Impacts of the National Forest Rehabilitation Plan and Human-Induced Environmental Changes on the Carbon and Nitrogen Balances of the South Korean Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Forest
2.2. Model Description
2.3. Database
2.4. Model Simulation
3. Results
3.1. Reliability of the Simulation
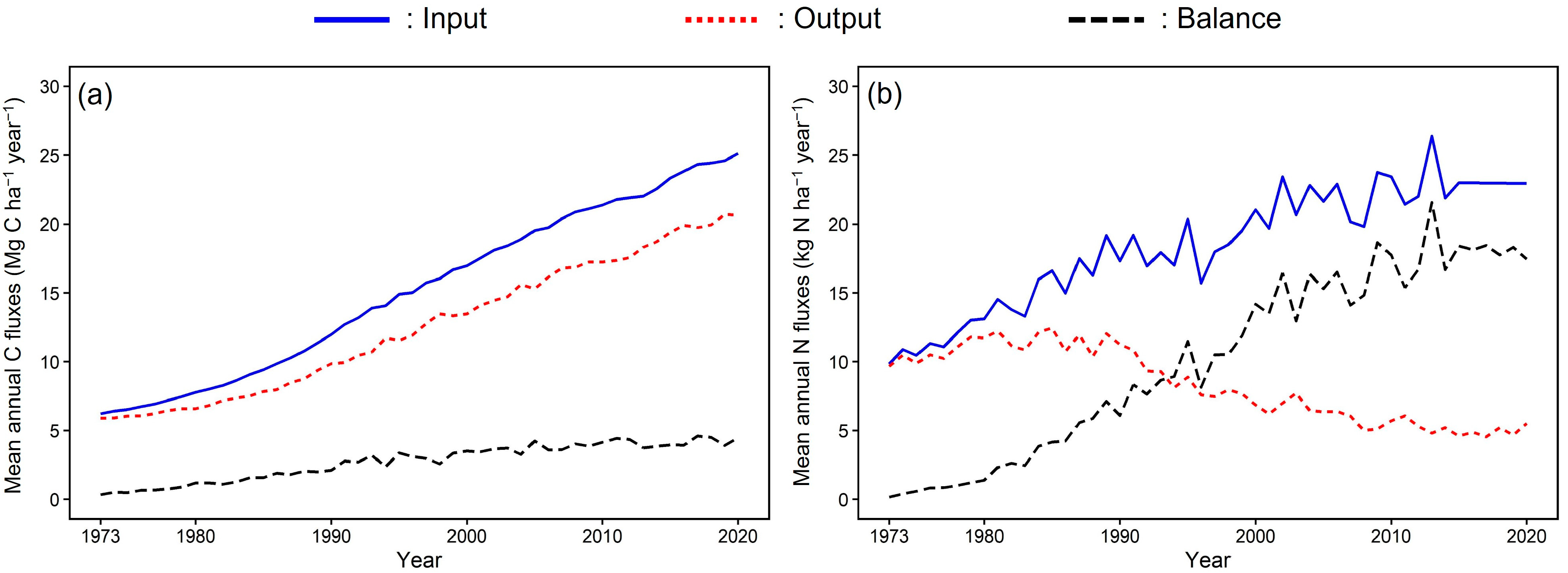
3.2. Carbon and Nitrogen Dynamics after the National Forest Rehabilitation Plan
3.3. Impacts of Human-Induced Environmental Changes on Carbon and Nitrogen Dynamics
4. Discussions
4.1. Carbon and Nitrogen Dynamics after the National Forest Rehabilitation Plan
4.2. Impacts of Human-Induced Environmental Changes on Carbon and Nitrogen Dynamics
4.3. Implications of the Findings in Managing South Korean Forests
4.4. Limitations of the Simulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Forcing | Forcing Type | Source | Resolution | |
|---|---|---|---|---|
| Spatial | Temporal | |||
| Soil depth | Spatial | Forest Site and Soil Map [35] | 100 ha × 60,564 units | – |
| Forest type | Spatial | Forest Type Map [36] | ||
| Initial stand age | Spatial | Forest Type Map [36] | ||
| Initial C and N stocks | Spatial | The spin-up process [28] | ||
| Solar radiation | Spatiotemporal | ArcGis Pro 2.6 [37] | 100 ha × 60,564 units | Annual, 1973–2020 |
| Precipitation | Spatiotemporal | KMA [25] | ||
| Air temperature | Spatiotemporal | KMA [25] | ||
| CO2 concentration | Spatiotemporal | KMA [25] | ||
| N deposition | Spatiotemporal | CCMI [39] | ||


References
- Grassi, G.; House, J.; Dentener, F.; Federici, S.; den Elzen, M.; Penman, J. The Key Role of Forests in Meeting Climate Targets Requires Science for Credible Mitigation. Nat. Clim. Chang. 2017, 7, 220–226. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human Alteration of the Global Nitrogen Cycle: Sources and Consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, R.K.; Ladha, J.K. Nitrate in Groundwater and Integration of Nitrogen-Catch Crop in Rice-Sweet Pepper Cropping System. Soil Sci. Soc. Am. J. 1998, 62, 1610–1619. [Google Scholar] [CrossRef]
- Likens, G.E.; Bormann, F.H.; Pierce, R.S.; Eaton, J.S.; Johnson, N.M. Biogeochemistry of a Forested Ecosystem; Springer: New York, NY, USA, 1977; pp. 1–13. [Google Scholar] [CrossRef]
- Bormann, F.H.; Likens, G.E. Nutrient Cycling. Science 1967, 155, 424–429. [Google Scholar] [CrossRef]
- Bianchi, T.S. The Evolution of Biogeochemistry: Revisited. Biogeochemistry 2021, 154, 141–181. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2007: Synthesis Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2007. [Google Scholar]
- Li, D.; Niu, S.; Luo, Y. Global Patterns of the Dynamics of Soil Carbon and Nitrogen Stocks Following Afforestation: A Meta-analysis. New Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The Global Nitrogen Cycle in the Twenty-First Century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef] [PubMed]
- Kanakidou, M.; Myriokefalitakis, S.; Daskalakis, N.; Fanourgakis, G.; Nenes, A.; Baker, A.R.; Tsigaridis, K.; Mihalopoulos, N. Past, Present, and Future Atmospheric Nitrogen Deposition. J. Atmos. Sci. 2016, 73, 2039–2047. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. Climate at a Glance: Global Time Series; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2021.
- Magnani, F.; Mencuccini, M.; Borghetti, M.; Berbigier, P.; Berninger, F.; Delzon, S.; Grelle, A.; Hari, P.; Jarvis, P.G.; Kolari, P.; et al. The Human Footprint in the Carbon Cycle of Temperate and Boreal Forests. Nature 2007, 447, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; DeLucia, E.H.; Gielen, B.; Calfapietra, C.; Giardina, C.P.; King, J.S.; Ledford, J.; McCarthy, H.R.; Moore, D.J.P.; Ceulemans, R.; et al. Forest Response to Elevated CO2 Is Conserved across a Broad Range of Productivity. Proc. Natl. Acad. Sci. USA 2005, 102, 18052–18056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Zhang, W.; Chen, H.; Mo, J. Impacts of Nitrogen Deposition on Soil Nitrogen Cycle in Forest Ecosystems: A Review. Acta Ecol. Sin. 2015, 35, 35–43. [Google Scholar] [CrossRef]
- Bonan, G. Climate Change and Terrestrial Ecosystem Modeling; Cambridge University Press: Cambridge, UK, 2019; pp. 391–428. [Google Scholar] [CrossRef]
- Fang, S.; He, Z.; Du, J.; Chen, L.; Lin, P.; Zhao, M. Carbon Mass Change and Its Drivers in a Boreal Coniferous Forest in the Qilian Mountains, China from 1964 to 2013. Forests 2018, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Luo, Y.; Noulèkoun, F.; Noh, N.J.; Lee, J.; Son, Y. Carbon and Nitrogen Turnover Times of South Korean Forests Estimated via Data-Model Fusion. J. Geophys Res. Biogeosciences 2021, submitted and under review. [Google Scholar]
- Shanin, V.N.; Komarov, A.S.; Mikhailov, A.V.; Bykhovets, S.S. Modelling Carbon and Nitrogen Dynamics in Forest Ecosystems of Central Russia under Different Climate Change Scenarios and Forest Management Regimes. Ecol. Model. 2011, 222, 2262–2275. [Google Scholar] [CrossRef]
- Kurz, W.A.; Dymond, C.C.; White, T.M.; Stinson, G.; Shaw, C.H.; Rampley, G.J.; Smyth, C.; Simpson, B.N.; Neilson, E.T.; Trofymow, J.A.; et al. CBM-CFS3: A Model of Carbon-Dynamics in Forestry and Land-Use Change Implementing IPCC Standards. Ecol. Model. 2009, 220, 480–504. [Google Scholar] [CrossRef]
- National Institute of Forest Science. Fifty Years of National Forest Service; National Institute of Forest Science: Seoul, Korea, 2017. (In Korean)
- Korea Forest Service. Lessons Learned from the Republic of Korea’s National Reforestation Programme; Korea Forest Service: Daejeon, Korea, 2014.
- Korea Forest Service. Statistical Yearbook of Forestry; Korea Forest Service: Daejeon, Korea, 2020. (In Korean)
- Korea Meteorological Administration. Climate Data Open Portal. 2021. Available online: Data.kma.go.kr/cmmn/main.do (accessed on 30 June 2021).
- Choi, E.; Kim, T. Estimated Nitrogen Discharge by a Mass Balance Approach. J. Environ. Policy 2004, 3, 95–117. [Google Scholar]
- Ministry of Environment. Environmental Statistics Yearbook; Ministry of Environment: Sejong, Korea, 2019.
- Lee, J.; Yoon, T.K.; Han, S.; Kim, S.; Yi, M.J.; Park, G.S.; Kim, C.; Son, Y.M.; Kim, R.; Son, Y. Estimating the Carbon Dynamics of South Korean Forests from 1954 to 2012. Biogeosciences 2014, 11, 4637–4650. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kraxner, F.; Son, Y.; Jeon, S.W.; Shvidenko, A.; Schepaschenko, D.; Ham, B.-Y.; Lim, C.-H.; Song, C.; Hong, M.; et al. Quantifying Impacts of National-Scale Afforestation on Carbon Budgets in South Korea from 1961 to 2014. Forests 2019, 10, 579. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lim, C.-H.; Kim, G.S.; Markandya, A.; Chowdhury, S.; Kim, S.J.; Lee, W.-K.; Son, Y. Economic Viability of the National-Scale Forestation Program: The Case of Success in the Republic of Korea. Ecosyst. Serv. 2018, 29, 40–46. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Vicca, S.; Janssens, I.A.; Luyssaert, S.; Campioli, M.; Sardans, J.; Estiarte, M.; Peñuelas, J. Spatial Variability and Controls over Biomass Stocks, Carbon Fluxes, and Resource-Use Efficiencies across Forest Ecosystems. Trees 2014, 28, 597–611. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Lee, J.; Chang, H.; Roh, Y.; An, J.; Son, Y. Development of a Forest Carbon and Nitrogen Model: Pilot Application for a Pinus Densiflora Forest in Central Korea. For. Sci. Technol. 2019, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Song, K.C.; Hyun, B.K.; Kang, H.J. Reclassification of Korean Soils According to Revised Soil Taxonomy. Korean J. Soil Sci. Fertil. 2019, 52, 93–104. [Google Scholar] [CrossRef]
- Smith, D.M.; Larson, B.C.; Kelty, M.J.; Ashton, P.M.S. The Practice of Silviculture: Applied Forest Ecology, 9th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Korea Forest Service. Forest Site and Soil Map; Korea Forest Service: Daejeon, Korea, 2020. (In Korean)
- Korea Forest Service. Forest Type Map; Korea Forest Service: Daejeon, Korea, 2020. (In Korean)
- Environmental Systems Research Institute. ArcGIS Pro 2.6; Environmental Systems Research Institute: Redlands, CA, USA, 2021. [Google Scholar]
- Hegglin, M.; Kinnison, D.; Lamarque, J. CCMI Nitrogen Surface Fluxes in Support of CMIP6-Version 2.0. Version 20161207. Earth Syst. Grid Fed. 2016. [Google Scholar] [CrossRef]
- Sellar, A.A.; Walton, J.; Jones, C.G.; Wood, R.; Abraham, N.L.; Andrejczuk, M.; Andrews, M.B.; Andrews, T.; Archibald, A.T.; Mora, L.; et al. Implementation of U.K. Earth System Models for CMIP6. J. Adv. Modeling Earth Syst. 2020, 12, e2019MS001946. [Google Scholar] [CrossRef]
- Shi, S.; Peng, C.; Wang, M.; Zhu, Q.; Yang, G.; Yang, Y.; Xi, T.; Zhang, T. A Global Meta-Analysis of Changes in Soil Carbon, Nitrogen, Phosphorus and Sulfur, and Stoichiometric Shifts after Forestation. Plant. Soil 2016, 407, 323–340. [Google Scholar] [CrossRef]
- Tang, X.; Liu, S.; Liu, J.; Zhou, G. Effects of Vegetation Restoration and Slope Positions on Soil Aggregation and Soil Carbon Accumulation on Heavily Eroded Tropical Land of Southern China. J. Soils Sediments 2010, 10, 505–513. [Google Scholar] [CrossRef]
- Davis, M. Soil Impacts of Afforestation in the High Country. N. Z. For. 1998, 42, 34–38. [Google Scholar]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Corre, M.D.; Veldkamp, E.; Arnold, J.; Wright, S.J. Impact of Elevated N Input on Soil N Cycling and Losses in Old-growth Lowland and Montane Forests in Panama. Ecology 2010, 91, 1715–1729. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, P.; Emmett, B.A.; Kjønaas, O.J.; Koopmans, C.J.; Tietema, A. Impact of Nitrogen Deposition on Nitrogen Cycling in Forests: A Synthesis of NITREX Data. For. Ecol. Manag. 1998, 101, 37–55. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial Ecosystem Carbon Dynamics and Climate Feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant Carbon Metabolism and Climate Change: Elevated CO2 and Temperature Impacts on Photosynthesis, Photorespiration and Respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschbaum, M.U.F. The Temperature Dependence of Organic Matter Decomposition: Seasonal Temperature Variations Turn a Sharp Short-term Temperature Response into a More Moderate Annually Averaged Response. Glob. Chang. Biol. 2010, 16, 2117–2129. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Cox, P.; Betts, R.; Bopp, L.; von Bloh, W.; Brovkin, V.; Cadule, P.; Doney, S.; Eby, M.; Fung, I.; et al. Climate–Carbon Cycle Feedback Analysis: Results from the C4MIP Model Intercomparison. J. Clim. 2006, 19, 3337–3353. [Google Scholar] [CrossRef]
- Luo, Y.; Su, B.; Currie, W.S.; Dukes, J.S.; Finzi, A.; Hartwig, U.; Hungate, B.; Murtrie, R.E.M.; Oren, R.; Parton, W.J.; et al. Progressive Nitrogen Limitation of Ecosystem Responses to Rising Atmospheric Carbon Dioxide. Bioscience 2004, 54, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Norby, R.J.; Kauwe, M.G.D.; Domingues, T.F.; Duursma, R.A.; Ellsworth, D.S.; Goll, D.S.; Lapola, D.M.; Luus, K.A.; MacKenzie, A.R.; Medlyn, B.E.; et al. Model–Data Synthesis for the next Generation of Forest Free-air CO2 Enrichment (FACE) Experiments. New Phytol. 2016, 209, 17–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.; Medlyn, B.E.; Drake, J.E.; Duursma, R.A.; Anderson, I.C.; Barton, C.V.M.; Boer, M.M.; Carrillo, Y.; Castañeda-Gómez, L.; Collins, L.; et al. The Fate of Carbon in a Mature Forest under Carbon Dioxide Enrichment. Nature 2020, 580, 227–231. [Google Scholar] [CrossRef]
- Hyvönen, R.; Ågren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The Likely Impact of Elevated [CO2], Nitrogen Deposition, Increased Temperature and Management on Carbon Sequestration in Temperate and Boreal Forest Ecosystems: A Literature Review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef]
- Fleischer, K.; Rebel, K.T.; Molen, M.K.; Erisman, J.W.; Wassen, M.J.; Loon, E.E.; Montagnani, L.; Gough, C.M.; Herbst, M.; Janssens, I.A.; et al. The Contribution of Nitrogen Deposition to the Photosynthetic Capacity of Forests. Glob. Biogeochem. Cycles 2013, 27, 187–199. [Google Scholar] [CrossRef]
- de Vries, W.; Du, E.; Butterbach-Bahl, K. Short and Long-Term Impacts of Nitrogen Deposition on Carbon Sequestration by Forest Ecosystems. Curr. Opin. Env. Sustain. 2014, 9, 90–104. [Google Scholar] [CrossRef]
- Iivonen, S.; Kaakinen, S.; Jolkkonen, A.; Vapaavuori, E.; Linder, S. Influence of Long-Term Nutrient Optimization on Biomass, Carbon, and Nitrogen Acquisition and Allocation in Norway Spruce. Can. J. For. Res. 2006, 36, 1563–1571. [Google Scholar] [CrossRef]
- Churkina, G.; Trusilova, K.; Vetter, M.; Dentener, F. Contributions of Nitrogen Deposition and Forest Regrowth to Terrestrial Carbon Uptake. Carbon Balance Manag. 2007, 2, 5. [Google Scholar] [CrossRef]
- He, L.; Chen, J.M.; Croft, H.; Gonsamo, A.; Luo, X.; Liu, J.; Zheng, T.; Liu, R.; Liu, Y. Nitrogen Availability Dampens the Positive Impacts of CO2 Fertilization on Terrestrial Ecosystem Carbon and Water Cycles. Geophys. Res. Lett. 2017, 44, 11590–11600. [Google Scholar] [CrossRef] [Green Version]
- Reich, P.B.; Hobbie, S.E.; Lee, T.; Ellsworth, D.S.; West, J.B.; Tilman, D.; Knops, J.M.H.; Naeem, S.; Trost, J. Nitrogen Limitation Constrains Sustainability of Ecosystem Response to CO2. Nature 2006, 440, 922–925. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W.; Solberg, S.; Dobbertin, M.; Sterba, H.; Laubhann, D.; van Oijen, M.; Evans, C.; Gundersen, P.; Kros, J.; Wamelink, G.W.W.; et al. The Impact of Nitrogen Deposition on Carbon Sequestration by European Forests and Heathlands. Forest Ecol. Manag. 2009, 258, 1814–1823. [Google Scholar] [CrossRef]
- Norby, R.J.; Warren, J.M.; Iversen, C.M.; Medlyn, B.E.; McMurtrie, R.E. CO2 Enhancement of Forest Productivity Constrained by Limited Nitrogen Availability. Proc. Natl. Acad. Sci. USA 2010, 107, 19368–19373. [Google Scholar] [CrossRef] [Green Version]
- Lovett, G.M.; Goodale, C.L. A New Conceptual Model of Nitrogen Saturation Based on Experimental Nitrogen Addition to an Oak Forest. Ecosystems 2011, 14, 615–631. [Google Scholar] [CrossRef]
- Lupon, A.; Gerber, S.; Sabater, F.; Bernal, S. Climate Response of the Soil Nitrogen Cycle in Three Forest Types of a Headwater Mediterranean Catchment. J. Geophys. Res. Biogeosci. 2015, 120, 859–875. [Google Scholar] [CrossRef] [Green Version]
- Melillo, J.M.; Steudler, P.A.; Aber, J.D.; Newkirk, K.; Lux, H.; Bowles, F.P.; Catricala, C.; Magill, A.; Ahrens, T.; Morrisseau, S. Soil Warming and Carbon-Cycle Feedbacks to the Climate System. Science 2002, 298, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.M.; Melillo, J.M.; Johnson, J.E.; Mohan, J.; Steudler, P.A.; Lux, H.; Burrows, E.; Smith, R.M.; Vario, C.L.; Scott, L.; et al. Soil Warming Alters Nitrogen Cycling in a New England Forest: Implications for Ecosystem Function and Structure. Oecologia 2012, 168, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, S.; Grime, J.P.; Harris, J.; McPherson, E. Evidence of a Feedback Mechanism Limiting Plant Response to Elevated Carbon Dioxide. Nature 1993, 364, 616–617. [Google Scholar] [CrossRef]
- Pepper, D.A.; Eliasson, P.E.; McMurtrie, R.E.; Corbeels, M.; Ågren, G.I.; Strömgren, M.; Linder, S. Simulated Mechanisms of Soil N Feedback on the Forest CO2 Response. Global Change Biol. 2007, 13, 1265–1281. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Currie, W.S.; Nadelhoffer, K.J.; Martin, M.E.; McDowell, W.H.; Melillo, J.M.; Steudler, P. Ecosystem Response to 15 Years of Chronic Nitrogen Additions at the Harvard Forest LTER, Massachusetts, USA. For. Ecol. Manag. 2004, 196, 7–28. [Google Scholar] [CrossRef]
- Aber, J.D.; Magill, A.; Mcnulty, S.G.; Boone, R.D.; Nadelhoffer, K.J.; Downs, M.; Hallett, R. Forest Biogeochemistry and Primary Production Altered by Nitrogen Saturation. Water Air Soil Pollut. 1995, 85, 1665–1670. [Google Scholar] [CrossRef] [Green Version]
- Greenhouse Gas Inventory and Research Center of Korea. National Greenhouse Gas. Inventory Report of Korea; Greenhouse Gas Inventory and Research Center of Korea: Seoul, Korea, 2021.
- Pregitzer, K.S.; Euskirchen, E.S. Carbon Cycling and Storage in World Forests: Biome Patterns Related to Forest Age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Amiro, B.D.; Barr, A.G.; Black, T.A.; Iwashita, H.; Kljun, N.; McCaughey, J.H.; Morgenstern, K.; Murayama, S.; Nesic, Z.; Orchansky, A.L.; et al. Carbon, Energy and Water Fluxes at Mature and Disturbed Forest Sites, Saskatchewan, Canada. Agric. For. Meteorol. 2006, 136, 237–251. [Google Scholar] [CrossRef]
- Goulden, M.L.; McMillan, A.M.S.; Winston, G.C.; Rocha, A.V.; Manies, K.L.; Harden, J.W.; Bond-Lamberty, B.P. Patterns of NPP, GPP, Respiration, and NEP during Boreal Forest Succession. Glob. Chang. Biol. 2011, 17, 855–871. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yu, G.; Wang, Q. Effects of Climate and Forest Age on the Ecosystem Carbon Exchange of Afforestation. J. For. Res. 2020, 31, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Pugh, T.A.M.; Lindeskog, M.; Smith, B.; Poulter, B.; Arneth, A.; Haverd, V.; Calle, L. Role of Forest Regrowth in Global Carbon Sink Dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 201810512. [Google Scholar] [CrossRef] [Green Version]
- Bose, A.K.; Weiskittel, A.; Kuehne, C.; Wagner, R.G.; Turnblom, E.; Burkhart, H.E. Does Commercial Thinning Improve Stand-Level Growth of the Three Most Commercially Important Softwood Forest Types in North America? For. Ecol. Manag. 2018, 409, 683–693. [Google Scholar] [CrossRef]
- Johnston, C.M.T.; Radeloff, V.C. Global Mitigation Potential of Carbon Stored in Harvested Wood Products. Proc. Natl. Acad. Sci. USA 2019, 116, 14526–14531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Büntgen, U.; Krusic, P.J.; Piermattei, A.; Coomes, D.A.; Esper, J.; Myglan, V.S.; Kirdyanov, A.V.; Camarero, J.J.; Crivellaro, A.; Körner, C. Limited Capacity of Tree Growth to Mitigate the Global Greenhouse Effect under Predicted Warming. Nat. Commun 2019, 10, 2171. [Google Scholar] [CrossRef] [PubMed]
- Lamba, S.; Hall, M.; Räntfors, M.; Chaudhary, N.; Linder, S.; Way, D.; Uddling, J.; Wallin, G. Physiological Acclimation Dampens Initial Effects of Elevated Temperature and Atmospheric CO2 Concentration in Mature Boreal Norway Spruce. Plant. Cell Environ. 2018, 41, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. What Have We Learned from 15 Years of Free-air CO2 Enrichment (FACE)? A Meta-analytic Review of the Responses of Photosynthesis, Canopy Properties and Plant Production to Rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Wei, X.; Rich, R.L.; Montgomery, R.A. Boreal and Temperate Trees Show Strong Acclimation of Respiration to Warming. Nature 2016, 531, 633–636. [Google Scholar] [CrossRef]



| Scenarios | Level of Environmental Forcing | ||
|---|---|---|---|
| Air Temperature | CO2 Concentration | N Deposition | |
| (1) T0CO0Nd0 | Ambient | Ambient | Ambient |
| T0CO0Nd1 | Ambient | Ambient | Increased |
| T0CO1Nd0 | Ambient | Increased | Ambient |
| T0CO1Nd1 | Ambient | Increased | Increased |
| T1CO0Nd0 | Increased | Ambient | Ambient |
| T1CO0Nd1 | Increased | Ambient | Increased |
| T1CO1Nd0 | Increased | Increased | Ambient |
| (2) T1CO1Nd1 | Increased | Increased | Increased |
| Scenarios | Relative Difference (%) | |||||
|---|---|---|---|---|---|---|
| C Fluxes | N Fluxes | |||||
| Input | Output | Balance | Input | Output | Balance | |
| T0CO0Nd0 | – | – | – | – | – | – |
| T0CO0Nd1 | +18.9 | +17.3 | +26.6 | +90.5 | +44.7 | +156 |
| T0CO1Nd0 | +2.03 | +1.90 | +2.64 | 0 | −0.53 | +0.76 |
| T0CO1Nd1 | +21.4 | +19.7 | +29.8 | +90.5 | +43.9 | +157 |
| T1CO0Nd0 | +2.95 | +3.90 | −1.53 | 0 | +1.54 | −2.19 |
| T1CO0Nd1 | +22.4 | +22.0 | +24.2 | +90.5 | +46.7 | +153 |
| T1CO1Nd0 | +5.10 | +5.94 | +1.12 | 0 | +0.95 | −1.35 |
| T1CO1Nd1 | +25.0 | +24.5 | +27.5 | +90.5 | +45.8 | +154 |
| Scenarios | Difference | |||||
|---|---|---|---|---|---|---|
| C Stocks (Mg C ha−1) | N Stocks (kg N ha−1) | |||||
| Tree Biomass | Primary DOM | Mineral Soil | Tree Biomass | Primary DOM | Mineral Soil | |
| T0CO0Nd0 | – | – | – | – | – | – |
| T0CO0Nd1 | +23.4 | +2.95 | +2.91 | +62.3 | +75.1 | +161 |
| T0CO1Nd0 | +2.36 | +0.25 | +0.27 | −1.97 | −0.43 | +3.87 |
| T0CO1Nd1 | +26.3 | +3.28 | +3.25 | +58.8 | +74.8 | +167 |
| T1CO0Nd0 | −0.67 | −0.55 | −0.67 | +6.64 | −6.17 | −4.66 |
| T1CO0Nd1 | +22.1 | +2.15 | +2.10 | +71.7 | +64.8 | +156 |
| T1CO1Nd0 | +1.71 | −0.31 | −0.41 | +4.64 | −6.50 | −0.71 |
| T1CO1Nd1 | +25.1 | +2.46 | +2.43 | +68.3 | +64.6 | +162 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-S.; Noulèkoun, F.; Noh, N.-J.; Son, Y.-W. Impacts of the National Forest Rehabilitation Plan and Human-Induced Environmental Changes on the Carbon and Nitrogen Balances of the South Korean Forests. Forests 2021, 12, 1150. https://doi.org/10.3390/f12091150
Kim H-S, Noulèkoun F, Noh N-J, Son Y-W. Impacts of the National Forest Rehabilitation Plan and Human-Induced Environmental Changes on the Carbon and Nitrogen Balances of the South Korean Forests. Forests. 2021; 12(9):1150. https://doi.org/10.3390/f12091150
Chicago/Turabian StyleKim, Hyung-Sub, Florent Noulèkoun, Nam-Jin Noh, and Yo-Whan Son. 2021. "Impacts of the National Forest Rehabilitation Plan and Human-Induced Environmental Changes on the Carbon and Nitrogen Balances of the South Korean Forests" Forests 12, no. 9: 1150. https://doi.org/10.3390/f12091150
APA StyleKim, H.-S., Noulèkoun, F., Noh, N.-J., & Son, Y.-W. (2021). Impacts of the National Forest Rehabilitation Plan and Human-Induced Environmental Changes on the Carbon and Nitrogen Balances of the South Korean Forests. Forests, 12(9), 1150. https://doi.org/10.3390/f12091150






