Soil Effects on Stem Growth and Wood Anatomy of Tamboril Are Mediated by Tree Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Plant Material
2.3. Sampling
2.4. Sample Preparation
2.5. Data Collection and Statistics
3. Results
3.1. Plant Size
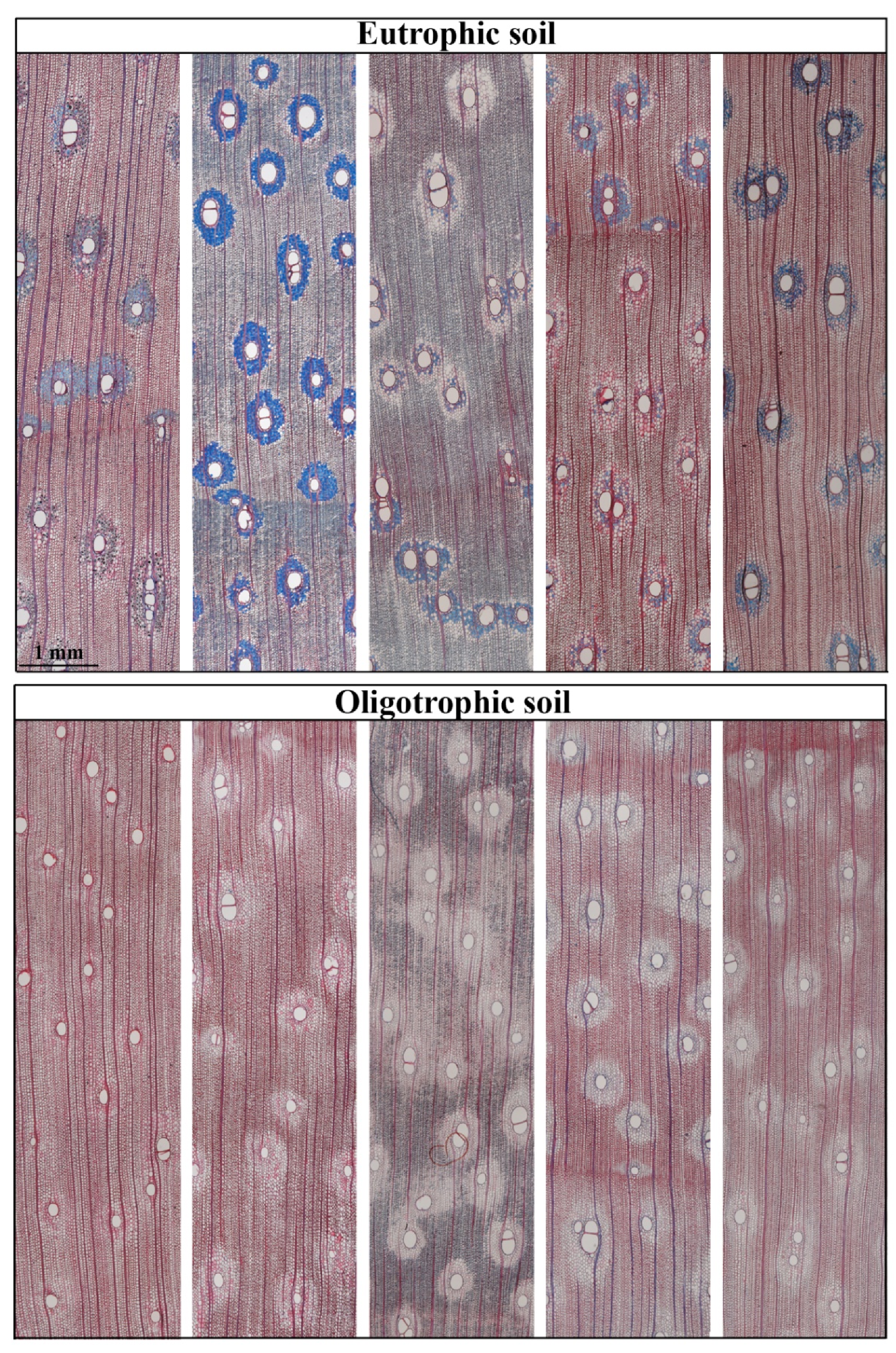
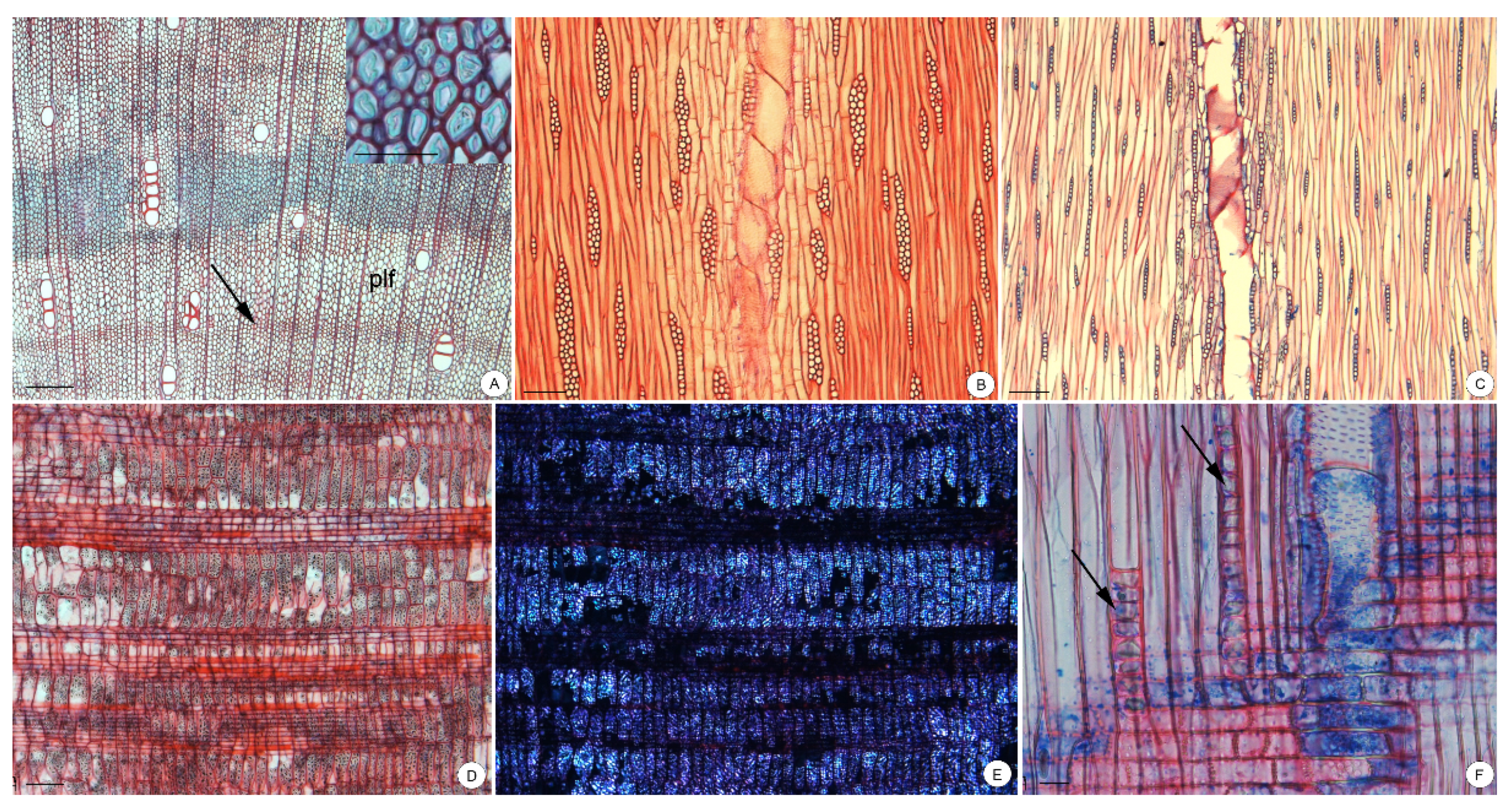
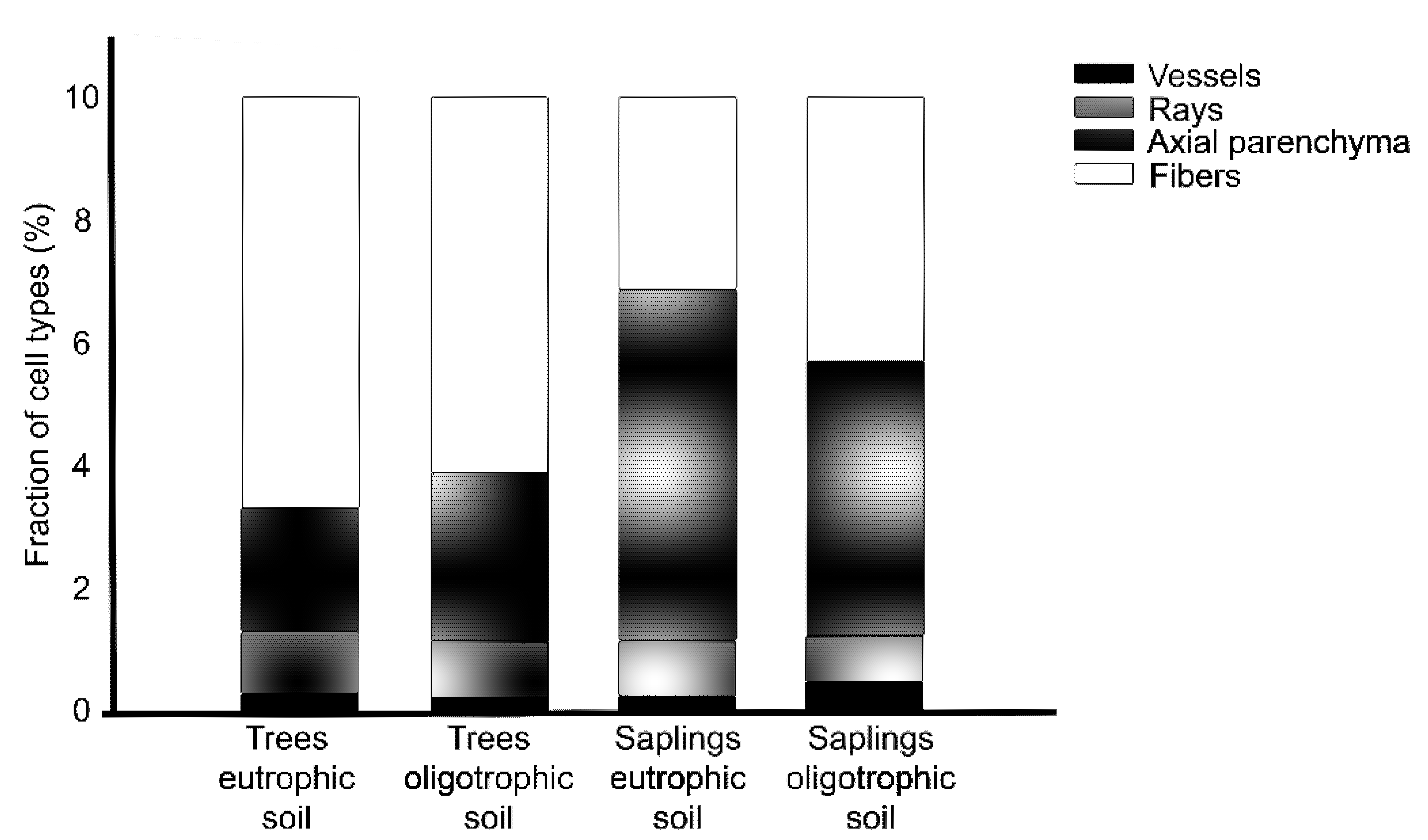
3.2. Wood Anatomy
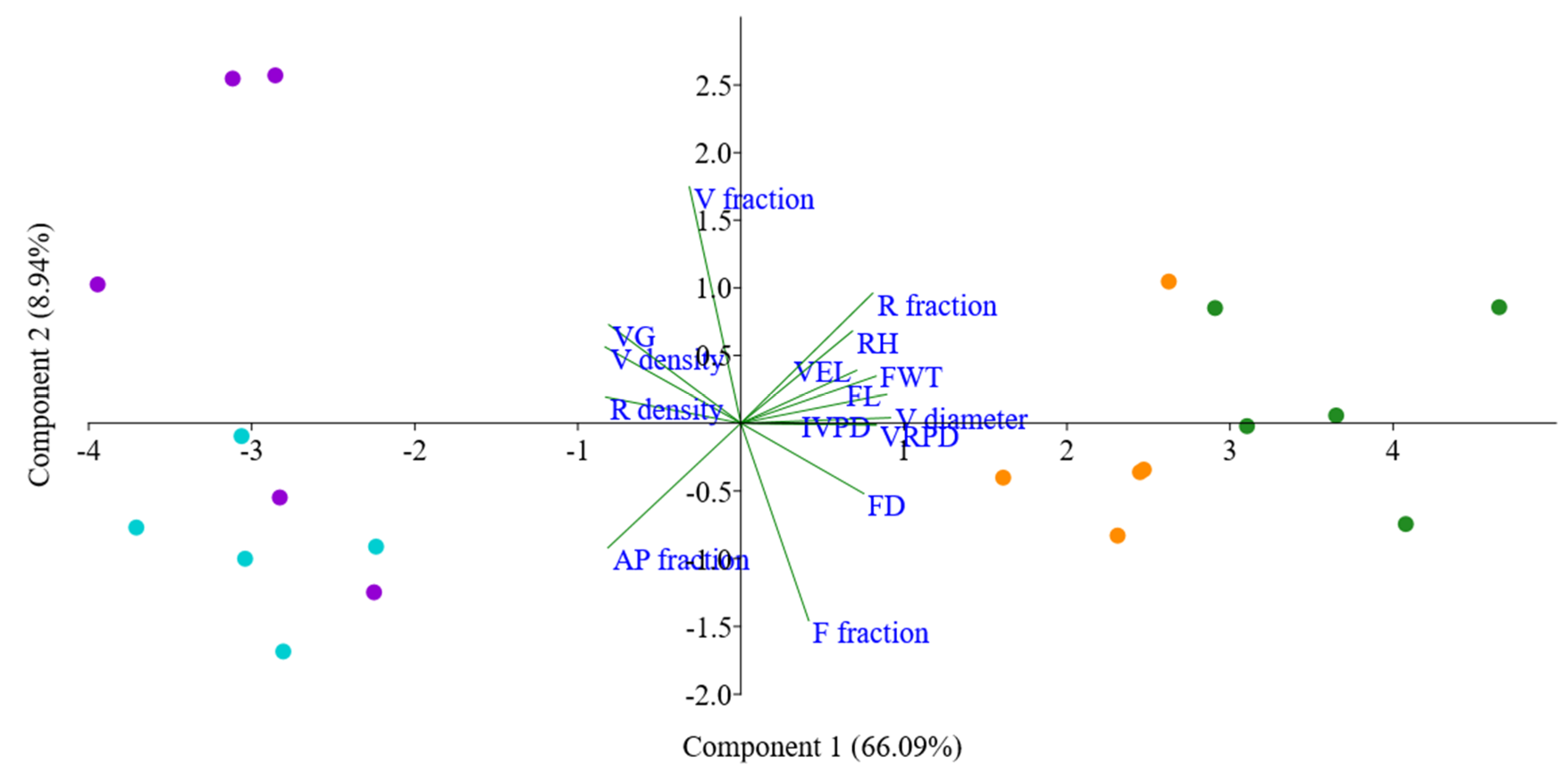
3.3. Statistical Analysis
4. Discussion
4.1. Allometric Features
4.2. Wood Anatomy in Response to Soil Type
4.3. Storage Compounds inside the Parenchyma
4.4. Age Effect on Wood Anatomy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorce, C.; Giovannelli, A.; Sebastiani, L.; Anfodillo, T. Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Rep. 2013, 32, 885–898. [Google Scholar] [CrossRef]
- Spannl, S.; Homeier, J.; Bräuning, A. Nutrient-Induced Modifications of Wood Anatomical Traits of Alchornea lojaensis (Euphorbiaceae). Front. Earth Sci. 2016, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Buttò, V.; Rozenberg, P.; Deslauriers, A.; Rossi, S.; Morin, H. Environmental and developmental factors driving xylem anatomy and micro-density in Black spruce. New Phytol. 2021, 230, 957–971. [Google Scholar] [CrossRef]
- Santiago, L.S. Nutrient limitation of eco-physiological processes in tropical trees. Trees 2015, 29, 1291–1300. [Google Scholar] [CrossRef] [Green Version]
- Maathuis, F.J.M.; Diatloff, E. Roles and Functions of Plant Mineral Nutrients. Methods Mol. Biol. 2013, 953, 1–21. [Google Scholar] [CrossRef]
- Ployet, R.; Labate, M.T.V.; Cataldi, T.R.; Christina, M.; Morel, M.; Clemente, H.S.; Denis, M.; Favreau, B.; Filho, M.T.; Laclau, J.; et al. A systems biology view of wood formation in Eucalyptus grandis trees submitted to different potassium and water regimes. New Phytol. 2019, 223, 766–782. [Google Scholar] [CrossRef] [Green Version]
- Santiago, L.S.; Wright, S.J.; Harms, K.E.; Yavitt, J.B.; Korine, C.; Garcia, M.N.; Turner, B.L. Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J. Ecol. 2012, 100, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Salinas-Peba, L.; Parra-Tabla, V.; Campo, J.; Munguía-Rosas, M.A. Survival and growth of dominant tree seedlings in seasonally tropical dry forests of Yucatan: Site and fertilization effects. J. Plant Ecol. 2014, 7, 470–479. [Google Scholar] [CrossRef] [Green Version]
- Razaq, M.; Zhang, P.; Shen, H.-L. Salahuddin Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heineman, K.D.; Turner, B.L.; Dalling, J.W. Variation in wood nutrients along a tropical soil fertility gradient. New Phytol. 2016, 211, 440–454. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.J. Plant responses to nutrient addition experiments conducted in tropical forests. Ecol. Monogr. Ecol. Soc. Am. 2019, 89, e01382. [Google Scholar] [CrossRef]
- Cunha-Blum, J.; Oki, Y.; Solar, R.; Fernandes, G.W. More is not always better: Responses of the endemic plant Vellozia nanuzae to additional nutrients. Acta Bot. Bras. 2020, 34, 487–496. [Google Scholar] [CrossRef]
- Goldstein, G.; Bucci, S.J.; Scholz, F.G. Why do trees adjust water relations and hydraulic architecture in response to nutrient availability? Tree Physiol. 2013, 33, 238–240. [Google Scholar] [CrossRef] [Green Version]
- Doria, L.C.; Podadera, D.S.; Batalha, M.A.; Lima, R.S.; Marcati, C.R. Do woody plants of the Caatinga show a higher degree of xeromorphism than in the Cerrado? Flora—Morphol. Distrib. Funct. Ecol. Plants 2016, 224, 244–251. [Google Scholar] [CrossRef] [Green Version]
- De Melo, J.C.F., Jr.; Amorim, M.W.; Soffiatti, P. Comparative wood anatomy of Ficus cestrifolia (Moraceae) in two distinct soil conditions. Rodriguesia 2018, 69, 2109–2118. [Google Scholar] [CrossRef]
- Costa, L.S.; De Moura, C.O.; Bucci, S.J.; Sonsin-Oliveira, J.; Gomes, S.M.; Bustamante, M.M.D.C. Nutrient enrichment changes water transport structures of savanna woody plants. Environ. Res. Lett. 2021, 16, 055021. [Google Scholar] [CrossRef]
- Soong, J.L.; Janssens, I.A.; Grau, O.; Margalef, O.; Stahl, C.; Van Langenhove, L.; Urbina, I.; Chave, J.; Dourdain, A.; Ferry, B.; et al. Soil properties explain tree growth and mortality, but not biomass, across phosphorus-depleted tropical forests. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fujii, K.; Shibata, M.; Kitajima, K.; Ichie, T.; Kitayama, K.; Turner, B.L. Plant–soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol. Res. 2018, 33, 149–160. [Google Scholar] [CrossRef]
- Rodriguez-Zaccaro, F.D.; Valdovinos-Ayala, J.; Percolla, M.I.; Venturas, M.D.; Pratt, R.B.; Jacobsen, A.L. Wood structure and function change with maturity: Age of the vascular cambium is associated with xylem changes in current-year growth. Plant Cell Environ. 2019, 42, 1816–1831. [Google Scholar] [CrossRef]
- Flora do Brasil—Enterolobium Contortisiliquum (Vell.) Morong. Available online: http://floradobrasil.jbrj.gov.br/reflora/listaBrasil/ConsultaPublicaUC/BemVindoConsultaPublicaConsultar.do?invalidatePageControl-Coun-ter=1&idsFilhosAlgas=%5B2%5D&idsFilhosFungos=%5B1%2C10%2C11%5D&lingua=&grupo=5&familia=null&genero=&especie=&autor=&nomeVernaculo=&nomeCompleto=Fabaceae+Enterolobium+Mart.&formaVida=null&substrato=null&ocorreBrasil=QUALQUER&ocorrencia=OCORRE&endemismo=TODOS&origem=TODOS®iao=QUALQUER&estado=QUALQUER&ilhaOceanica=32767&domFitogeograficos=QUALQUER&bacia=QUALQUER&vegetacao=TODOS&mostrarAte=SUBESP_VAR&opcoesBusca=TODOS_OS_NOMES&loginUsuario=Visitante&senhaUsuario=&contexto=consulta-publica (accessed on 16 June 2021).
- Luz, A.R.M.; Rocha, L.; De Queiroz, L.P.; De Souza, R. Flora da Bahia: Leguminosae—Enterolobium (Clado Mimosoide: Ingeae). SITIENTIBUS Sér. Ciênc. Biol. 2021, 21, 1–9. [Google Scholar] [CrossRef]
- Engel, V.L.; Parrotta, J.A. An evaluation of direct seeding for reforestation of degraded lands in central São Paulo state, Brazil. For. Ecol. Manag. 2001, 152, 169–181. [Google Scholar] [CrossRef]
- De Oliveira Gonçalves, P.J.R.; Vieira, L.C.; Nogueira, A.V.; Corseuil, H.X.; Mezzari, M.P. Tolerance of Tree Reforestation Species (Schizolobium parahyba, Mimosa scabrella and Enterolobium contortisiliquum) to Gasoline and Diesel Phytotoxicity Assays. J. Bioremediat. Biodegrad. 2011, S7, 2. [Google Scholar] [CrossRef] [Green Version]
- Bueno, M.L.; Dexter, K.G.; Pennington, R.T.; Pontara, V.; Neves, D.M.; Ratter, J.A.; de Oliveira-Filho, A.T. The Environmental Triangle of the Cerrado Domain: Ecological Factors Driving Shifts in Tree Species Composition between Forests and Savannas. J. Ecol. 2018, 106, 2109–2120. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Filho, A.T.; Ratter, J.A. The Cerrados of Brazil. In The Cerrados of Brazil; Oliveira, P.S., Marquis, R.J., Eds.; Columbia University Press: New York, NY, USA; Chichester, UK; West Sussex, UK, 2002. [Google Scholar] [CrossRef] [Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Carvalho, W.A. Levantamento Semidetalhado dos Solos da Fazenda Experimental Edgárdia—Município de Botucatu—SP; UNESP—Faculdade de Ciencias Agronomicas: Botucatu, Sao Paulo, Brazil, 1991; Available online: https://books.google.com.br/books?id=V2-OkgEACAAJ (accessed on 2 July 2021).
- Nogueira, L.R., Jr. Caracterização de Solos Degradadados Pela Atividade Agrícola e Alterações Biológicas Após Reflorestamentos Com Diferentes Associações de Espécies da Mata Atlântica. Doctoral Dissertation, Universidade de São Paulo, Piracicaba, Brazil, 2000. [Google Scholar]
- Empresa Brasileira De Pesquisa Agropecuária—EMBRAPA. Sistema Brasileiro de Classificação de Solos, 5th ed.; EMBRAPA: Brasília, Brazil, 2018. [Google Scholar]
- Instituto Agronômico de Campinas. Available online: http://www.iac.sp.gov.br/produtoseservicos/analisedosolo/interpretacaoanalise.php (accessed on 21 June 2021).
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company, Inc.: New York, NY, USA; London, UK, 1940. [Google Scholar]
- Bukatsch, F. Bemerkungen Zur Doppelfärbung: Astrablau–Safranin. Mikrokosmos 1972, 61, 255. [Google Scholar]
- Franklin, G.L. Preparation of Thin Sections of Synthetic Resins and Wood-Resin Composites, and a New Macerating Method for Wood. Nature 1945, 155, 51. [Google Scholar] [CrossRef]
- Kraus, J.E.; Arduin, M. Manual Básico de Métodos Em Morfologia Vegetal; EDUR: Seropédica, Brazil, 1997. [Google Scholar]
- Berlyn, G.P.; Miksche, J.P. Botanical Microtechnique and Cytochemistry; Iowa State University Press: Iowa City, IA, USA, 1976. [Google Scholar]
- IAWA Committee. List of microscopic features for hardwood identification. IAWA Bull. 1989, 10, 220–332. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Carlquist, S. Comparative Wood Anatomy, 2nd ed.; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Barnett, J.R.; Jeronimidis, G. Wood Quality and Its Biological Basis; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Bowling, A.J.; Vaughn, K.C. Gelatinous Fibers Are Widespread in Coiling Tendrils and Twining Vines. Am. J. Bot. 2009, 96, 719–727. [Google Scholar] [CrossRef]
- Lens, F.; Sperry, J.S.; Christman, M.A.; Choat, B.; Rabaey, D.; Jansen, S. Testing Hypotheses That Link Wood Anatomy to Cavitation Resistance and Hydraulic Conductivity in the Genus Acer. New Phytol. 2011, 190, 709–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, J.R.; Rossi, S.; Khare, S.; Longui, E.L.; Marcati, C.R. Disentangling the Effects of Genotype and Environment on Growth and Wood Features of Balfourodendron riedelianum Trees by Common Garden Experiments in Brazil. Forests 2020, 11, 905. [Google Scholar] [CrossRef]
- Cary, K.L.; Ranieri, G.M.; Pittermann, J. Xylem Form and Function under Extreme Nutrient Limitation: An Example from California’s Pygmy Forest. New Phytol. 2020, 226, 760–769. [Google Scholar] [CrossRef]
- Dickson, R.E. Carbon and Nitrogen Allocation in Trees. Ann. Sci. For. 1989, 46, 631s–647s. [Google Scholar] [CrossRef] [Green Version]
- Fichtler, E.; Worbes, M. Wood Anatomical Variables in Tropical Trees and Their Relation to Site Conditions and Individual Tree Morphology. IAWA J. 2012, 33, 119–140. [Google Scholar] [CrossRef]
- Sonsin, J.O.; Gasson, P.E.; Barros, C.F.; Marcati, C.R. A Comparison of the Wood Anatomy of 11 Species from Two Cerrado Habitats (Cerrado s.s. and Adjacent Gallery Forest). Bot. J. Linn. Soc. 2012, 170, 257–276. [Google Scholar] [CrossRef] [Green Version]
- Morris, H.; Plavcová, L.; Gorai, M.; Klepsch, M.M.; Kotowska, M.; Jochen Schenk, H.; Jansen, S. Vessel-Associated Cells in Angiosperm Xylem: Highly Specialized Living Cells at the Symplast–Apoplast Boundary. Am. J. Bot. 2018, 105, 151–160. [Google Scholar] [CrossRef] [Green Version]
- de Lima, R.S.; de Oliveira, P.L.; Rodrigues, L.R. Anatomia Do Lenho de Enterolobium contortisiliquum (Vell.) Morong (Leguminosae-Mimosoideae) Ocorrente Em Dois Ambientes. Rev. Bras. Bot. 2009, 32, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Morris, H.; Plavcová, L.; Cvecko, P.; Fichtler, E.; Gillingham, M.A.F.; Martínez-Cabrera, H.I.; Mcglinn, D.J.; Wheeler, E.; Zheng, J.; Ziemińska, K.; et al. A Global Analysis of Parenchyma Tissue Fractions in Secondary Xylem of Seed Plants. New Phytol. 2016, 209, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Spatial Variation of Vessel Grouping in the Xylem of Betula platyphylla Roth. J. Plant Res. 2016, 129, 29–37. [Google Scholar] [CrossRef]
- Carlquist, S. Fibre Dimorphism: Cell Type Diversification as an Evolutionary Strategy in Angiosperm Woods. Bot. J. Linn. Soc. 2014, 174, 44–67. [Google Scholar] [CrossRef] [Green Version]
- Evert, R.F. Anatomia Das Plantas de Esau; Blucher: São Paulo, Brazil, 2013. [Google Scholar]
- Dickison, W. Integrative Plant Anatomy, 1st ed.; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]

| Chemical Attributes | Eutrophic Soil | Oligotrophic Soil |
|---|---|---|
| pH CaCl2 | 5.6 (moderately acid) | 4.3 (highly acid) |
| Organic matter (g dm−3) | 26 (medium) | 3.1 (low) |
| Presin (mg dm−3) | 13 (medium) | 2.6 (low) |
| S-SO4−2 (mg dm−3) | 22 (high) | 5.5 (medium) |
| Base-cation saturation (%) | 72 (eutrophic) | 36 (oligotrophic) |
| K (mmolc dm−3) | 1.8 (medium) | 1.2 (low) |
| Ca (mmolc dm−3) | 64 (high) | 6.6 (low) |
| Mg (mmolc dm−3) | 15 (high) | 1.8 (low) |
| Al (mmolc dm−3) | 0.4 (low) | 2.5 (high) |
| B (g dm−3) | 0.24 (medium) | 0.42 (medium) |
| Cu (g dm−3) | 13.2 (high) | 0.56 (medium) |
| Fe (g dm−3) | 13.8 (high) | 22.45 (high) |
| Mn (g dm−3) | 176 (high) | 34 (high) |
| Zn (g dm−3) | 2.06 (high) | 0.44 (low) |
| Soil | 9-Year-Old Trees | 2-Year-Old Saplings | |||
|---|---|---|---|---|---|
| Individual | DBH (cm) | H (m) | CD (cm) | H (cm) | |
| Eutrophic | 1 | 29.6 | 11 | 2.73 | 75.5 |
| 2 | 25.5 | 10 | 2.59 | 82.7 | |
| 3 | 28.7 | 16 | 2.76 | 90 | |
| 4 | 29 | 13 | 2.46 | 65 | |
| 5 | 43 | 15 | 2.71 | 74 | |
| Oligotrophic | 1 | 16.9 | 3.75 | 3.30 | 99.7 |
| 2 | 22 | 5.75 | 1.96 | 50 | |
| 3 | 14.9 | 4.5 | 2.55 | 55.9 | |
| 4 | 14.6 | 7.5 | 2.53 | 65.6 | |
| 5 | 19.7 | 7 | 1.47 | 41 | |
| Features/Soil | Trees | Saplings | ||
|---|---|---|---|---|
| Eutrophic | Oligotrophic | Eutrophic | Oligotrophic | |
| Vessel density (n mm2) | 3.24 ± 0.27 | 3.07 ± 0.56 | 14.25 ± 3.83 | 15.95 ± 7.18 |
| Vessel grouping | 2.17 ± 0.13 | 2.21 ± 0.20 | 4.18 ± 0.63 | 4.79 ± 1.56 |
| Vessel element diameter (µm) | 143.25 ± 13.07 | 122.72 ± 20.27 | 57.00 ± 7.42 | 58.24 ± 8.81 |
| Vessel element length (µm) | 315.08 ± 33.75 | 291.88 ± 28.07 | 229.38 ± 19.40 | 229.82 ± 37.91 |
| Intervessel pit diameter (µm) | 8.57 ± 0.40 | 7.51 ± 0.75 | 6.51 ± 0.53 | 6.20 ± 0.63 |
| Vessel-ray pit diameter (µm) | 8.18 ± 0.52 | 8.38 ± 0.73 | 5.76 ± 0.51 | 5.34 ± 0.96 |
| Fiber length (µm) | 874.39 ± 47.68 | 849.14 ± 100.42 | 439.92 ± 6.36 | 407.15 ± 45.18 |
| Fiber diameter (µm) | 31.10 ± 4.38 | 27.62 ± 4.59 | 22.01 ± 1.50 | 22.36 ± 3.62 |
| Fiber wall thickness (µm) | 6.55 ± 1.12 | 4.46 ± 0.12 | 2.91 ± 0.29 | 2.67 ± 0.40 |
| Rays height (µm) | 148.58 ± 22.73 | 144.26 ± 7.10 | 112.51 ± 5.39 | 123.25 ± 19.02 |
| Rays density (n mm2) | 5.24 ± 0.36 | 5.41 ± 0.23 | 7.27 ± 0.58 | 6.70 ± 0.54 |
| Vessels fraction (mm2) | 30,227.05 ± 5739.13 | 24,082.29 ± 7480.81 | 27,501.15 ± 6212.37 | 49,836.43 ± 25,224.47 |
| Fibers fraction (mm2) | 665,599.27 ± 62,255.31 | 609,342.54 ± 66,568.52 | 312,430.42 ± 71,249.96 | 426,151.15 ± 102,494.11 |
| Axial parenchyma fraction (mm2) | 202,790.99 ± 50,710.13 | 275,121.46 ± 69,229.71 | 574,358.50 ± 70,979.53 | 452,113.57 ± 101,125.87 |
| Rays fraction (mm2) | 101,382.70 ± 33,542.00 | 91,453.71 ± 16,913.73 | 85,709.94 ± 10,271.79 | 71,898.85 ± 14,087.33 |
| Feature | Age | Soil | Age × Soil | |||
|---|---|---|---|---|---|---|
| F-Value | p | F-Value | p | F-Value | p | |
| Diameter of stem | 168.1 | <0.0001 | 16.78 | 0.0008 | 15.41 | 0.0012 |
| Height | 164.4 | <0.0001 | 30.48 | <0.0001 | 28.08 | <0.0001 |
| Vessel density | 42.95 | <0.0001 | 0.1789 | 0.6779 | 0.2677 | 0.612 |
| Vessel grouping | 36.32 | <0.0001 | 0.7258 | 0.4068 | 0.5581 | 0.4658 |
| Vessel element diameter | 158.9 | <0.0001 | 2.638 | 0.1238 | 3.284 | 0.0888 |
| Vessel element length | 28.95 | <0.0001 | 0.804 | 0.3832 | 0.6581 | 0.4291 |
| Intervessel pit diameter | 43.05 | <0.0001 | 7.074 | 0.01713 | 1.988 | 0.1777 |
| Vessel-ray pit diameter | 76.32 | <0.0001 | 0.1881 | 0.6703 | 1.172 | 0.295 |
| Fiber length | 266 | <0.0001 | 1.166 | 0.2963 | 0.0196 | 0.8905 |
| Fiber diameter | 18.52 | 0.0005 | 0.8778 | 0.3627 | 1.329 | 0.2659 |
| Fiber wall thickness | 97.95 | <0.0001 | 17.89 | 0.0006 | 11.3 | 0.0039 |
| Rays height | 13.59 | 0.002 | 0.1753 | 0.6811 | 0.9354 | 0.3479 |
| Rays density | 47.14 | <0.0001 | 0.1752 | 0.6826 | 1.482 | 0.2412 |
| Vessels fraction | 3.472 | 0.0809 | 1.716 | 0.2087 | 5.31 | 0.0349 |
| Fibers fraction | 3.618 | 0.0753 | 1.643 | 0.2182 | 0.0439 | 0.8366 |
| Parenchyma fraction | 66.49 | <0.0001 | 0.5505 | 0.4689 | 8.365 | 0.0106 |
| Rays fraction | 60.21 | <0.0001 | 0.6911 | 0.418 | 6.047 | 0.0257 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angélico, T.d.S.; Marcati, C.R.; Rossi, S.; da Silva, M.R.; Sonsin-Oliveira, J. Soil Effects on Stem Growth and Wood Anatomy of Tamboril Are Mediated by Tree Age. Forests 2021, 12, 1058. https://doi.org/10.3390/f12081058
Angélico TdS, Marcati CR, Rossi S, da Silva MR, Sonsin-Oliveira J. Soil Effects on Stem Growth and Wood Anatomy of Tamboril Are Mediated by Tree Age. Forests. 2021; 12(8):1058. https://doi.org/10.3390/f12081058
Chicago/Turabian StyleAngélico, Talita dos Santos, Carmen Regina Marcati, Sergio Rossi, Magali Ribeiro da Silva, and Júlia Sonsin-Oliveira. 2021. "Soil Effects on Stem Growth and Wood Anatomy of Tamboril Are Mediated by Tree Age" Forests 12, no. 8: 1058. https://doi.org/10.3390/f12081058
APA StyleAngélico, T. d. S., Marcati, C. R., Rossi, S., da Silva, M. R., & Sonsin-Oliveira, J. (2021). Soil Effects on Stem Growth and Wood Anatomy of Tamboril Are Mediated by Tree Age. Forests, 12(8), 1058. https://doi.org/10.3390/f12081058








