Responses of Sap Flux Densities of Different Plant Functional Types to Environmental Variables Are Similar in Both Dry and Wet Seasons in a Subtropical Mixed Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Measurement of Sap Flux Density
2.3. Measurement of Environmental Parameters
2.4. Data and Statistical Analysis
3. Results
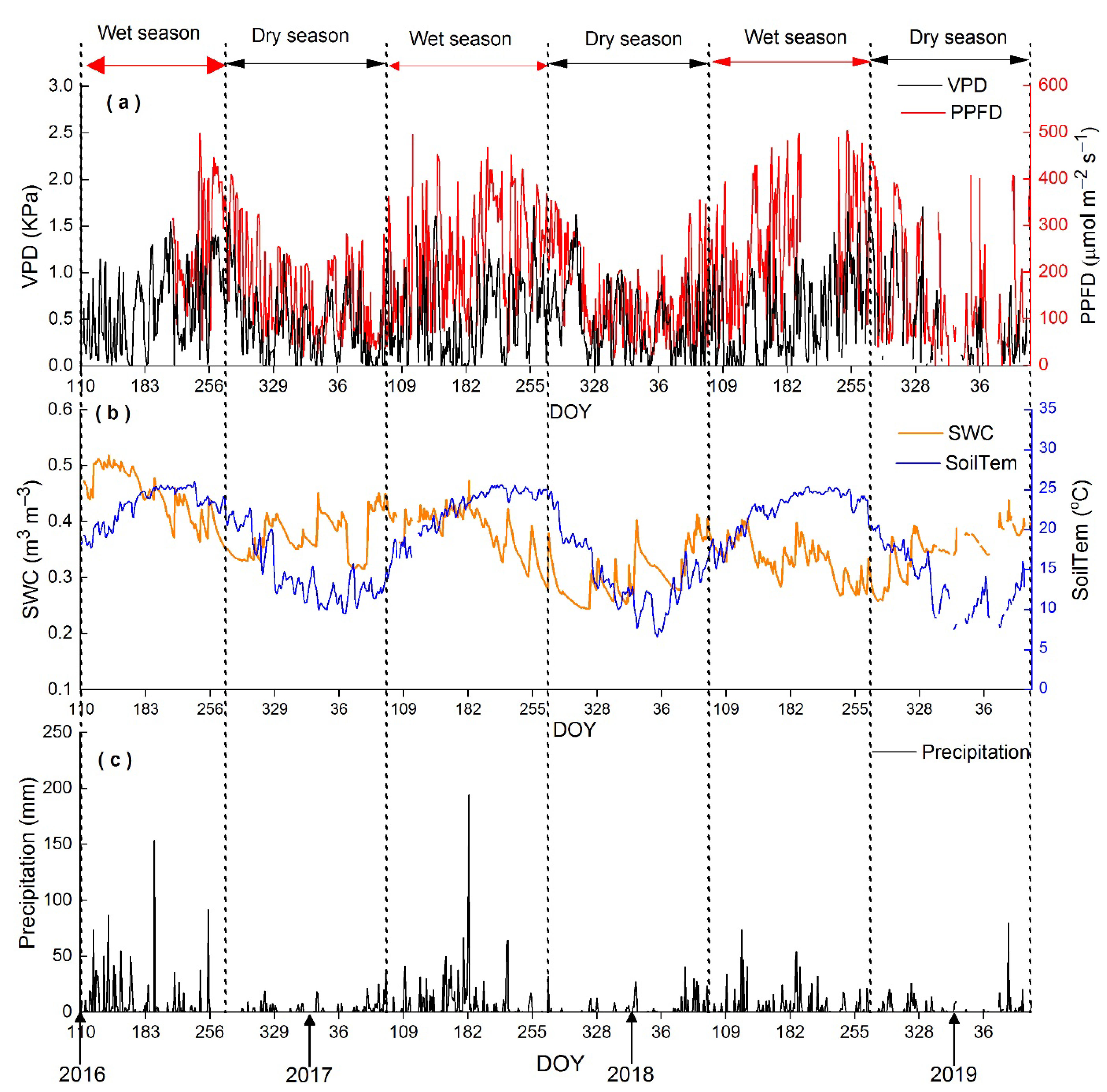
3.1. Seasonal Variations in Microclimate
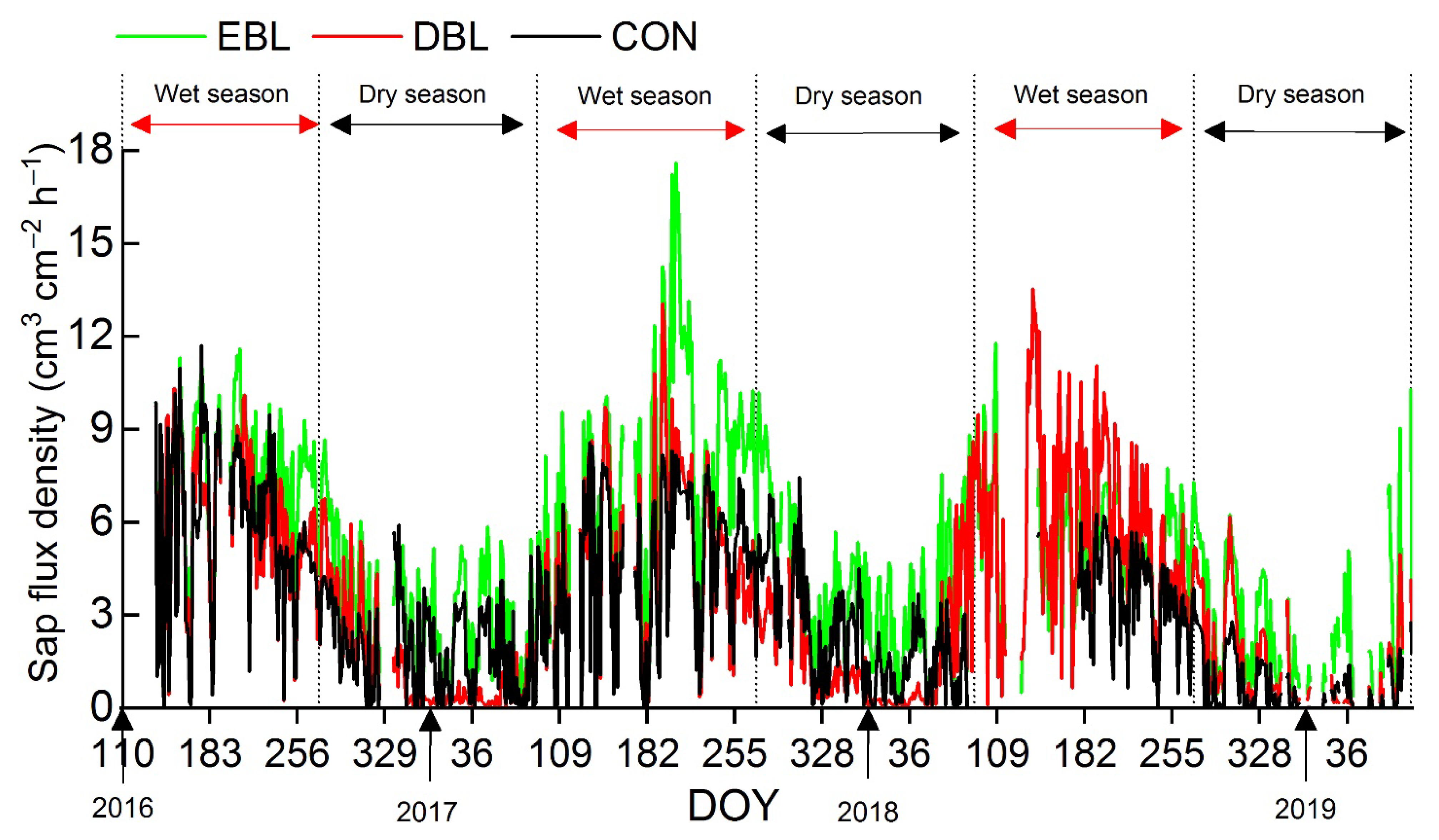
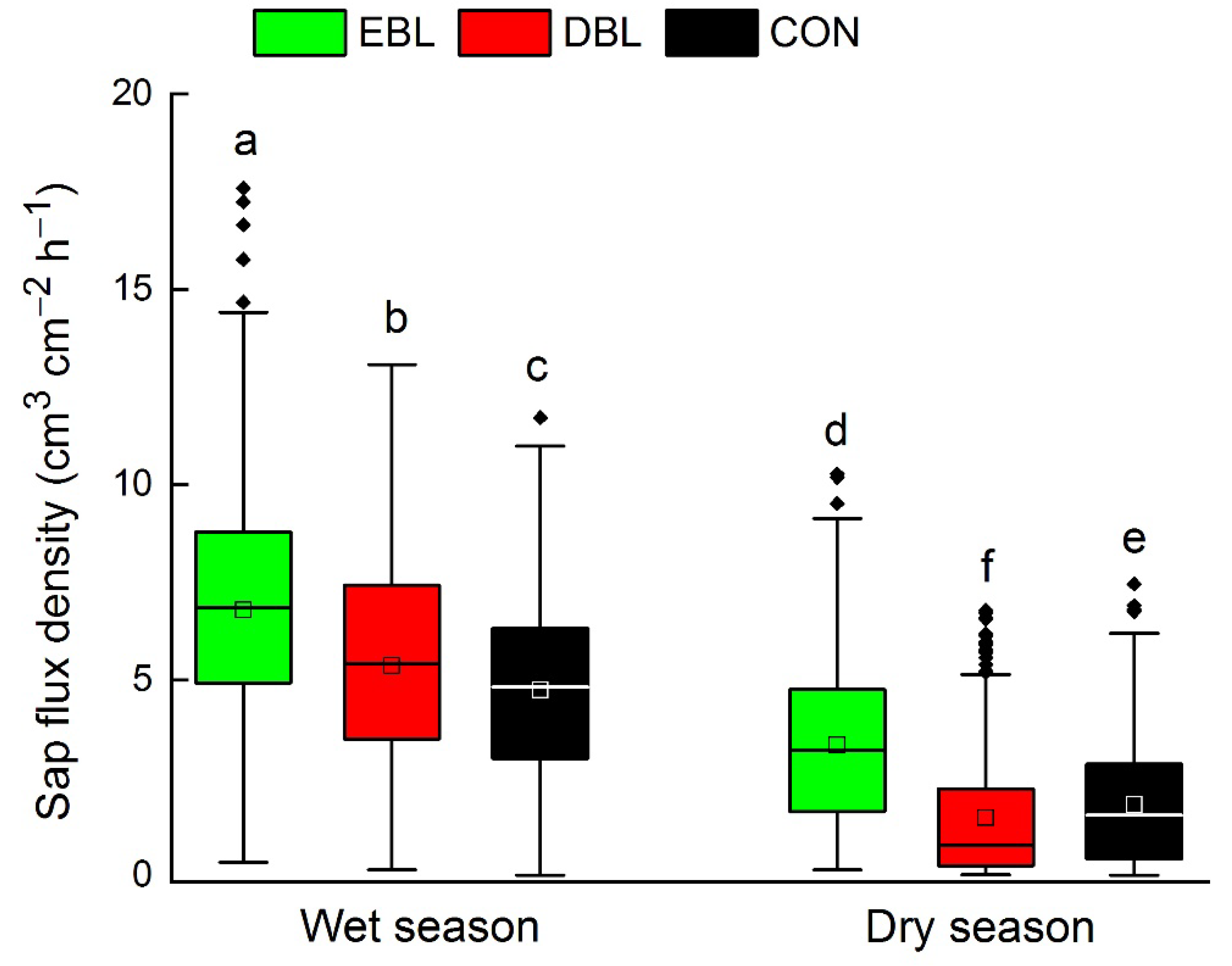
3.2. Seasonal Variations in Sap Flux Densities of Different Functional Types
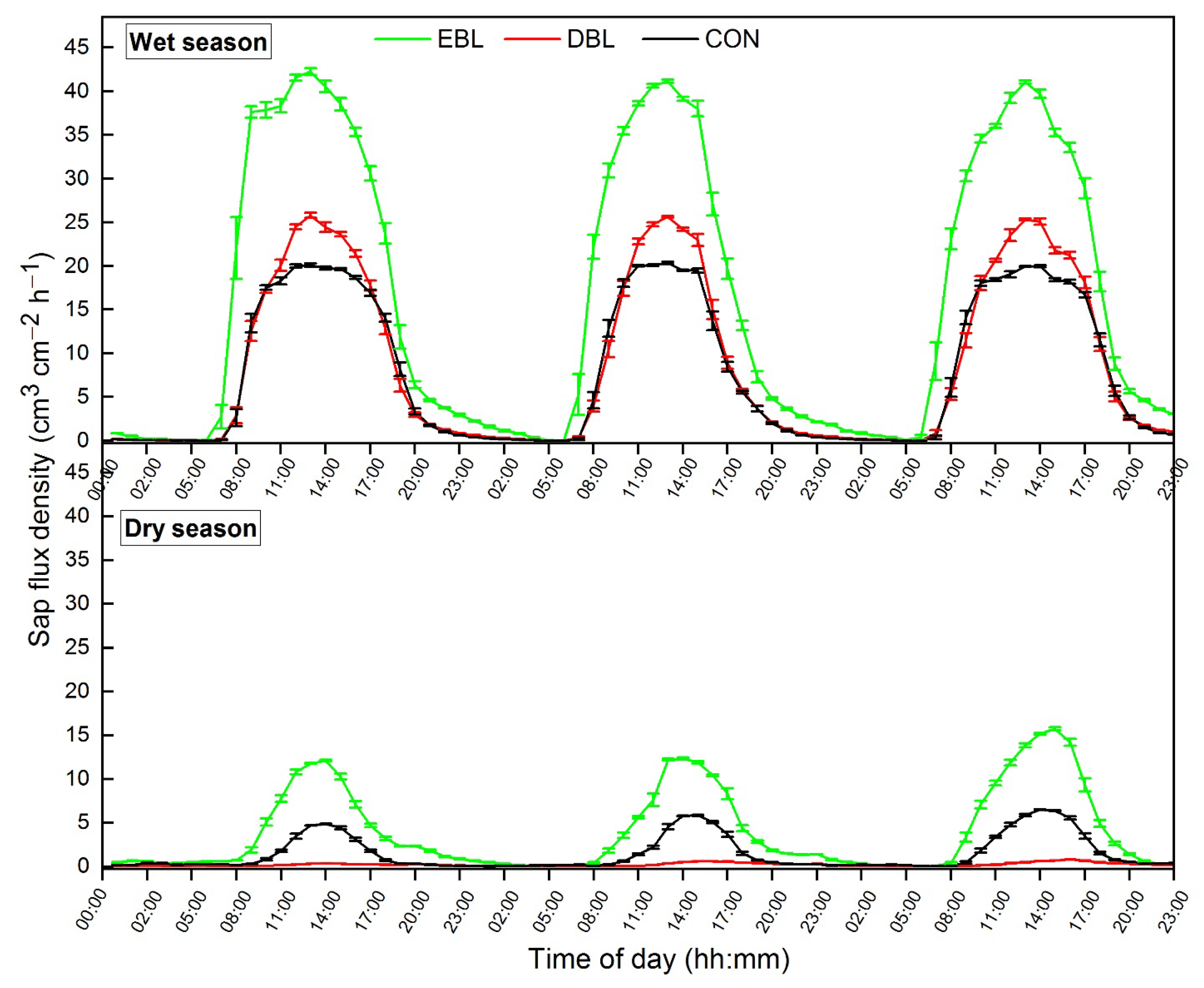
3.3. Diurnal Patterns of Sap Flux Densities of Different Plant Functional Types in Wet and Dry Seasons
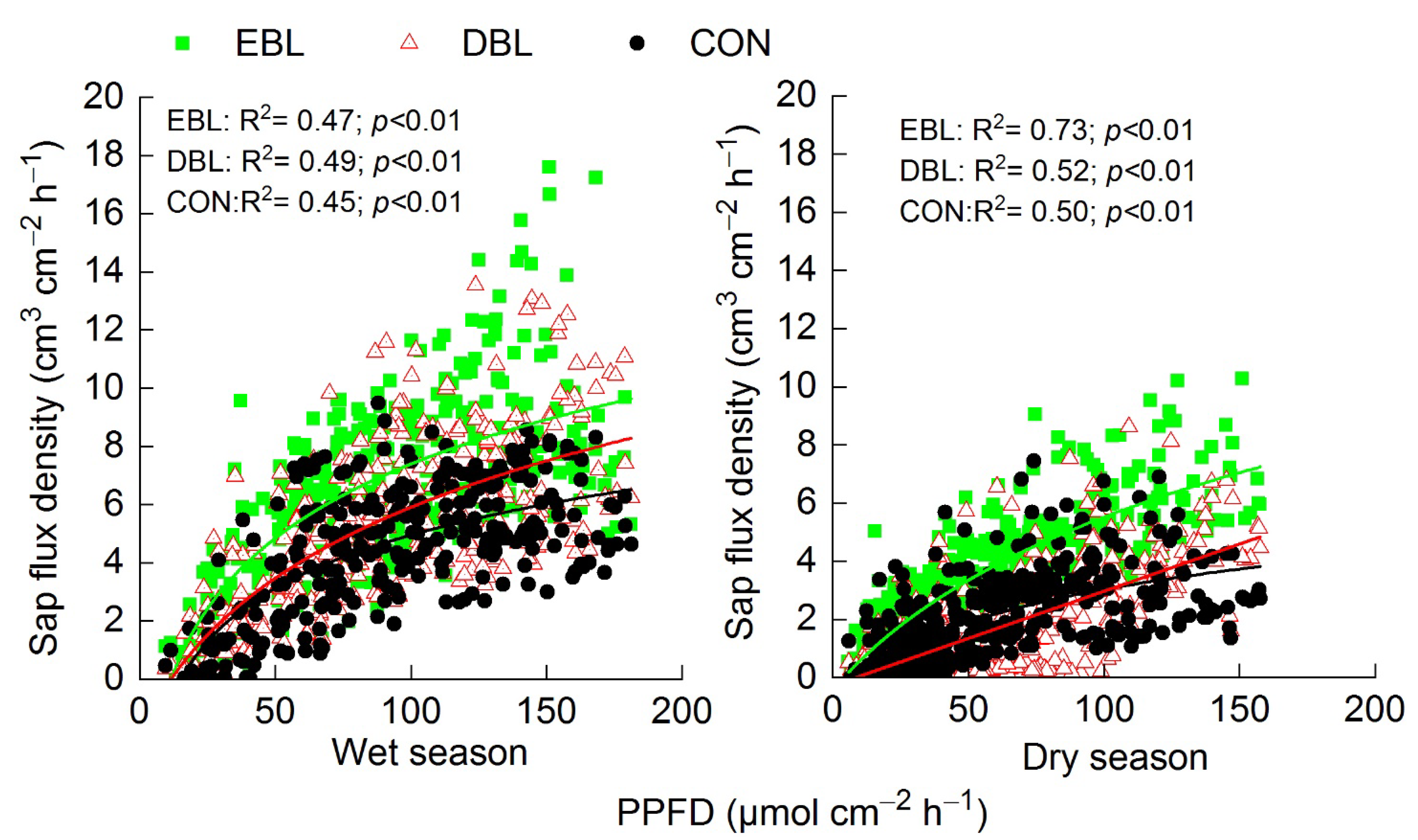
3.4. Seasonal Trends of Sap Flux Densities to Environmental Variables
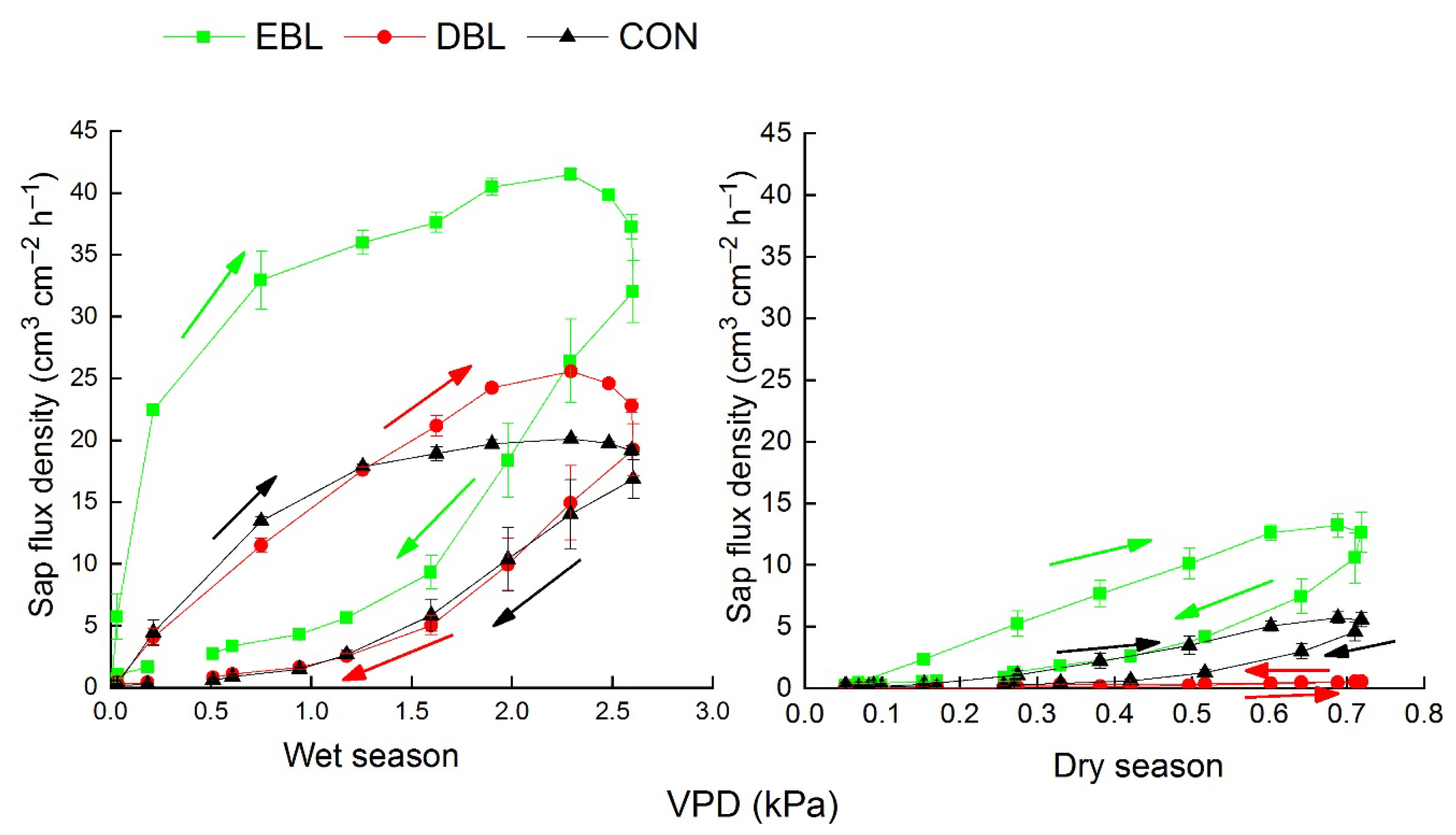
3.5. Diurnal Responses of Sap Flux Densities to Environmental Variables
4. Discussion
4.1. Seasonal Changes in Sap Flux Density
4.2. Regulation of Sap Flux Density in Subtropical Mixed Forest
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Box, E.O. Plant functional types and climate at the global scale. J. Veg. Sci. 1996, 7, 309–320. [Google Scholar] [CrossRef]
- Takahashi, K.; Okuhara, I. Comparison of climatic effects on radial growth of evergreen broad-leaved trees at their northern distribution limit and co-dominating deciduous broad-leaved trees and evergreen conifers. Ecol. Res. 2012, 27, 125–132. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M.; Zak, M.; Martínez Carretero, E.; Araníbar, J. Plant functional traits, ecosystem structure and land-use history along a climatic gradient in central-western Argentina. J. Veg. Sci. 1999, 10, 651–660. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Xu, L.; Kiang, N. How plant functional-type, weather, seasonal drought, and soil physical properties alter water and energy fluxes of an oak-grass savanna and an annual grassland. Agric. For. Meteorol. 2004, 123, 13–39. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Doh, S.; Lee, D.; Lee, D.; Jin, V.L.; Kimball, J.S. Topographic and climatic controls on soil respiration in six temperate mixed-hardwood forest slopes, Korea. Glob. Chang. Biol. 2003, 9, 1427–1437. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Xie, Z. Geographical and climatic gradients of evergreen versus deciduous broad-leaved tree species in subtropical China: Implications for the definition of the mixed forest. Ecol. Evol. 2017, 7, 3636–3644. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Song, T.; Wang, K. Relationships between woody plants and environmental factors in karst mixed evergreen-deciduous broadleaf forest, southwest China. J. Food Agric. Environ. 2012, 10, 890–896. [Google Scholar]
- Yang, H.; Guo, Z.; Chu, X.; Man, R.; Chen, J.; Liu, C.; Tao, J.; Jiang, Y. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef]
- Werden, L.K.; Waring, B.G.; Smith-Martin, C.M.; Powers, J.S. Tropical dry forest trees and lianas differ in leaf economic spectrum traits but have overlapping water-use strategies. Tree Physiol. 2018, 38, 517–530. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Fitzgerald, D.B.; Bower, L.M.; Pianka, E.R. Functional traits, convergent evolution, and periodic tables of niches. Ecol. Lett. 2015, 18, 737–751. [Google Scholar] [CrossRef]
- Cristiano, P.M.; Campanello, P.I.; Bucci, S.J.; Rodriguez, S.A.; Lezcano, O.A.; Scholz, F.G.; Madanes, N.; di Francescantonio, D.; Carrasco, L.O.; Zhang, Y.J.; et al. Evapotranspiration of subtropical forests and tree plantations: A comparative analysis at different temporal and spatial scales. Agric. For. Meteorol. 2015, 203, 96–106. [Google Scholar] [CrossRef]
- Ouyang, L.; He, W.; Huang, K.C.; Zhou, C.; Gu, D.X.; Huang, Y.Q.; Zhao, P. Seasonal water use strategy of canopy tree species and possible implication for their coexistence in a subtropical secondary forest. Ecohydrology 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Song, Q.H.; Fei, X.H.; Zhang, Y.P.; Sha, L.Q.; Liu, Y.T.; Zhou, W.J.; Wu, C.S.; Lu, Z.Y.; Luo, K.; Gao, J.B.; et al. Water use efficiency in a primary subtropical evergreen forest in Southwest China. Sci. Rep. 2017, 7, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhao, P.; Hu, Y.; Zhao, X.; Ouyang, L.; Zhu, L.; Ni, G. The sap flow-based assessment of atmospheric trace gas uptake by three forest types in subtropical China on different timescales. Environ. Sci. Pollut. Res. 2018, 25, 28431–28444. [Google Scholar] [CrossRef]
- Cristiano, P.M.; Díaz Villa, M.V.E.; de Diego, M.S.; Lacoretz, M.V.; Madanes, N.; Goldstein, G. Carbon assimilation, water consumption and water use efficiency under different land use types in subtropical ecosystems: From native forests to pine plantations. Agric. For. Meteorol. 2020, 291, 108094. [Google Scholar] [CrossRef]
- Yi, K.; Maxwell, J.T.; Wenzel, M.K.; Roman, D.T.; Sauer, P.E.; Phillips, R.P.; Novick, K.A. Linking variation in intrinsic water-use efficiency to isohydricity: A comparison at multiple spatiotemporal scales. New Phytol. 2019, 221, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, H.; Tanaka, N.; Kume, T. Do coniferous forests evaporate more water than broad-leaved forests in Japan? J. Hydrol. 2007, 336, 361–375. [Google Scholar] [CrossRef]
- Soh, W.K.; Yiotis, C.; Murray, M.; Parnell, A.; Wright, I.J.; Spicer, R.A.; Lawson, T.; Caballero, R.; McElwain, J.C. Rising CO2 drives divergence in water use efficiency of evergreen and deciduous plants. Sci. Adv. 2019, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.; Chen, H.; Wang, K.; Tan, W.; Deng, P.; Yang, J. Seasonal water use patterns of woody species growing on the continuous dolostone outcrops and nearby thin soils in subtropical China. Plant Soil 2011, 341, 399–412. [Google Scholar] [CrossRef]
- Liu, W.; Su, J.; Li, S.; Lang, X.; Huang, X. Non-structural carbohydrates regulated by season and species in the subtropical monsoon broad-leaved evergreen forest of Yunnan Province, China. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Huang, J.-G.; Guo, X.; Rossi, S.; Zhai, L.; Yu, B.; Zhang, S.; Zhang, M. Intra-annual wood formation of subtropical Chinese red pine shows better growth in dry season than wet season. Tree Physiol. 2018, 38, 1225–1236. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Ostergaard, K.T.; Guyot, A.; Fujiwara, S.; Lockington, D.A. Estimating groundwater evapotranspiration by a subtropical pine plantation using diurnal water table fluctuations: Implications from night-time water use. J. Hydrol. 2016, 542, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Økland, T.; Halvorsen, R.; Gao, J.; Liu, Q.; Eilertsen, O.; Bratli, H. Gradients analyses of forests ground vegetation and its relationships to environmental variables in five subtropical forest areas, S and SW China. Sommerfeltia 2008, 32, 3. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; López-Moreno, J.I. Hydrological response to different time scales of climatological drought: An evaluation of the Standardized Precipitation Index in a mountainous Mediterranean basin. Hydrol. Earth Syst. Sci. 2005, 9, 523–533. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wang, Y.; Liu, S.; Wei, X.; Wang, X. Response of relative sap flow to meteorological factors under different soil moisture conditions in rainfed jujube (Ziziphus jujuba Mill.) plantations in semiarid Northwest China. Agric. Water Manag. 2014, 136, 23–33. [Google Scholar] [CrossRef]
- Bovard, B.D.; Curtis, P.S.; Vogel, C.S.; Su, H.B.; Schmid, H.P. Environmental controls on sap flow in a northern hardwood forest. Tree Physiol. 2005, 25, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Prior, L.D.; Bowman, D.M.J.S.; Eamus, D. Seasonal differences in leaf attributes in Australian tropical tree species: Family and habitat comparisons. Funct. Ecol. 2004, 18, 707–718. [Google Scholar] [CrossRef]
- Mathias, J.M.; Thomas, R.B. Global tree intrinsic water use efficiency is enhanced by increased atmospheric CO2 and modulated by climate and plant functional types. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Granier, A. Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol. 1987, 3, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Tie, Q.; Hu, H.; Tian, F.; Guan, H.; Lin, H. Environmental and physiological controls on sap flow in a subhumid mountainous catchment in North China. Agric. For. Meteorol. 2017, 240–241, 46–57. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Oberbauer, S.F.; Clark, D.B. Whole tree xylem sap flow responses to multiple environmental variables in a wet tropical forest. Plant Cell Environ. 2004, 27, 551–567. [Google Scholar] [CrossRef]
- Zheng, C.L.; Wang, Q. Water-use response to climate factors at whole tree and branch scale for a dominant desert species in central Asia: Haloxylon ammodendron. Ecohydrology 2014, 7, 56–63. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, J.; Sun, Z.; Niu, J.; Zhou, C.; Gu, D.; Huang, Y.; Zhao, P. Suppression of nighttime sap flux with lower stem photosynthesis in Eucalyptus trees. Int. J. Biometeorol. 2016, 60, 545–556. [Google Scholar] [CrossRef]
- Lu, P.; Urban, L.; Zhao, P. Granier’s thermal dissipation probe (TDP) method for measuring sap flow in trees: Theory and practice. ACTA Bot. Sin. Ed. 2004, 46, 631–646. [Google Scholar]
- Wang, X.P. The phytocoenological features of mixed needleleaf and broadleaf forest on subtropical mountain in Guangxi. J. Wuhan Bot. Res. 1990, 8, 243–253. [Google Scholar]
- Huang, K.C.; He, W.; Zhou, C.M.; Huang, Y.Q.; Zhou, L.W. Foliar physiological characteristics of Schima superba along a vertical canopy gradient. Chin. J. Ecol. 2017, 36, 2751–2758. [Google Scholar] [CrossRef]
- Gu, D.X.; He, W.; Huang, K.C.; Otieno, D.; Zhou, C.M.; He, C.X.; Huang, Y.Q. Transpiration of Moso bamboo in southern China is influenced by ramet age, phenology, and drought. For. Ecol. Manag. 2019, 450, 117526. [Google Scholar] [CrossRef]
- Pasqualotto, G.; Carraro, V.; Menardi, R.; Anfodillo, T. Calibration of granier-type (TDP) sap flow probes by a high precision electronic potometer. Sensors 2019, 19, 2419. [Google Scholar] [CrossRef] [Green Version]
- Pataki, D.E.; McCarthy, H.R.; Litvak, E.; Pincetl, S. Transpiration of urban forests in the Los Angeles metropolitan area. Ecol. Appl. 2011, 21, 661–677. [Google Scholar] [CrossRef] [Green Version]
- Kutscha, N.P.; Sachs, I.B. Color Tests for Differentiating Heartwood and Sapwood in Certain Softwood Tree Species; United States Department of Agriculture: Madison, WI, USA, 1962; Voulme 2246, p. 17.
- Campbell, G.S.; Norman, J.M. An Introduction to Environmental Biophysics; Springer: Berlin/Heidelberg, Germany, 1998; p. 286. [Google Scholar] [CrossRef]
- Breslow, N. A generalized Kruskal-Wallis test for comparing K samples subject to unequal patterns of censorship. Biometrika 1970, 57, 579–594. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Song, G.M.; Wang, Q.; Jin, J. Leaf photosynthetic capacity of sunlit and shaded mature leaves in a deciduous forest. Forests 2020, 11, 318. [Google Scholar] [CrossRef] [Green Version]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-3. 2015. Available online: https://mran.revolutionanalytics.com/snapshot/2016-01-01/web/packages/agricolae/agricolae.pdf#page=74 (accessed on 18 June 2021).
- Oguntunde, P.G. Whole-plant water use and canopy conductance of cassava under iimited available soil water and varying evaporative demand. Plant Soil 2005, 278, 371–383. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought-and shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. J. Exp. Bot. 2013, 64, 4053–4080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, M.; Yu, G.; He, N.; Hou, J. Leaf morphological and anatomical traits from tropical to temperate coniferous forests: Mechanisms and influencing factors. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-Alonzo, R.; Paz, H.; Zuluaga, R.C.; Rosell, J.A.; Olson, M.E. Coordinated evolution of leaf and stem economics in tropical dry forest trees. Ecology 2012, 93, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Somavilla, N.S.; Kolb, R.M.; Rossatto, D.R. Leaf anatomical traits corroborate the leaf economic spectrum: A case study with deciduous forest tree species. Braz. J. Bot. 2014, 37, 69–82. [Google Scholar] [CrossRef]
- Huang, D.W.; Zhang, D.Q.; Zhou, G.Y.; Liu, S.Z.; Dennis, O.; Lin, L.Y. Characteristics of dominant tree species stem sap flow and their relationships with environmental factors in a mixed conifer-broadleaf forest in Dinghushan, Guangdong Province of South China. Chin. J. Appl. Ecol. 2012, 23, 1159–1166. [Google Scholar]
- Meinzer, F.C.; Goldstein, G.; Franco, A.C.; Bustamante, M.; Igler, E.; Jackson, P.; Caldas, L.; Rundel, P.W. Atmospheric and hydraulic limitations on transpiration in Brazilian cerrado woody species. Funct. Ecol. 1999, 13, 273–282. [Google Scholar] [CrossRef]
- Sperry, J.S.; Pockman, W.T. Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis. Plant Cell Environ. 1993, 16, 279–287. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Brodribb, T.J. Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 2012, 24, 1510–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshifuji, N.; Komatsu, H.; Kumagai, T.; Tanaka, N.; Tantasirin, C.; Suzuki, M. Interannual variation in transpiration onset and its predictive indicator for a tropical deciduous forest in northern Thailand based on 8-year sap-flow records. Ecohydrology 2011, 4, 225–235. [Google Scholar] [CrossRef]
- Niinemets, Ü. Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiol. 2002, 22, 515–535. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Z.; Zhang, J.X.; Si, Y.; Zhao, P. Conduits anatomical structure and leaf traits of diffuse- and ring-porous stems in subtropical evergreen broad-leaved forests. Chin. J. Plant Ecol. 2019, 43, 131–138. [Google Scholar] [CrossRef]
- Brinkmann, N.; Eugster, W.; Zweifel, R.; Buchmann, N.; Kahmen, A. Temperate tree species show identical response in tree water deficit but different sensitivities in sap flow to summer soil drying. Tree Physiol. 2016, 36, 1508–1519. [Google Scholar] [CrossRef] [Green Version]
- Haberle, K.-H.; Reiter, I.; Patzner, K.; Heyne, C.; Matyssek, R. Switching the light off: A break in photosynthesis and sap flow of forest trees under total solar eclipse. Meteorol. Z. 2001, 10, 201–206. [Google Scholar] [CrossRef]
- Niinemets, Ü. The controversy over traits conferring shade-tolerance in trees: Ontogenetic changes revisited. J. Ecol. 2006, 94, 464–470. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Hurtado, V.H.; Poorter, L. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct. Ecol. 2006, 20, 207–216. [Google Scholar] [CrossRef]
- Roderick, M.L.; Berry, S.L.; Noble, I.R. A framework for understanding the relationship between environment and vegetation based on the surface area to volume ratio of leaves. Funct. Ecol. 2000, 14, 423–437. [Google Scholar] [CrossRef]
- Yamane, T.; Hamana, Y.; Nakano, M. Detection of water-deficit stress from daily sap flow profiles in peach. J. Jpn. Soc. Hortic. Sci. 2011, 80, 383–389. [Google Scholar] [CrossRef]
- Granier, A.; Claustres, J.P. Water relations of a Norway spruce (Picea abies) tree growing in natural condition: Variation within the tree. Acta Oecologica 1989, 10, 295–310. [Google Scholar]
- Pataki, D.E.; Oren, R.; Smith, W.K. Sap flux of co-occurring species in a western subalpine forest during seasonal soil drought. Ecology 2000, 81, 2557–2566. [Google Scholar] [CrossRef]
- Gonzalez-Benecke, C.A.; Martin, T.A.; Cropper, W.P. Whole-tree water relations of co-occurring mature Pinus palustris and Pinus elliottii var. elliottii. Can. J. For. Res. 2011, 41, 509–523. [Google Scholar] [CrossRef] [Green Version]
- Zinnert, J.C.; Shiflett, S.A.; Vick, J.K.; Young, D.R. Plant functional traits of a shrub invader relative to sympatric native shrubs. Ecosphere 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Williams, M.; Bond, B.J.; Ryan, M.G. Evaluating different soil and plant hydraulic constraints on tree function using a model and sap flow data from ponderosa pine. Plant. Cell Environ. 2001, 24, 679–690. [Google Scholar] [CrossRef]
- Phillips, N.; Nagchaudhuri, A.; Oren, R.; Katul, G. Time constant for water transport in loblolly pine trees estimated from time series of evaporative demand and stem sapflow. Trees 1997, 11, 412–419. [Google Scholar] [CrossRef]
- Granier, A.; Biron, P.; Lemoine, D. Water balance, transpiration and canopy conductance in two beech stands. Agric. For. Meteorol. 2000, 100, 291–308. [Google Scholar] [CrossRef]
- Chuang, Y.L.; Oren, R.; Bertozzi, A.L.; Phillips, N.; Katul, G.G. The porous media model for the hydraulic system of a conifer tree: Linking sap flux data to transpiration rate. Ecol. Modell. 2006, 191, 447–468. [Google Scholar] [CrossRef]
- Ferreras, A.E.; Whitworth-Hulse, J.I.; Tecco, P.A.; Marcora, P.I.; Funes, G. Environmental constraints to native woody species recruitment in invaded mountain woodlands of central Argentina. For. Ecol. Manag. 2019, 440, 189–201. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Murray, B.R.; Barton, C.; Eamus, D. Seasonal responses of xylem sap velocity to VPD and solar radiation during drought in a stand of native trees in temperate Australia. Funct. Plant Biol. 2004, 31, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Tyree, M.T. Plant hydraulic conductance measured by the high pressure flow meter in crop plants. J. Exp. Bot. 2000, 51, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Kellomäki, S.; Wang, K. Sap flow in Scots pines growing under conditions of year-round carbon dioxide enrichment and temperature elevation. Plant Cell Environ. 1998, 21, 969–981. [Google Scholar] [CrossRef]
- Gindaba, J.; Rozanov, A.; Negash, L. Response of seedlings of two Eucalyptus and three deciduous tree species from Ethiopia to severe water stress. For. Ecol. Manag. 2004, 201, 119–129. [Google Scholar] [CrossRef]
- Kröber, W.; Heklau, H.; Bruelheide, H. Leaf morphology of 40 evergreen and deciduous broadleaved subtropical tree species and relationships to functional ecophysiological traits. Plant Biol. 2015, 17, 373–383. [Google Scholar] [CrossRef]
| Plant Functional Types | Species | Tree NO | Tree Diameters (DBH) | Sapwood Depth | Sapwood Depth Formula | ||||
|---|---|---|---|---|---|---|---|---|---|
| May 2016 (cm) | December 2016 (cm) | January 2018 (cm) | May 2016 (cm) | December 2016 (cm) | January 2018 (cm) | ||||
| Evergreen broad-leaved trees (EBL) | C. fissa | 1 | 12.4 | 13.2 | 14.3 | 6.01 | 6.01 | 6.19 | y = 0.0029 X2 + 0.0975x + 4.2639 |
| 2 | 20.2 | 20.5 | 21 | 7.44 | 7.51 | 7.62 | |||
| 3 | 21.8 | 24.2 | 25.2 | 7.8 | 8.34 | 8.56 | |||
| S. superba | 1 | 12.7 | 13.1 | 14 | 3.61 | 3.73 | 4 | y = 0.3017x − 0.2219 | |
| 2 | 12.2 | 12.6 | 13.1 | 3.46 | 3.58 | 3.73 | |||
| 3 | 17.6 | 18.9 | 19.2 | 5.09 | 5.48 | 5.57 | |||
| Deciduous broad-leaved trees (DBL) | C. henryi | 1 | 20.1 | 20.5 | 21 | 4.9 | 5.08 | 5.31 | y = 0.0095X2 + 0.0533x − 0.0111 |
| 2 | 9.5 | 10.3 | 11 | 1.35 | 1.55 | 1.73 | |||
| 3 | 11 | 11.6 | 12.2 | 1.73 | 1.89 | 2.06 | |||
| L. formosana | 1 | 11.7 | 18 | 18.8 | 4.63 | 7.13 | 7.46 | y = 0.0046X2 + 0.2447x + 1.174 | |
| 2 | 11.1 | 11.7 | 12.4 | 4.41 | 4.63 | 4.88 | |||
| 3 | 14.3 | 13.5 | 14 | 5.62 | 5.30 | 5.5 | |||
| Coniferous (CON) | P. massoniana | 1 | 33.5 | 33.7 | 38 | 8.87 | 8.95 | 10.67 | y = 0.0032X2 + 0.1691x − 0.4093 |
| 2 | 35.9 | 37 | 37.7 | 9.82 | 10.26 | 10.55 | |||
| 3 | 26.4 | 28.1 | 28.8 | 6.3 | 6.89 | 7.13 | |||
| Seasons | Environment Variables | Plant Functional Types | SWC | SoilTem | PPFD | VPD |
|---|---|---|---|---|---|---|
| Wet season | SWC | EBL | 0.29 | −0.03 | −0.12 | −0.06 |
| DBL | 0.22 | 0.00 | −0.16 | −0.03 | ||
| CON | 0.41 | −0.03 | −0.12 | −0.07 | ||
| SoilTem | EBL | −0.06 | 0.13 | 0.12 | 0.05 | |
| DBL | −0.04 | 0.03 | 0.16 | 0.03 | ||
| CON | −0.08 | 0.15 | 0.11 | 0.06 | ||
| PPFD | EBL | −0.08 | 0.03 | 0.46 | 0.23 | |
| DBL | −0.06 | 0.01 | 0.61 | 0.13 | ||
| CON | −0.11 | 0.04 | 0.43 | 0.25 | ||
| VPD | EBL | −0.06 | 0.02 | 0.34 | 0.31 | |
| DBL | −0.04 | 0.00 | 0.45 | 0.17 | ||
| CON | −0.08 | 0.03 | 0.32 | 0.34 | ||
| Dry season | SWC | EBL | −0.03 | −0.05 | −0.12 | −0.02 |
| DBL | 0.11 | −0.12 | −0.08 | −0.02 | ||
| CON | −0.13 | −0.01 | −0.07 | −0.09 | ||
| SoilTem | EBL | 0.01 | 0.16 | 0.32 | 0.02 | |
| DBL | −0.04 | 0.39 | 0.23 | 0.02 | ||
| CON | 0.04 | 0.04 | 0.19 | 0.09 | ||
| PPFD | EBL | 0.01 | 0.07 | 0.72 | 0.04 | |
| DBL | −0.02 | 0.17 | 0.53 | 0.04 | ||
| CON | 0.02 | 0.02 | 0.44 | 0.18 | ||
| VPD | EBL | 0.01 | 0.06 | 0.52 | 0.06 | |
| DBL | −0.04 | 0.15 | 0.38 | 0.05 | ||
| CON | 0.04 | 0.02 | 0.31 | 0.26 |
| Seasons | Environment Variables | Plant Functional Types | SWC | SoilTem | PPFD | VPD |
|---|---|---|---|---|---|---|
| Wet season | SWC | EBL | −0.38 | 0.55 | 0.01 | −0.23 |
| DBL | 0.07 | 0.04 | 0.02 | −0.22 | ||
| CON | −0.16 | 0.32 | 0.01 | −0.26 | ||
| SoilTem | EBL | 0.35 | −0.60 | −0.08 | 0.24 | |
| DBL | −0.06 | −0.05 | −0.10 | 0.22 | ||
| CON | 0.15 | −0.34 | −0.07 | 0.27 | ||
| PPFD | EBL | −0.01 | 0.12 | 0.40 | 0.41 | |
| DBL | 0.00 | 0.01 | 0.51 | 0.39 | ||
| CON | 0.00 | 0.07 | 0.36 | 0.46 | ||
| VPD | EBL | 0.14 | −0.23 | 0.26 | 0.62 | |
| DBL | −0.02 | −0.02 | 0.34 | 0.58 | ||
| CON | 0.06 | −0.13 | 0.24 | 0.70 | ||
| Dry season | SWC | EBL | −0.07 | 0.17 | −0.03 | −0.27 |
| DBL | −0.14 | 0.02 | 0.02 | −0.36 | ||
| CON | −0.14 | 0.21 | −0.03 | −0.27 | ||
| SoilTem | EBL | 0.06 | −0.19 | −0.02 | 0.27 | |
| DBL | 0.13 | −0.02 | 0.01 | 0.36 | ||
| CON | 0.13 | −0.22 | −0.01 | 0.27 | ||
| PPFD | EBL | 0.01 | 0.01 | 0.38 | 0.43 | |
| DBL | 0.01 | 0.00 | −0.18 | 0.57 | ||
| CON | 0.01 | 0.01 | 0.32 | 0.43 | ||
| VPD | EBL | 0.03 | −0.07 | 0.23 | 0.71 | |
| DBL | 0.06 | −0.01 | −0.11 | 0.94 | ||
| CON | 0.05 | −0.09 | 0.19 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Wang, Q.; Otieno, D. Responses of Sap Flux Densities of Different Plant Functional Types to Environmental Variables Are Similar in Both Dry and Wet Seasons in a Subtropical Mixed Forest. Forests 2021, 12, 1007. https://doi.org/10.3390/f12081007
Huang K, Wang Q, Otieno D. Responses of Sap Flux Densities of Different Plant Functional Types to Environmental Variables Are Similar in Both Dry and Wet Seasons in a Subtropical Mixed Forest. Forests. 2021; 12(8):1007. https://doi.org/10.3390/f12081007
Chicago/Turabian StyleHuang, Kechao, Quan Wang, and Dennis Otieno. 2021. "Responses of Sap Flux Densities of Different Plant Functional Types to Environmental Variables Are Similar in Both Dry and Wet Seasons in a Subtropical Mixed Forest" Forests 12, no. 8: 1007. https://doi.org/10.3390/f12081007
APA StyleHuang, K., Wang, Q., & Otieno, D. (2021). Responses of Sap Flux Densities of Different Plant Functional Types to Environmental Variables Are Similar in Both Dry and Wet Seasons in a Subtropical Mixed Forest. Forests, 12(8), 1007. https://doi.org/10.3390/f12081007






