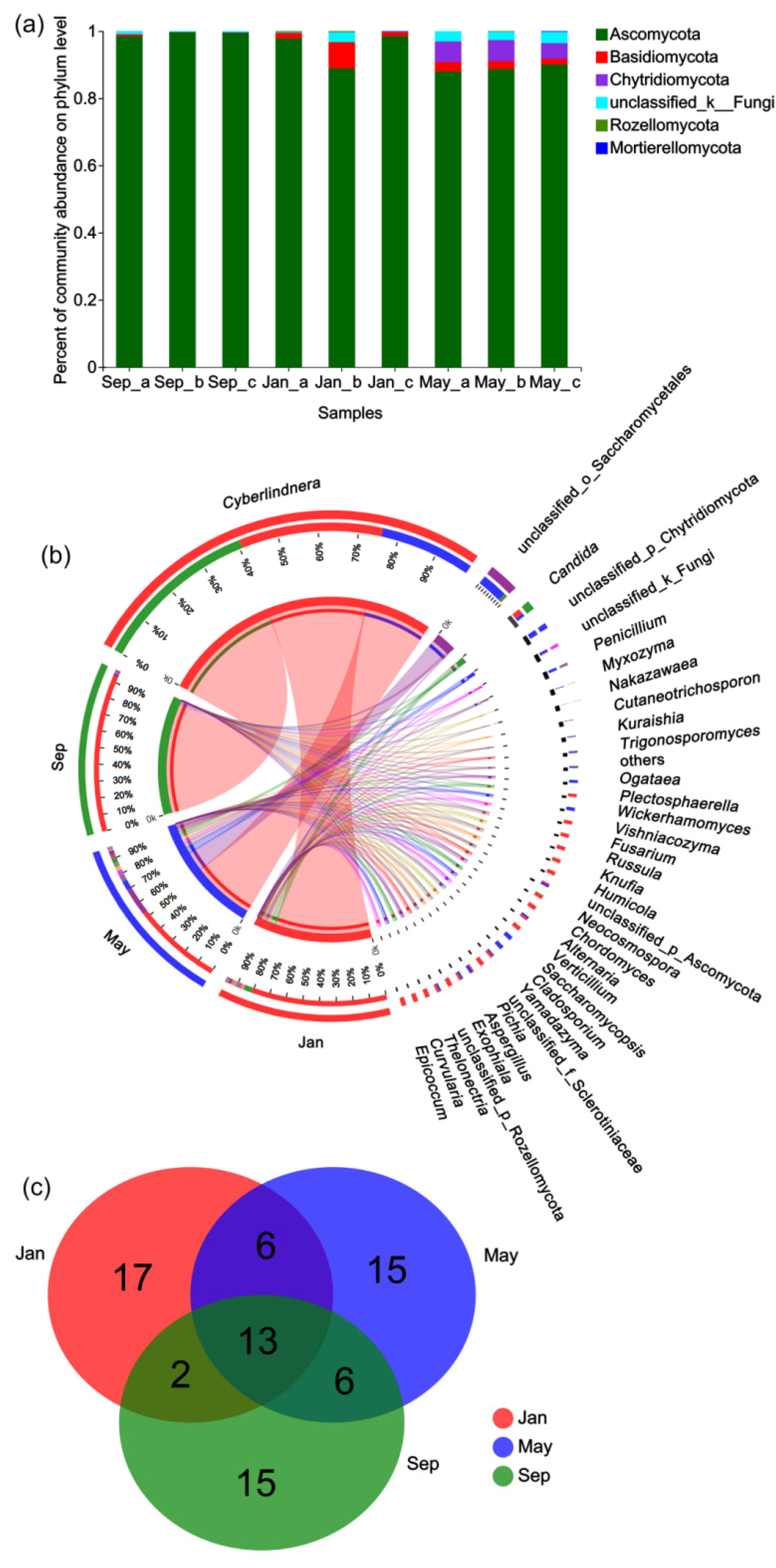
3.3. Taxonomic Profiling of the Fungal Community
3.4. Seasonal Changes in the Composition of the Gut Microbiome
3.5. Seasonal Changes in the Cold Tolerance of D. valens Larvae
3.6. Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engel, P.; Moran, N.A. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.C.; Kim, S.H.; You, H.; Kim, B.; Kim, A.C.; Lee, K.A.; Yoon, J.H.; Ryu, J.H.; Lee, W.J. Drosophila microbiome regulates modulates host development and homeostasis via insulin signaling. Science 2011, 334, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Ge, S.; Shi, F.; Pei, J.; Hou, Z.; Zong, S.; Ren, L. Gut Bacteria Associated With Monochamus saltuarius (Coleoptera: Cerambycidae) and Their Possible Roles in Host Plant Adaptations. Front. Microbiol. 2021, 12, 687211. [Google Scholar] [CrossRef]
- Williams, C.M.; Henry, H.A.; Sinclair, B.J. Cold truths: How winter drives responses of terrestrial organisms to climate change. Biol. Rev. 2015, 90, 214–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.M.; Duman, J.G. Maintenance of the supercooled state in overwintering pyrochroid beetle larvae, Dendroides canadensis: Role of hemolymph ice nucleators and antifreeze proteins. J. Comp. Physiol. B 1997, 167, 105–113. [Google Scholar] [CrossRef]
- Ferguson, L.V.; Sinclair, B.J. Insect immunity varies idiosyncratically during overwintering. J. Exp. Zool. Part A 2017, 327, 222–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denlinger, D.L.; Lee, R.E. Low Temperature Biology of Insects; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Carey, H.V.; Duddleston, K.N. Animal-microbial symbioses in changing environments. J. Therm. Biol. 2014, 44, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, H.; Tang, M. Community structure of gut bacteria of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae) larvae during overwintering stage. Sci. Rep. 2017, 7, 14242. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.V.; Dhakal, P.; Lebenzon, J.E.; Heinrichs, D.E.; Bucking, C.; Sinclair, B.J. Seasonal shifts in the insect gut microbiome are concurrent with changes in cold tolerance and immunity. Funct. Ecol. 2018, 32, 2357–2368. [Google Scholar] [CrossRef]
- Owen, D.R.; Smith, S.L.; Seybold, S.J. Red turpentine beetle. Forest Insect & Disease Leaflet; US Department of Agriculture, Forest Service: Washington, DC, USA, 2010; Volume 55, pp. 1–8. [Google Scholar]
- Sun, J.; Lu, M.; Gillette, N.E.; Wingfield, M.J. Red turpentine beetle: Innocuous native becomes invasive tree killer in China. Annu. Rev. Entomol. 2013, 58, 293–311. [Google Scholar] [CrossRef] [Green Version]
- Lou, Q.Z.; Lu, M.; Sun, J.H. Yeast diversity associated with invasive Dendroctonus valens killing Pinus tabuliformis in China using cultural and molecular methods. Microb. Ecol. 2014, 68, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.W.; Chou, W.M.; Huo, F.Y.; Wang, X.L.; Fang, J.X.; Zhao, M.M. Biology of Dendroctonus valens in Shanxi Province. Shanxi For. Sci. Technol. 2001, 23, 34–37. (In Chinese) [Google Scholar]
- Zhan, Z.; Yu, L.; Li, Z.; Ren, L.; Gao, B.; Wang, L.; Luo, Y. Combining GF-2 and Sentinel-2 images to detect tree mortality caused by red turpentine beetle during the early outbreak stage in north China. Forests 2020, 11, 172. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Zhao, D.; Xu, Y.; Shi, F.; Zong, S.; Tao, J. Reference gene selection for expression analyses by qRT-PCR in Dendroctonus valens. Insects 2020, 11, 328. [Google Scholar] [CrossRef]
- Zhao, D.; Zheng, C.; Shi, F.; Xu, Y.; Zong, S.; Tao, J. Expression analysis of genes related to cold tolerance in Dendroctonus valens. PeerJ 2021, 9, e10864. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, J.H.; Don, O.; Zhang, Z. The red turpentine beetle, Dendroctonus valens LeConte (Scolytidae): An exotic invasive pest of pine in China. Biodivers. Conserv. 2005, 14, 1735–1760. [Google Scholar] [CrossRef]
- Xu, L.T.; Lu, M.; Sun, J.H. Invasive bark beetle-associated microbes degrade a host defensive monoterpene. Insect Sci. 2016, 23, 183–190. [Google Scholar] [CrossRef]
- He, S.Y.; Ge, X.Z.; Wang, T.; Wen, J.B.; Zong, S.X. Areas of potential suitability and survival of Dendroctonus valens in China under extreme climate warming scenario. Bull. Entomol. Res. 2015, 105, 477–484. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, X.D.; de Beer, Z.W.; Wingfield, M.J.; Sun, J.H. Ophiostomatoid fungi associated with the invasive pine-infesting bark beetle, Dendroctonus valens, in China. Fungal Divers. 2009, 38, 133–145. [Google Scholar]
- Liu, F.; Wickham, J.D.; Cao, Q.; Lu, M.; Sun, J. An invasive beetle-fungus complex is maintained by fungal nutritional-compensation mediated by bacterial volatiles. ISME J. 2020, 14, 2829–2842. [Google Scholar] [CrossRef] [PubMed]
- Marincowitz, S.; Duong, T.A.; Taerum, S.J.; de Beer, Z.W.; Wingfield, M.J. Fungal associates of an invasive Pine—Infesting bark beetle, Dendroctonus valens, including seven new Ophiostomatalean fungi. Persoonia 2020, 45, 177–195. [Google Scholar] [CrossRef]
- Cannon, R.J.C.; Block, W. Cold tolerance of microarthropods. Biol. Rev. 1988, 63, 23–77. [Google Scholar] [CrossRef]
- Lee, R. Principles of insect low temperature tolerance. In Insects at Low Temperature; Lee, R., Denlinger, D., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 17–46. [Google Scholar]
- Lee, R.E.; Lee, M.R.; Strong-Gunderson, J.M. Insect cold-hardiness and ice nucleating active microorganisms including their potential use for biological control. J. Insect Physiol. 1993, 39, 1–12. [Google Scholar] [CrossRef]
- Dong, Y.; Pei, J.; Shao, Y.; Zong, S.; Hou, Z. Cold tolerance and cold tolerant substances of larva and adult of Dendroctonus valens LeConte (Coleoptera). J. Environ. Entomol. 2021, (in press). [Google Scholar]
- Lee, R.E.; Strong-Gunderson, J.M.; Lee, M.R.; Grove, K.S.; Riga, T.J. Isolation of ice nucleating active bacteria from insects. J. Exp. Zool. 1991, 257, 124–127. [Google Scholar] [CrossRef]
- Tsumuki, H.; Konno, H.; Maeda, T.; Okamoto, Y. An ice-nucleating active fungus isolated from the gut of the rice stem borer, Chilo suppressalis Walker (Lepidoptera: Pyralidae). J. Insect Physiol. 1992, 38, 119–125. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Pardini, R.S. Mechanisms for regulating oxygen toxicity in phytophagous insects. Free Radic. Biol. Med. 1990, 8, 401–413. [Google Scholar] [CrossRef]
- Barbehenn, R.V. Gut-based antioxidant enzymes in a polyphagous and a graminivorous grasshopper. J. Chem. Ecol. 2002, 28, 1329–1347. [Google Scholar] [CrossRef]
- Hou, Z.; Wei, C. De novo comparative transcriptome analysis of a rare cicada, with identification of candidate genes related to adaptation to a novel host plant and drier habitats. BMC Genom. 2019, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Shi, F.; Ge, S.; Tao, J.; Ren, L.; Wu, H.; Zong, S. Comparative transcriptome analysis of the newly discovered insect vector of the pine wood nematode in China, revealing putative genes related to host plant adaptation. BMC Genom. 2021, 22, 189. [Google Scholar] [CrossRef]
- Lai, Y.P.; Tao, K.; Hou, T.P. Preliminary analysis of geographical distribution based on cold hardiness for Evergestis extimalis (Scopoli) (Lepidoptera:Pyralidae) on Qinghai-Tibet Plateau. Entomol. Res. 2019, 49, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Grubor-Lajsic, G.; Block, W.; Telesmanic, M.; Javanovic, A.; Stevanovic, D.; Baca, F. Effect of cold acclimation on the antioxidant defense system of two larval Lepidoptera (Noctuidae). Arch. Insect Biochem. Physiol. 1997, 36, 1–10. [Google Scholar] [CrossRef]
- Morales-Jiménez, J.; Zúñiga, G.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Bacterial community and nitrogen fixation in the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Curculionidae: Scolytinae). Microb. Ecol. 2009, 58, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.S.; Adams, S.M.; Currie, C.R.; Gillette, N.E.; Raffa, K.F. Geographic variation in bacterial communities associated with the red turpentine beetle (Coleoptera: Curculionidae). Environ. Entomol. 2010, 39, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Dohet, L.; Grégoire, J.C.; Berasategui, A.; Kaltenpoth, M.; Biedermann, P.H.W. Bacterial and fungal symbionts of parasitic Dendroctonus bark beetles. FEMS Microbiol. Ecol. 2016, 92, fiw129. [Google Scholar] [CrossRef] [Green Version]
- Hernández-García, J.A.; Briones-Roblero, C.I.; Rivera-Orduña, F.N.; Zúñiga, G. Revealing the gut bacteriome of Dendroctonus bark beetles (Curculionidae: Scolytinae): Diversity, core members and co-evolutionary patterns. Sci. Rep. 2017, 7, 13864. [Google Scholar] [CrossRef]
- Cao, Q.; Wickham, J.D.; Chen, L.; Ahmad, F.; Lu, M.; Sun, J. Effect of oxygen on verbenone conversion from cis-verbenol by gut facultative anaerobes of Dendroctonus valens. Front. Microbiol. 2018, 9, 464. [Google Scholar] [CrossRef]
- Hernández-García, J.A.; Gonzalez-Escobedo, R.; Briones-Roblero, C.I.; Cano-Ramírez, C.; Rivera-Orduña, F.N.; Zúñiga, G. Gut bacterial communities of Dendroctonus valens and D. mexicanus (Curculionidae: Scolytinae): A metagenomic analysis across different geographical locations in Mexico. Int. J. Mol. Sci. 2018, 19, 2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Xu, L.; Zhou, F.; Wang, B.; Wang, S.; Lu, M.; Sun, J. Gut bacterial communities of Dendroctonus valens and monoterpenes and carbohydrates of Pinus tabuliformis at different attack densities to host pines. Front. Microbiol. 2018, 9, 1251. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Yu, Z.J.; Wang, D.; Bronislava, V.; Branislav, P.; Liu, J.Z. The bacterial microbiome of field-collected Dermacentor marginatus and Dermacentor reticulatus from Slovakia. Parasit. Vectors 2019, 12, 325. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, C.; Ahmad, F.; Yin, X.; Hu, Y.; Mo, J. Exploring the effect of plant substrates on bacterial community structure in termite fungus-combs. PLoS ONE 2020, 15, e0232329. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for Basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [Green Version]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A place for DNA–DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.G.; Storey, J.D. subSeq: Determining appropriate sequencing depth through efficient read subsampling. Bioinformatics 2014, 30, 3424–3426. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Feng, Y.; Tursun, R.; Xu, Z.; Ouyang, F.; Zong, S. Effect of three species of host tree on the cold hardiness of overwintering larvae of Anoplophora glabripennis (Coleoptera: Cerambycidae). Eur. J. Entomol. 2016, 113, 212–216. [Google Scholar] [CrossRef] [Green Version]
- McCord, J.M.; Fridovich, I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet B and ozone induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant. Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Meth. Enzymol. 1955, 2, 764–775. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lysenko, O. Non-sporeforming bacteria pathogenic to insects: Incidence and mechanisms. Annu. Rev. Microbiol. 1985, 39, 673–695. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, J.; Yoshida, T.; Owada, T.; Kita, K.; Tanno, K. Erwiniu herbicola: Ice nucleation active bacteria isolated from diamondback moth, Plutella xylostella L. pupae. Jpn. J. Appl. Entomol. Z. 1991, 35, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Sato, M. Gut colonization by an ice nucleation active bacterium, Erwinia (Pantoea) ananas reduces the cold hardiness of mulberry pyralid larvae. Cryobiology 1999, 38, 281–289. [Google Scholar] [CrossRef]
- Takahashi, K.; Watanabe, K.; Sato, M. Survival and characteristics of ice nucleation-active bacteria on Mulberry trees (Morus spp.) and in Mulberry pyralid (Glyphodes pyloalis). Ann. Phytopathol. Soc. Jpn. 1995, 61, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Strong-Gunderson, J.M.; Lee, R.E.; Lee, M.R.; Riga, T.J. Ingestion of ice nucleating active bacteria increases the supercooling point of the lady beetle Hippodamia convergens. J. Insect Physiol. 1990, 36, 153–157. [Google Scholar] [CrossRef]
- Tang, C.; Sun, F.; Zhang, X.; Zhao, T.; Qi, J. Transgenic ice nucleation-active Enterobacter cloacae reduces cold hardiness of corn borer and cotton bollworm larvae. FEMS Microbiol. Ecol. 2004, 51, 79–86. [Google Scholar] [CrossRef]
- Rider, M.H.; Hussain, N.; Dilworth, S.M.; Storey, J.M.; Storey, K.B. AMP-activated protein kinase and metabolic regulation in cold-hardy insects. J. Insect Physiol. 2011, 57, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, B.J.; Ferguson, L.V.; Salehipour-shirazi, G.; MacMillan, H.A. Cross-tolerance and cross-talk in the cold: Relating low temperatures to desiccation and immune stress in insects. Integr. Comp. Biol. 2013, 53, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.M.; Teakle, D.S.; Macrae, I.C. Kinetics of colonization of adult Queensland fruit-flies Bactrocera tryoni by dinitrogen-fixing alimentary-tract bacteria. Appl. Environ. Microbiol. 1994, 60, 2508–2517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauzon, C.R.; Bussert, T.G.; Sjogren, R.E.; Prokopy, R.J. Serratia marcescens as a bacterial pathogen of Rhagoletis pomonella flies (Diptera: Tephritidae). Eur. J. Entomol. 2003, 100, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Ohkuma, M.; Noda, S.; Kudo, T. Phylogenetic diversity of nitrogen fixation genes in the symbiotic microbial community in the gut of diverse termites. Appl. Environ. Microbiol. 1999, 65, 4926–4934. [Google Scholar] [CrossRef] [Green Version]
- Dillon, R.J.; Vennard, C.T.; Charnley, A.K. A Note: Gut bacteria produce components of a locust cohesion pheromone. J. Appl. Microbiol. 2002, 92, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, A.; Crotti, E.; Borruso, L.; Jucker, C.; Lupi, D.; Colombo, M.; Daffonchio, D. Characterization of the bacterial community associated with larvae and adults of Anoplophora chinensis collected in Italy by culture and culture-independent methods. BioMed. Res. Int. 2013, 2013, 420287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaltenpoth, M.; Flórez, L.V. Versatile and dynamic symbioses between insects and Burkholderia bacteria. Annu. Rev. Entomol. 2020, 65, 145–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, Y.; Hosokawa, T.; Fukatsu, T. An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J. 2011, 5, 446–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flórez, L.V.; Kaltenpoth, M. Symbiont dynamics and strain diversity in the defensive mutualism between Lagria beetles and Burkholderia. Environ. Microbiol. 2017, 19, 3674–3688. [Google Scholar] [CrossRef]
- Takeshita, K.; Kikuchi, Y. Riptortus pedestris and Burkholderia symbiont: An ideal model system for Insect—Microbe symbiotic associations. Res. Microbiol. 2017, 168, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Soto-Robles, L.V.; Torres-Banda, V.; Rivera-Orduña, F.N.; Curiel-Quesada, E.; Hidalgo-Lara, M.E.; Zúñiga, G. An overview of genes from Cyberlindnera americana, a symbiont yeast isolated from the gut of the bark beetle Dendroctonus rhizophagus (Curculionidae: Scolytinae), involved in the detoxification process using genome and transcriptome data. Front. Microbiol. 2019, 10, 2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, F.N.; González, E.; Gómez, Z.; López, N.; Hernández-Rodríguez, C.; Berkov, A.; Zúñiga, G. Gut—Associated yeast in bark beetles of the genus Dendroctonus Erichson (Coleoptera: Curculionidae: Scolytinae). Biol. J. Linn. Soc. 2009, 98, 325–342. [Google Scholar] [CrossRef] [Green Version]
- Briones-Roblero, C.I.; Rodríguez-Díaz, R.; Santiago-Cruz, J.A.; Zúñiga, G.; Rivera-Orduña, F.N. Degradation capacities of bacteria and yeasts isolated from the gut of Dendroctonus rhizophagus (Curculionidae: Scolytinae). Folia. Microbiol. 2017, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S. The ecology of yeast in the bark beetle holobiont: A century of research revisited. Microb. Ecol. 2015, 69, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, F.; Briones-Roblero, C.I.; Nelson, D.R.; Rivera-Orduña, F.N.; Zúñiga, G. Cytochrome P450 complement (CYPome) of Candida oregonensis, a gut-associated yeast of bark beetle, Dendroctonus rhizophagus. Fungal Biol. 2016, 120, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Passoth, V.; Olstorpe, M.; Schnürer, J. Past, present and future research directions with Pichia anomala. Antonie Van Leeuwenhoek 2011, 99, 121–125. [Google Scholar] [CrossRef]
- Zacchi, L.; Vaughan-Martini, A. Yeasts associated with insects in agricultural areas of Perugia, Italy. Ann. Microbiol. 2002, 52, 237–244. [Google Scholar]
- Toki, W.; Tanahashi, M.; Togashi, K.; Fukatsu, T. Fungal farming in a non-social beetle. PLoS ONE 2012, 7, e41893. [Google Scholar] [CrossRef]
- Ricci, I.; Mosca, M.; Valzano, M.; Damiani, C.; Scuppa, P.; Rossi, P.; Crotti, E.; Cappelli, A.; Ulissi, U.; Capone, A.; et al. Different mosquito species host Wickerhamomyces anomalus (Pichia anomala): Perspectives on vector-borne diseases symbiotic control. Antonie Van Leeuwenhoek 2011, 99, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelli, A.; Ulissi, U.; Valzano, M.; Damiani, C.; Epis, S.; Gabrielli, M.G.; Conti, S.; Polonelli, L.; Bandi, C.; Favia, G.; et al. A Wickerhamomyces anomalus killer strain in the malaria vector Anopheles stephensi. PLoS ONE 2014, 9, e95988. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Bongiorno, G.; Giovati, L.; Montagna, M.; Crotti, E.; Damiani, C.; Gradoni, L.; Polonelli, L.; Ricci, I.; Favia, G.; et al. Isolation of a Wickerhamomyces anomalus yeast strain from the sandfly Phlebotomus perniciosus, displaying the killer phenotype. Med. Vet. Entomol. 2016, 30, 101–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Z.; Dong, Y.; Shi, F.; Xu, Y.; Ge, S.; Tao, J.; Ren, L.; Zong, S. Seasonal Shifts in Cold Tolerance and the Composition of the Gut Microbiome of Dendroctonus valens LeConte Occur Concurrently. Forests 2021, 12, 888. https://doi.org/10.3390/f12070888
Hou Z, Dong Y, Shi F, Xu Y, Ge S, Tao J, Ren L, Zong S. Seasonal Shifts in Cold Tolerance and the Composition of the Gut Microbiome of Dendroctonus valens LeConte Occur Concurrently. Forests. 2021; 12(7):888. https://doi.org/10.3390/f12070888
Chicago/Turabian StyleHou, Zehai, Yaxin Dong, Fengming Shi, Yabei Xu, Sixun Ge, Jing Tao, Lili Ren, and Shixiang Zong. 2021. "Seasonal Shifts in Cold Tolerance and the Composition of the Gut Microbiome of Dendroctonus valens LeConte Occur Concurrently" Forests 12, no. 7: 888. https://doi.org/10.3390/f12070888
APA StyleHou, Z., Dong, Y., Shi, F., Xu, Y., Ge, S., Tao, J., Ren, L., & Zong, S. (2021). Seasonal Shifts in Cold Tolerance and the Composition of the Gut Microbiome of Dendroctonus valens LeConte Occur Concurrently. Forests, 12(7), 888. https://doi.org/10.3390/f12070888






