Chronically Low Nutrient Concentrations in Tree Rings Are Linked to Greater Tree Vulnerability to Drought in Nothofagus dombeyi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Species
2.2. Field Sampling and Dendrochronological Data
2.3. Tree-Ring Isotopic Composition
2.4. Nutrient Analyses
2.5. Data Analyses
3. Results
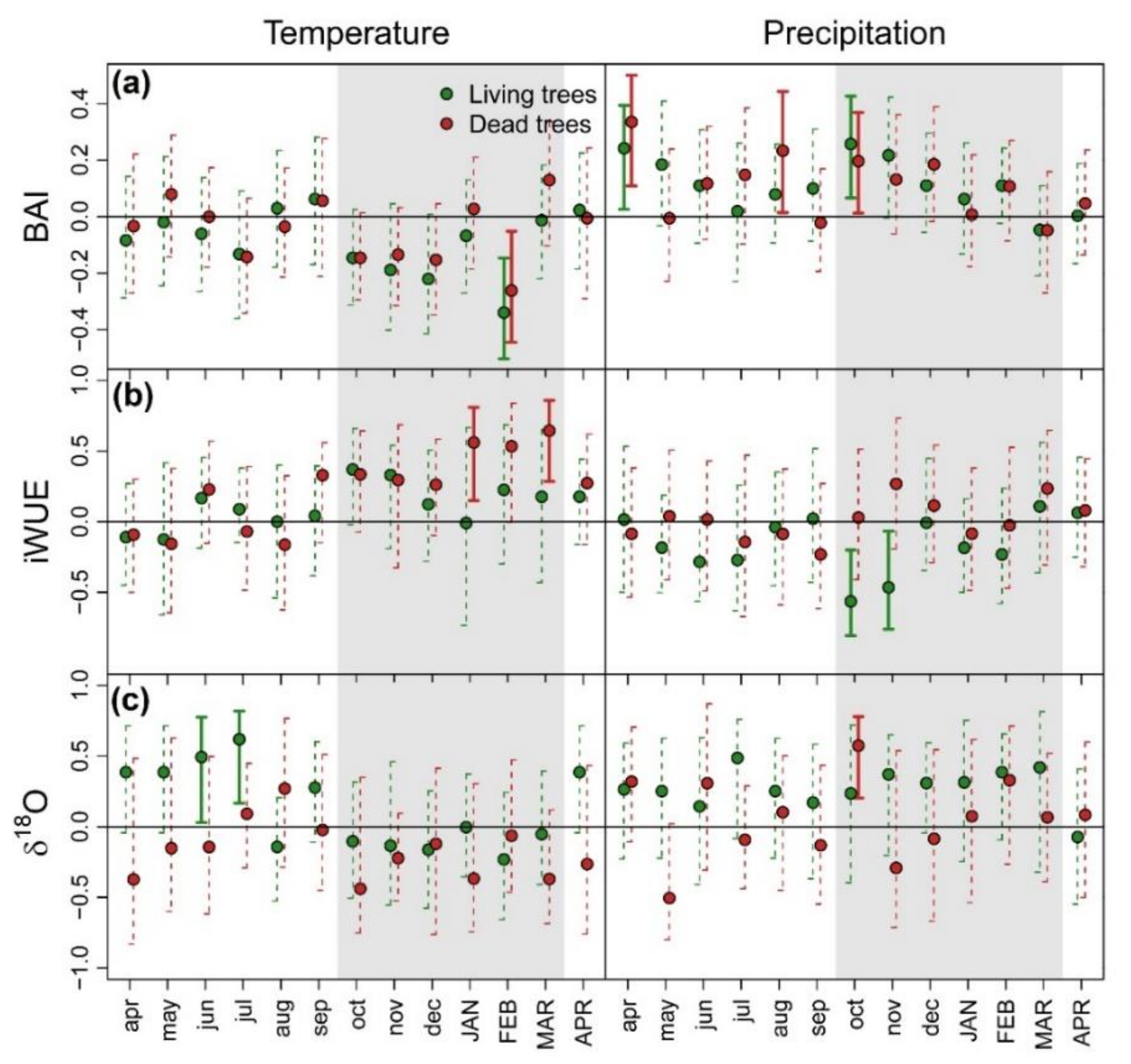
3.1. Patterns and Climate-Drivers of Growth and Tree-Ring Isotope Signature
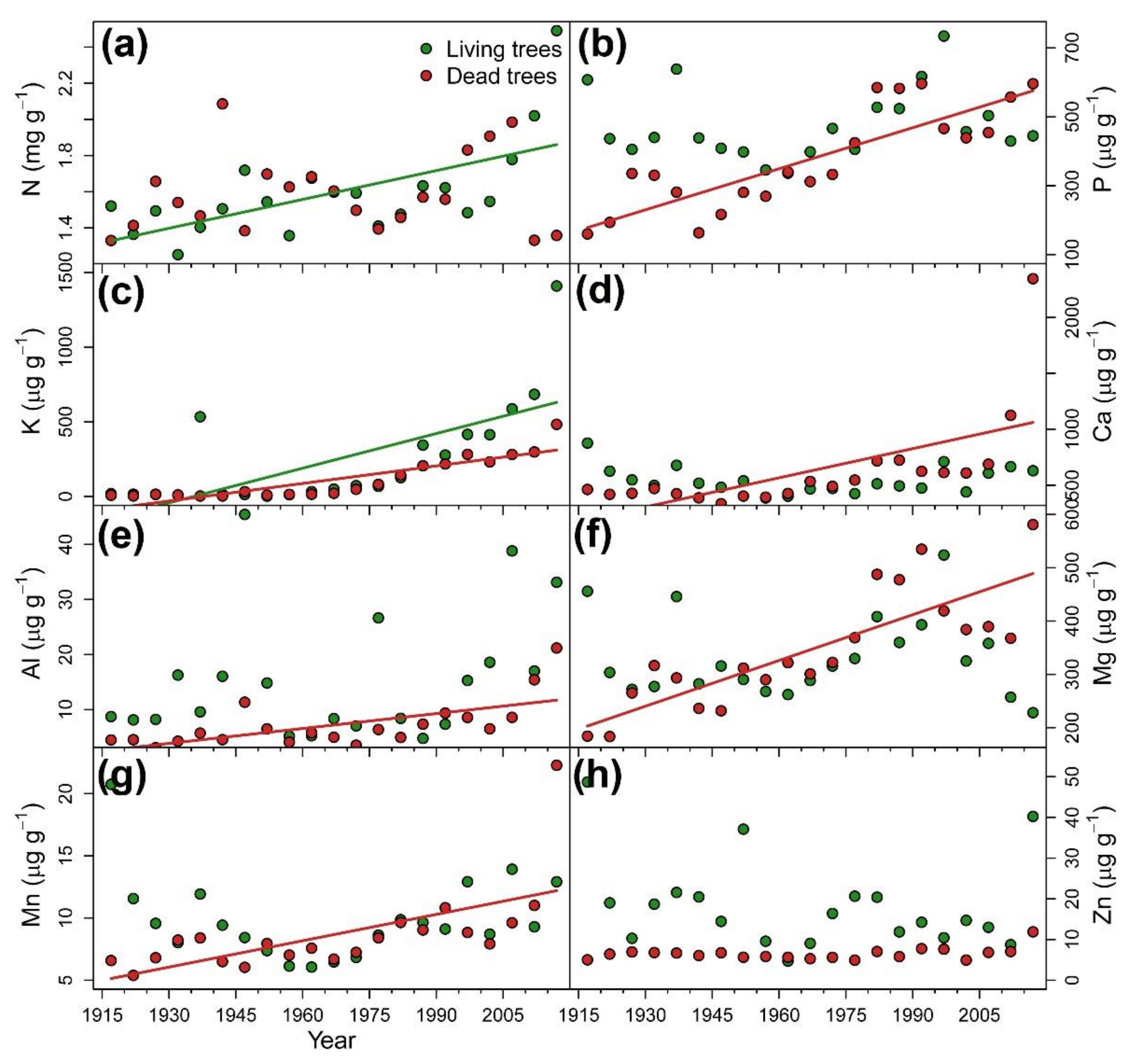
3.2. Long-Term Reconstruction of Tree Nutrient Status
3.3. Integration of Growth, Isotopic Signals and Nutrient Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Climatic Trends

References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Kharuk, V.I.; Im, S.T.; Petrov, I.A.; Dvinskaya, M.L.; Shushpanov, A.S.; Golyukov, A.S. Climate-driven conifer mortality in Siberia. Glob. Ecol. Biogeogr. 2021, 30, 543–556. [Google Scholar] [CrossRef]
- van Mantgem, P.J.; Stephenson, N.L. Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol. Lett. 2007, 909–916. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Cantero, A.; Sánchez-Salguero, R.; Sánchez-Miranda, A.; Granda, E.; Serra-Maluquer, X.; Ibáñez, R. Forest growth responses to drought at short- and long-term scales in Spain: Squeezing the stress memory from tree rings. Front. Ecol. Evol. 2018, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Phillips, O.L.; Aragão, L.E.O.C.; Lewis, S.L.; Fisher, J.B.; Lloyd, J.; López-gonzález, G.; Malhi, Y.; Monteagudo, A.; Peacock, J.; Quesada, C.A.; et al. Drought sensitivity of the Amazon rainforest. Science 2009, 323, 1344–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC AR5 The Physical Science Basis. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Zhou, S.; Zhang, Y.; Williams, A.P.; Gentine, P. Projected increases in intensity, frequency, and terrestrial carbon costs of compound drought and aridity events. Sci. Adv. 2019, 5, eaau5740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christidis, N.; Jones, G.S.; Stott, P.A. Dramatically increasing chance of extremely hot summers since the 2003 European heatwave. Nat. Clim. Chang. 2015, 5, 46–50. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, art129. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.L.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; Mcdowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef]
- Gessler, A.; Schaub, M.; McDowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2017, 214, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Kreuzwieser, J.; Gessler, A. Global climate change and tree nutrition: Influence of water availability. Tree Physiol. 2010, 30, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, W.H.; Dietze, M.C.; Jackson, R.B.; Phillips, R.P.; Rhoades, C.C.; Rustad, L.E.; Vose, J.M. Forest biogeochemistry in response to drought. Glob. Chang. Biol. 2016, 22, 2318–2328. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Estiarte, M.; Ogaya, R.; Peñuelas, J. Field-simulated droughts affect elemental leaf stoichiometry in Mediterranean forests and shrublands. Acta Oecologica 2013, 50, 20–31. [Google Scholar] [CrossRef]
- Sardans, J.; Grau, O.; Chen, H.Y.H.; Janssens, I.A.; Ciais, P.; Piao, S.; Peñuelas, J. Changes in nutrient concentrations of leaves and roots in response to global change factors. Glob. Chang. Biol. 2017, 23, 3849–3856. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Tortosa, D.; Castro, J.; Villar-salvador, P.; Viñegla, B.; Matías, L.; Michelsen, A.; Rubio de Casas, R.; Querejeta, J.I. The “isohydric trap”: A proposed feedback between water shortage, stomatal regulation, and nutrient acquisition drives differential growth and survival of European pines under climatic dryness. Glob. Chang. Biol. 2018, 24, 4069–4083. [Google Scholar] [CrossRef] [PubMed]
- Voltas, J.; Lucabaugh, D.; Chambel, M.R.; Ferrio, J.P. Intraspecific variation in the use of water sources by the circum-Mediterranean conifer Pinus halepensis. New Phytol. 2015, 208, 1031–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobbágy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Scheidegger, Y.; Saurer, M.; Bahn, M.; Siegwolf, R. Linking stable oxygen and carbon isotopes with stomatal conductance and photosynthetic capacity: A conceptual model. Oecologia 2000, 125, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; O’Leary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Saurer, M.; Siegwolf, R.T.W.; Schweingruber, F.H. Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Chang. Biol. 2004, 10, 2109–2120. [Google Scholar] [CrossRef]
- Peñuelas, J.; Canadell, J.G.; Ogaya, R. Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 2011, 20, 597–608. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Tardif, J.C.; Conciatori, F. Attributing forest responses to global-change drivers: Limited evidence of a CO2-fertilization effect in Iberian pine growth. J. Biogeogr. 2015, 42, 2220–2233. [Google Scholar] [CrossRef] [Green Version]
- Gessler, A.; Löw, M.; Heerdt, C.; De Beeck, M.O.; Schumacher, J.; Grams, T.E.E.; Bahnweg, G.; Ceulemans, R.; Werner, H.; Matyssek, R.; et al. Within-canopy and ozone fumigation effects on δ13C and Δ18O in adult beech (Fagus sylvatica) trees: Relation to meteorological and gas exchange parameters. Tree Physiol. 2009, 29, 1349–1365. [Google Scholar] [CrossRef] [Green Version]
- Roden, J.; Siegwolf, R. Is the dual-isotope conceptual model fully operational? Tree Physiol. 2013, 32, 1179–1182. [Google Scholar] [CrossRef]
- Gessler, A.; Ferrio, J.P.; Hommel, R.; Treydte, K.; Werner, R.A.; Monson, R.K. Stable isotopes in tree rings: Towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol. 2014, 34, 796–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treydte, K.; Boda, S.; Pannatier, E.G.; Fonti, P.; Frank, D.; Ullrich, B.; Saurer, M.; Siegwolf, R.; Battipaglia, G.; Werner, W.; et al. Seasonal transfer of oxygen isotopes from precipitation and soil to the tree ring: Source water versus needle water enrichment. New Phytol. 2014, 202, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Siegwolf, R.; Körner, C. Inter- and intra-annual stable carbon and oxygen isotope signals in response to drought in Mediterranean pines. Agric. For. Meteorol. 2013, 168, 59–68. [Google Scholar] [CrossRef]
- Shestakova, T.A.; Aguilera, M.; Ferrio, J.P.; Gutiérrez, E.; Voltas, J. Unravelling spatiotemporal tree-ring signals in Mediterranean oaks: A variance–covariance modelling approach of carbon and oxygen isotope ratios. Tree Physiol. 2014, 34, 819–838. [Google Scholar] [CrossRef]
- Barbeta, A.; Peñuelas, J. Increasing carbon discrimination rates and depth of water uptake favor the growth of Mediterranean evergreen trees in the ecotone with temperate deciduous forests. Glob. Chang. Biol. 2017, 23, 5054–5068. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Dawson, T.E.; Adams, H.D.; Collins, A.D.; Dickman, L.T.; Newman, B.D.; Stockton, E.A.; McDowell, N.G. Warming combined with more extreme precipitation regimes modifies the water sources used by trees. New Phytol. 2017, 213, 584–596. [Google Scholar] [CrossRef]
- Hevia, A.; Sánchez-Salguero, R.; Camarero, J.J.; Querejeta, J.I.; Sangüesa-Barreda, G.; Gazol, A. Long-term nutrient imbalances linked to drought-triggered forest dieback. Sci. Total Environ. 2019, 690, 1254–1267. [Google Scholar] [CrossRef]
- Houle, D.; Tremblay, S.; Ouimet, R. Foliar and wood chemistry of sugar maple along a gradient of soil acidity and stand health. Plant Soil 2007, 300, 173–183. [Google Scholar] [CrossRef]
- Veblen, T.T.; Hill, R.S.; Read, J. The Ecology and Biogeography of Nothofagus Forests; Yale University Press: London, UK, 1996. [Google Scholar]
- Suarez, M.L.; Ghermandi, L.; Kitzberger, T. Factors predisposing episodic drought-induced tree mortality in Nothofagus—Site, climatic sensitivity and growth trends. J. Ecol. 2004, 92, 954–966. [Google Scholar] [CrossRef]
- Suarez, M.L.; Kitzberger, T. Differential effects of climate variability on forest dynamics along a precipitation gradient in northern Patagonia. J. Ecol. 2010, 98, 1023–1034. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G. Strong hydraulic segmentation and leaf senescence due to dehydration may trigger die-back in Nothofagus dombeyi under severe droughts: A comparison with the co-occurring Austrocedrus chilensis. Trees Struct. Funct. 2014, 28, 1475–1487. [Google Scholar] [CrossRef]
- Suarez, M.L.; Villalba, R.; Mundo, I.A.; Schroeder, N. Sensitivity of Nothofagus dombeyi tree growth to climate changes along a precipitation gradient in northern Patagonia, Argentina. Trees Struct. Funct. 2015, 29, 1053–1067. [Google Scholar] [CrossRef]
- De Fina, A.L. El clima de la región de los bosques Andino-Patagónicos. In La Región de Los Bosques Andino-Patagónicos, Sinopsis General; Dimitri, M.J., Ed.; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 1972; pp. 35–58. [Google Scholar]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef] [Green Version]
- Beguería, S.; Vicente-Serrano, S.M. SPEI: Calculation of the Standardised Precipitation-Evapotranspiration Index. R Package Version 1.7. 2017. Available online: https://CRAN.R-project.org/package=SPEI (accessed on 3 May 2021).
- R Foundation for Statistical Computing. R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- IAEA/WMO Global Network of Isotopes in Precipitation. The GNIP Database. 2021. Available online: https://nucleus.iaea.org/ (accessed on 13 May 2021).
- Villalba, R.; Veblen, T.T. Influences of large-scale climatic variability on episodic tree mortality in northern Patagonia. Ecology 1998, 79, 2624–2640. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1977. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Suarez, M.L. Tree-ring records from Nothofagus dombeyi: A preliminary chronology network in Northern Patagonia, Argentina. Dendrochronologia 2010, 28, 65–72. [Google Scholar] [CrossRef]
- Biondi, F.; Qeadan, F. A theory-driven approach to tree-ring standardization: Defining the biological trend from expected basal area increment. Tree Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef] [Green Version]
- Sharp, Z. Principles of Stable Isotope Geochemistry; Prentice Hall: Hoboken, NJ, USA, 2005. [Google Scholar]
- Belmecheri, S.; Lavergne, A. Compiled records of atmospheric CO2 concentrations and stable carbon isotopes to reconstruct climate and derive plant ecophysiological indices from tree rings. Dendrochronologia 2020, 63, 125748. [Google Scholar] [CrossRef]
- Kuang, Y.W.; Wen, D.Z.; Zhou, G.Y.; Chu, G.W.; Sun, F.F.; Li, J. Reconstruction of soil pH by dendrochemistry of Masson pine at two forested sites in the Pearl River Delta, South China. Ann. For. Sci. 2008, 65, 804. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.E.; Wu, Z. A review on Hilbert-Huang transform: Method and its applications. Rev. Geophys. 2008, 46, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Guan, B.T.; Wright, W.E.; Cook, E.R. Ensemble empirical mode decomposition as an alternative for tree-ring chronology development. Tree Ring Res. 2018, 74, 28–38. [Google Scholar] [CrossRef]
- Lo, Y.; Blanco, J.A.; Guan, B.T. Douglas-fir radial growth in interior British Columbia can be linked to long-term oscillations in Pacific and Atlantic sea surface temperatures. Can. J. For. Res. 2017, 381, 371–381. [Google Scholar] [CrossRef]
- Guan, B.T. Ensemble empirical mode decomposition for analyzing phenological responses to warming. Agric. For. Meteorol. 2014, 194, 1–7. [Google Scholar] [CrossRef]
- González de Andrés, E.; Blanco, J.A.; Imbert, J.B.; Guan, B.T.; Lo, Y.H.; Castillo, F.J. ENSO and NAO affect long-term leaf litter dynamics and stoichiometry of Scots pine and European beech mixedwoods. Glob. Chang. Biol. 2019, 25, 3070–3090. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.-C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, N.E. Ensemble empirical mode decomposition: A noise-assisted data analysis method. Adv. Adapt. Data Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C. dplR: Dendrochronology Program Library in R. R Package Version 1.7.1. 2020. Available online: https://CRAN.R-project.org/package=dplR (accessed on 15 April 2021).
- McLeod, A.I. Kendall: Kendall Rank Correlation and Mann-Kendall Trend Test. R Package Version 2.2. 2011. Available online: https://CRAN.R-project.org/package=Kendall (accessed on 3 May 2021).
- Zeileis, A.; Kleiber, C.; Walter, K.; Hornik, K. Testing and dating of structural changes in practice. Comput. Stat. Data Anal. 2003, 44, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Helske, J.; Luukko, P. _Rlibeemd: Ensemble Empirical Mode Decomposition (EEMD) and Its Complete Variant (CEEMDAN)_. R Package Version 1.4.1. 2018. Available online: https://CRAN.R-project.org/package=Rlibeemd (accessed on 10 May 2021).
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Al, E. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 April 2021).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. _nlme: Linear and Nonlinear Mixed Effects Models_. R Package Version 3.1-145. 2020. Available online: https://CRAN.R-project.org/package=nlme (accessed on 15 April 2021).
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.43.15. 2019. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 15 April 2021).
- Rodríguez-Catón, M.; Villalba, R.; Srur, A.; Williams, A.P. Radial growth patterns associated with tree mortality in Nothofagus pumilio forest. Forests 2019, 10, 489. [Google Scholar] [CrossRef] [Green Version]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Estrada-Medina, H.; Allen, M.F.; Jiménez-Osornio, J.J. Water source partitioning among trees growing on shallow karst soils in a seasonally dry tropical climate. Oecologia 2007, 152, 26–36. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Anchukaitis, K.J.; Evans, M.N.; del Río, M.; Beeckman, H.; Cañellas, I. Effects of drought on xylem anatomy and water-use efficiency of two co-occurring pine species. Forests 2017, 8, 332. [Google Scholar] [CrossRef] [Green Version]
- Camarero, J.J.; Colangelo, M.; Gazol, A.; Azorín-molina, C. Drought and cold spells trigger dieback of temperate oak and beech forests in northern Spain. Dendrochronologia 2021, 66, 125812. [Google Scholar] [CrossRef]
- Sun, S.; Qiu, L.; He, C.; Li, C.; Zhang, J.; Meng, P. Drought-affected Populus simonii Carr. show lower growth and long-term increases in intrinsic water-use efficiency prior to tree mortality. Forests 2018, 9, 564. [Google Scholar] [CrossRef] [Green Version]
- Pellizzari, E.; Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Carrer, M. Wood anatomy and carbon-isotope discrimination support long-term hydraulic deterioration as a major cause of drought-induced dieback. Glob. Chang. Biol. 2016, 22, 2125–2137. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Battipaglia, G.; Borghetti, M.; De Micco, V.; Gentilesca, T.; Ripullone, F. A multi-proxy assessment of dieback causes in a Mediterranean oak species. Tree Physiol. 2017, 37, 617–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González de Andrés, E.; Camarero, J.J. Disentangling mechanisms of drought-induced dieback in Pinus nigra Arn. from growth and wood isotope patterns. Forests 2020, 11, 1339. [Google Scholar] [CrossRef]
- Ding, Y.; Nie, Y.; Chen, H.; Wang, K.; Querejeta, J.I. Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 2021, 229, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Allison, G.B.; Barnes, C.J.; Hughes, M.W. The distribution of deuterium and 18O in dry soils 2. Experimental. J. Hydrol. 1983, 64, 377–397. [Google Scholar] [CrossRef]
- Sprenger, M.; Leistert, H.; Gimbel, K.; Weiler, M. Illuminating hydrological processes at the soil-vegetation- atmosphere interface with water stable isotopes. Rev. Geophys. 2016, 54, 674–704. [Google Scholar] [CrossRef] [Green Version]
- Renée Brooks, J.; Barnard, H.R.; Coulombe, R.; McDonnell, J.J. Ecohydrologic separation of water between trees and streams in a Mediterranean climate. Nat. Geosci. 2010, 3, 100–104. [Google Scholar] [CrossRef]
- Offermann, C.; Ferrio, J.P.; Holst, J.; Grote, R.; Siegwolf, R.; Kayler, Z.; Gessler, A. The long way down—Are carbon and oxygen isotope signals in the tree ring uncoupled from canopy physiological processes? Tree Physiol. 2011, 31, 1088–1102. [Google Scholar] [CrossRef] [Green Version]
- Querejeta, J.I.; Ren, W.; Prieto, I. Vertical decoupling of soil nutrients and water under climate warming reduces plant cumulative nutrient uptake, water-use efficiency and productivity. New Phytol. 2021, 230, 1378–1393. [Google Scholar] [CrossRef]
- Camarero, J.J.; Sangüesa-Barreda, G.; Vergarechea, M. Prior height, growth, and wood anatomy differently predispose to drought-induced dieback in two Mediterranean oak speciesk. Ann. For. Sci. 2016, 73, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Fasanella, M.; Suarez, M.L.; Hasbún, R.; Premoli, A.C. Individual-based dendrogenomic analysis of forest dieback driven by extreme droughts. Can. J. For. Res. 2021, 51, 420–432. [Google Scholar] [CrossRef]
- Nardini, A.; Salleo, S.; Jansen, S. More than just a vulnerable pipeline: Xylem physiology in the light of ion-mediated regulation of plant water transport. J. Exp. Bot. 2011, 62, 4701–4718. [Google Scholar] [CrossRef]
- Trifilò, P.; Nardini, A.; Raimondo, F.; Lo Gullo, M.A.; Salleo, S. Ion-mediated compensation for drought-induced loss of xylem hydraulic conductivity in field-growing plants of Laurus nobilis. Funct. Plant Biol. 2011, 38, 606–613. [Google Scholar] [CrossRef]
- León-Sánchez, L.; Nicolás, E.; Goberna, M.; Prieto, I.; Maestre, F.T.; Querejeta, J.I. Poor plant performance under simulated climate change is linked to mycorrhizal responses in a semi-arid shrubland. J. Ecol. 2018, 106, 960–976. [Google Scholar] [CrossRef]
- Sette, C.R.; Laclau, J.P.; Tomazello Filho, M.; Moreira, R.M.; Bouillet, J.P.; Ranger, J.; Almeida, J.C.R. Source-driven remobilizations of nutrients within stem wood in Eucalyptus grandis plantations. Trees Struct. Funct. 2013, 27, 827–839. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef]
- Lambers, H.; Clements, J.C.; Nelson, M.N. How aphosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 2013, 100, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Hayes, P.E.; Laliberté, E.; Oliveira, R.S.; Turner, B.L. Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci. 2015, 20, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, T.; Emori, K.; Ito, S.I. Manganese hyperaccumulation from non-contaminated soil in Chengiopanax sciadophylloides Franch. et Sav. and its correlation with calcium accumulation. Soil Sci. Plant Nutr. 2013, 59, 591–602. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size matters a lot: Drought-affected Italian oaks are smaller and show lower growth prior to tree death. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Living Trees | Dead Trees | ||||
|---|---|---|---|---|---|
| Mean ± SE | Trend | Mean ± SE | Trend | ||
| Nutrient concentrations | N (mg g−1) | 1.6 ± 0.01 a | 0.438 ** | 1.6 ± 0.01 a | 0.076 |
| P (µg g−1) | 474.4 ± 22.19 a | 0.124 | 376.9 ± 31.9 b | 0.638 *** | |
| K (µg g−1) | 243.8 ± 75.73 a | 0.638 *** | 114.8 ± 30.09 a | 0.829 *** | |
| Ca (µg g−1) | 546.1 ± 26.04 a | −0.076 | 627.6 ± 94.05 a | 0.600 *** | |
| Al (µg g−1) | 15.4 ± 2.51 a | 0.200 | 7.2 ± 0.94 b | 0.543 *** | |
| Mg (µg g−1) | 331.5 ± 16.59 a | 0.029 | 346.1 ± 23.56 a | 0.667 *** | |
| Mn (µg g−1) | 9.9 ± 0.73 a | 0.067 | 8.7 ± 0.75 a | 0.600 *** | |
| Zn (µg g−1) | 18.3 ± 2.42 a | −0.200 | 6.5 ± 0.33 b | 0.133 | |
| Stoichiometric ratios | N:P | 7.7 ± 0.43 a | 0.143 | 11.0 ± 1.17 b | −0.328 |
| N:K | 198.2 ± 77.08 a | −0.619 *** | 318.3 ± 94.62 b | −0.790 *** | |
| P:Mn | 89.3 ± 3.96 a | 0.114 | 77.6 ± 4.38 b | 0.448 ** | |
| K:Ca | 0.4 ± 0.12 a | 0.762 *** | 0.2 ± 0.03 a | 0.733 *** | |
| Ca:Mn | 78.6 ± 2.73 a | −0.133 | 94.5 ± 4.44 b | 0.390 * | |
| Ca:Al | 34.9 ± 4.06 a | −0.286 | 62.1 ± 4.06 b | −0.067 | |
| Mg:Mn | 79.9 ± 3.98 a | 0.095 | 91.9 ± 3.51 b | 0.371 * | |
| iWUE | δ18O | ||||
|---|---|---|---|---|---|
| L Trees | D Trees | L Trees | D Trees | ||
| Nutrient concentrations | N (mg g−1) | 0.131 | −0.232 | 0.162 | 0.068 |
| P (µg g−1) | −0.126 | 0.490 * | −0.265 | 0.473 * | |
| K (µg g−1) | −0.139 | 0.548 * | −0.190 | 0.458 * | |
| Ca (µg g−1) | −0.100 | 0.317 | 0.347 | −0.432 * | |
| Al (µg g−1) | −0.074 | 0.713 *** | 0.293 | 0.311 | |
| Mg (µg g−1) | −0.016 | −0.016 | −0.321 | 0.579 * | |
| Mn (µg g−1) | 0.017 | 0.457 * | 0.009 | 0.473 * | |
| Zn (µg g−1) | 0.164 | 0.576 ** | 0.288 | 0.144 | |
| Stoichiometric ratios | N:P | 0.138 | −0.473 * | 0.362 + | −0.423 * |
| N:K | 0.095 | −0.541 * | 0.213 | −0.394 + | |
| P:Mn | −0.121 | 0.221 | −0.321 | 0.165 | |
| K:Ca | −0.166 | 0.468 * | 0.243 | 0.393 + | |
| Ca:Mn | −0.306 | 0.329 | −0.140 | −0.351 | |
| Ca:Al | 0.134 | −0.343 | 0.282 | 0.034 | |
| Mg:Mn | −0.029 | −0.029 | −0.345 | 0.364 | |
| Variables | Living Trees | Dead Trees | ||
|---|---|---|---|---|
| Coef. ± SE | F-Value | Coef. ± SE | F-Value | |
| DBH | 0.350 ± 0.038 | 72.868 *** | 0.499 ± 0.050 | 78.751 *** |
| iWUE | −0.035 ± 0.046 | 0.763 | −0.117 ± 0.060 | 3.950 * |
| δ18O | 0.051 ± 0.038 | 2.569 | 0.004 ± 0.028 | 0.025 |
| NMDS1Concentration | −0.280 ± 0.161 | 0.328 | −0.265 ± 0.183 | 29.072 *** |
| NMDS2Concentration | −0.094 ± 0.059 | 0.208 | 0.101 ± 0.161 | 0.256 |
| NMDS1Ratio | 0.397 ± 0.075 | 16.830 *** | 0.059 ± 0.045 | 2.991 |
| NMDS2Ratio | 0.143 ± 0.039 | 7.986 ** | 0.016 ± 0.036 | 0.505 |
| R_GLMM(m)2 | 0.319 | 0.482 | ||
| R_GLMM(c)2 | 0.387 | 0.523 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González de Andrés, E.; Suárez, M.L.; Querejeta, J.I.; Camarero, J.J. Chronically Low Nutrient Concentrations in Tree Rings Are Linked to Greater Tree Vulnerability to Drought in Nothofagus dombeyi. Forests 2021, 12, 1180. https://doi.org/10.3390/f12091180
González de Andrés E, Suárez ML, Querejeta JI, Camarero JJ. Chronically Low Nutrient Concentrations in Tree Rings Are Linked to Greater Tree Vulnerability to Drought in Nothofagus dombeyi. Forests. 2021; 12(9):1180. https://doi.org/10.3390/f12091180
Chicago/Turabian StyleGonzález de Andrés, Ester, María Laura Suárez, José Ignacio Querejeta, and J. Julio Camarero. 2021. "Chronically Low Nutrient Concentrations in Tree Rings Are Linked to Greater Tree Vulnerability to Drought in Nothofagus dombeyi" Forests 12, no. 9: 1180. https://doi.org/10.3390/f12091180
APA StyleGonzález de Andrés, E., Suárez, M. L., Querejeta, J. I., & Camarero, J. J. (2021). Chronically Low Nutrient Concentrations in Tree Rings Are Linked to Greater Tree Vulnerability to Drought in Nothofagus dombeyi. Forests, 12(9), 1180. https://doi.org/10.3390/f12091180









