Vegetation Type and Soil Moisture Drive Variations in Leaf Litter Decomposition Following Secondary Forest Succession
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Sampling
2.3. Indoor Controlled Experiment
2.4. Litter and Soil Property Assay
2.5. Data Analysis
3. Results
3.1. Dynamics of Leaf Litter Biomass Loss and Leaf Litter Decomposition Rate
3.2. Dynamics of Leaf Litter Quality
3.3. Effects of Factors on Leaf Litter Decomposition
4. Discussion
4.1. Interactions of Vegetation Type and Soil Moisture on Leaf Litter Quality
4.2. Interactions of Vegetation Type and Soil Moisture on Leaf Litter Decomposition
4.3. Effect of Leaf Litter Quality on Litter Decomposition Rate under Vegetation Type and Soil Moisture
4.4. Effect of Soil Properties on Litter Decomposition Rate under Different Vegetation Types and Soil Moisture
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, K.; Trivedi, P.; Singh, G.; Singh, B.; Patra, D.D. Effect of different leaf litters on carbon, nitrogen and microbial activities of sodic soils. Land Degrad. Dev. 2014, 27, 1215–1226. [Google Scholar] [CrossRef]
- Fang, X.M.; Wang, G.G.; Xu, Z.J.; Zong, Y.Y.; Zhang, X.L.; Li, J.J.; Wang, H.; Chen, F.S. Litter addition and understory removal influenced soil organic carbon quality and mineral nitrogen supply in a subtropical plantation forest. Plant Soil 2021, 460, 527–540. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J. An Earth-system perspective of the global nitrogen cycle. Nat. Cell Biol. 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Jia, T.; Wang, Y.; Chai, B. Bacterial community characteristics and enzyme activities in Bothriochloa ischaemum litter over progressive phytoremediation years in a copper tailings dam. Front. Microbiol. 2020, 11, 3344. [Google Scholar] [CrossRef]
- Taylor, P.G.; Cleveland, C.C.; Wieder, W.; Sullivan, B.W.; Doughty, C.E.; Dobrowski, S.; Townsend, A.R. Temperature and rainfall interact to control carbon cycling in tropical forests. Ecol. Lett. 2017, 20, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Campos, X.; Germino, M.J.; De Graaff, M.A. Enhanced precipitation promotes decomposition and soil C stabilization in semiarid ecosystems, but seasonal timing of wetting matters. Plant Soil 2017, 416, 427–436. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.Y.; Song, X.; Wang, X.; Suri, G.; Baoyin, T. Changes in litter decomposition rate of dominant plants in a semi-arid steppe across different land-use types: Soil moisture, not home-field advantage, plays a dominant role. Agric. Ecosyst. Environ. 2020, 303, 107119. [Google Scholar] [CrossRef]
- Otaki, M.; Tsuyuzaki, S. Succession of litter-decomposing microbial organisms in deciduous birch and oak forests, northern Japan. Acta Oecol. 2019, 101, 103485. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.C.; Kathryn, A.; Ellen, D.; Eviner, V.T.; Oscar, G.; Hobbie, S.E.; Bart, H.; Hiroko, K.; Natalia, P.H. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2010, 11, 1065–1071. [Google Scholar] [CrossRef]
- Urbanová, M.; Šnajdr, J.; Brabcová, V.; Merhautová, V.; Dobiášová, P.; Cajthaml, T.; Vaněk, D.; Frouz, J.; Šantrůčková, H.; Baldrian, P. Litter decomposition along a primary post-mining chronosequence. Biol. Fertil. Soils 2014, 50, 827–837. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J. Plant litter quality influences the contribution of soil fauna to litter decomposition in humid tropical forests, southwestern China. Soil Biol. Biochem. 2009, 41, 910–918. [Google Scholar] [CrossRef]
- Castro, H.; Fortunel, C.; Freitas, H. Effects of land abandonment on plant litter decomposition in a montado system: Relation to litter chemistry and community functional parameters. Plant Soil 2010, 333, 181–190. [Google Scholar] [CrossRef]
- Bradford, M.; Ii, R.W.; Baldrian, P.; Crowther, T.W.; Maynard, D.; Oldfield, E.E.; Wieder, W.; Wood, S.; King, J.R. Climate fails to predict wood decomposition at regional scales. Nat. Clim. Chang. 2014, 4, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, X.; Yang, N.; Xin, X.; Qu, Y.; Zhao, N.; Gao, Y. Effects of litter diversity and composition on litter decomposition characteristics and soil microbial community. Acta Ecol. Sin. 2019, 39, 6264–6272. [Google Scholar]
- Deng, L.; Wang, K.; Zhu, G.; Liu, Y.; Chen, L.; Shangguan, Z. Changes of soil carbon in five land use stages following 10 years of vegetation succession on the Loess Plateau, China. Catena 2018, 171, 185–192. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Liu, J.; Cheng, G.; Lu, X. Soil C and N dynamics and their non-additive responses to litter mixture under different moisture conditions from an alpine steppe soil, Northern Tibet. Soil Biol. Biochem. 2018, 125, 231–238. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Xu, M. Effect of revegetation on soil organic carbon concentration in deep soil layers in the hilly Loess Plateau of China. Acta Ecol. Sin. 2010, 30, 3947–3952. [Google Scholar]
- Zhou, Z.; Zhang, Z.; Zha, T.; Luo, Z.; Zheng, J.; Sun, O.J. Predicting soil respiration using carbon stock in roots, litter and soil organic matter in forests of Loess Plateau in China. Soil Biol. Biochem. 2013, 57, 135–143. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Y.; Zhang, H.; An, S. Fast bacterial succession associated with the decomposition of Quercus wutaishanica litter on the Loess Plateau. Biogeochemistry 2019, 144, 119–131. [Google Scholar] [CrossRef]
- Williams, R.A. Afforestation potential in the Wana region to sequester carbon and improve soil quality. In Climate Change and Food Security in West Asia and North Africa; Springer: Berlin/Heidelberg, Germany, 2013; pp. 281–297. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s ‘Grain-for-Green’ program: A synthesis. Glob. Chang. Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall-manipulation experiments. Biogeosciences 2013, 10, 5245–5272. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Gao, D.; Chen, Z.; Li, H.; Deng, J.; Qiao, W.; Han, X.; Yang, G.; Feng, Y.; Huang, J. Substrate quality and soil environmental conditions predict litter decomposition and drive soil nutrient dynamics following afforestation on the Loess Plateau of China. Geoderma 2018, 325, 152–161. [Google Scholar] [CrossRef]
- Han, M.Y.; Zhang, L.X.; Fan, C.H.; Liu, L.H.; Zhang, L.S.; Li, B.Z.; Alva, A.K. Release of nitrogen, phosphorus, and potassium during the decomposition of apple (Malus domestica) leaf litter under different fertilization regimes in Loess Plateau, China. Soil Sci. Plant Nutr. 2011, 57, 549–557. [Google Scholar] [CrossRef]
- Tateno, R.; Tokuchi, N.; Yamanaka, N.; Du, S.; Otsuki, K.; Shimamura, T.; Xue, Z.; Wang, S.; Hou, Q. Comparison of litterfall production and leaf litter decomposition between an exotic black locust plantation and an indigenous oak forest near Yan’an on the Loess Plateau, China. For. Ecol. Manag. 2007, 241, 84–90. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Hai, X.; Shangguan, Z.; Deng, L. Dynamics of soil microbial C:N:P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Sci. Total Environ. 2019, 693, 133613. [Google Scholar] [CrossRef]
- Wang, K.B.; Ren, Z.P.; Deng, L.; Zhou, Z.C.; Shangguan, Z.P.; Shi, W.Y.; Chen, Y.P. Profile distributions and controls of soil inorganic carbon along a 150-year natural vegetation restoration chronosequence. Soil Sci. Soc. Am. J. 2016, 80, 193–202. [Google Scholar] [CrossRef]
- Robertson, G.P.; Coleman, D.C.; Sollins, P.; Bledsoe, C.S. Standard Soil Methods for Long-Term Ecological Research; Oxford University Press: Oxford, UK, 1999; Volume 2. [Google Scholar]
- Rowland, A.P.; Roberts, J.D. Lignin and cellulose fractionation in decomposition studies using acid-detergent fibre methods. Commun. Soil Sci. Plant Anal. 1994, 25, 269–277. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. Methods Soil Anal. 1982, 9, 961–1010. [Google Scholar]
- Huffman, E.W., Jr. Performance of a new automatic carbon dioxide coulometer. Microchem. J. 1977, 22, 567–573. [Google Scholar] [CrossRef]
- Duan, J.; Wang, S.; Zhang, Z.; Xu, G.; Luo, C.; Chang, X.; Zhu, X.; Cui, S.; Zhao, X.; Wang, W.; et al. Non-additive effect of species diversity and temperature sensitivity of mixed litter decomposition in the alpine meadow on Tibetan Plateau. Soil Biol. Biochem. 2013, 57, 841–847. [Google Scholar] [CrossRef]
- Bosco, T.; Beatriz, M.; Bertiller, M.B.; Carrera, A.L. Combined effects of litter features, UV radiation, and soil water on litter decomposition in denuded areas of the arid Patagonian Monte. Plant Soil 2016, 406, 71–82. [Google Scholar] [CrossRef]
- Gingerich, R.T.; Anderson, J.T. Litter decomposition in created and reference wetlands in West Virginia, USA. Wetl. Ecol. Manag. 2011, 19, 449–458. [Google Scholar] [CrossRef]
- Mackintosh, T.J.; Davis, J.A.; Thompson, R.M. Impacts of multiple stressors on ecosystem function: Leaf decomposition in constructed urban wetlands. Environ. Pollut. 2016, 208, 221–232. [Google Scholar] [CrossRef]
- Fuell, A.K.; Entrekin, S.A.; Owen, G.S.; Owen, S.K. Drivers of leaf decomposition in two wetland types in the Arkansas River Valley, USA. Wetlands 2013, 33, 1127–1137. [Google Scholar] [CrossRef]
- Gingerich, R.T.; Merovich, G.; Anderson, J.T. Influence of environmental parameters on litter decomposition in wetlands in West Virginia, USA. J. Freshw. Ecol. 2014, 29, 535–549. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Tan, S.; Wang, Z.; Zhang, Q. Vegetation community and soil characteristics of abandoned agricultural land and pine plantation in the Qinling Mountains, China. For. Ecol. Manag. 2010, 259, 2036–2047. [Google Scholar] [CrossRef]
- Montané, F.; Romanyà, J.; Rovira, P.; Casals, P. Aboveground litter quality changes may drive soil organic carbon increase after shrub encroachment into mountain grasslands. Plant Soil 2010, 337, 151–165. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, X.; Dang, H.; Ye, C.; Zhang, Y.; Zhang, Q. Linking litter production, quality and decomposition to vegetation succession following agricultural abandonment. Soil Biol. Biochem. 2013, 57, 803–813. [Google Scholar] [CrossRef]
- Zukswert, J.M.; Prescott, C.E. Relationships among leaf functional traits, litter traits, and mass loss during early phases of leaf litter decomposition in 12 woody plant species. Oecologia 2017, 185, 305–316. [Google Scholar] [CrossRef]
- Pei, G.; Liu, J.; Peng, B.; Gao, D.; Wang, C.; Dai, W.; Jiang, P.; Bai, E. Nitrogen, lignin, C/N as important regulators of gross nitrogen release and immobilization during litter decomposition in a temperate forest ecosystem. For. Ecol. Manag. 2019, 440, 61–69. [Google Scholar] [CrossRef]
- Orians, C.M.; Schweiger, R.; Dukes, J.S.; Scott, E.; Müller, C. Combined impacts of prolonged drought and warming on plant size and foliar chemistry. Ann. Bot. 2019, 124, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Carrera, A.L.; Bertiller, M.B. Combined effects of leaf litter and soil microsite on decomposition process in arid rangelands. J. Environ. Manag. 2013, 114, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Chomel, M.; Fernandez, C.; Bousquet-Mélou, A.; Gers, C.; Monnier, Y.; Santonja, M.; Gauquelin, T.; Gros, R.; Lecareux, C.; Baldy, V. Secondary metabolites of Pinus halepensis alter decomposer organisms and litter decomposition during afforestation of abandoned agricultural zones. J. Ecol. 2014, 102, 411–424. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ball, B. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob. Chang. Biol. 2014, 21, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, W.; Zhang, J.; Deng, R. Litter decomposition in two subalpine forests during the freeze–thaw season. Acta Oecologica 2010, 36, 135–140. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Jian, Z.; Zhou, H.; Zhao, Y.; Wei, D. Litter decomposition and the degradation of recalcitrant components in Pinus massoniana plantations with various canopy densities. J. For. Res. 2019, 30, 1395–1405. [Google Scholar] [CrossRef] [Green Version]
- Klotzbücher, T.; Kaiser, K.; Filley, T.R.; Kalbitz, K. Processes controlling the production of aromatic water-soluble organic matter during litter decomposition. Soil Biol. Biochem. 2013, 67, 133–139. [Google Scholar] [CrossRef]
- Yue, K.; Peng, C.; Yang, W.; Peng, Y.; Zhang, C.; Huang, C.; Wu, F. Degradation of lignin and cellulose during foliar litter decomposition in an alpine forest river. Ecosphere 2016, 7, 01523. [Google Scholar] [CrossRef]
- Cortez, J.; Garnier, E.; Pérez-Harguindeguy, N.; Debussche, M.; Gillon, D. Plant traits, litter quality and decomposition in a Mediterranean old-field succession. Plant Soil 2007, 296, 19–34. [Google Scholar] [CrossRef]
- Kazakou, E.; Violle, C.; Roumet, C.; Pintor, C.; Gimenez, O.; Garnier, E. Litter quality and decomposability of species from a Mediterranean succession depend on leaf traits but not on nitrogen supply. Ann. Bot. 2009, 104, 1151–1161. [Google Scholar] [CrossRef] [Green Version]
- Overbeek, C.C.; Van Der Geest, H.G.; Van Loon, E.E.; Admiraal, W. Decomposition of standing litter biomass in newly constructed wetlands associated with direct effects of sediment and water characteristics and the composition and activity of the decomposer community using phragmites australis as a single standard substrate. Wetlands 2019, 39, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Giweta, M. Role of litter production and its decomposition, and factors affecting the processes in a tropical forest ecosystem: A review. J. Ecol. Environ. 2020, 44, 1–9. [Google Scholar] [CrossRef]
- Xiao, L.; Bi, Y.; Du, S.; Wang, Y.; Guo, C.; Christie, P. Response of ecological stoichiometry and stoichiometric homeostasis in the plant-litter-soil system to re-vegetation type in arid mining subsidence areas. J. Arid. Environ. 2021, 184, 104298. [Google Scholar] [CrossRef]
- Horodecki, P.; Jagodziński, A.M. Tree species effects on litter decomposition in pure stands on afforested post-mining sites. For. Ecol. Manag. 2017, 406, 1–11. [Google Scholar] [CrossRef]
- Celentano, D.; Zahawi, R.A.; Finegan, B.; Casanoves, F.; Ostertag, R.; Cole, R.J.; Holl, K.D. Restauración ecológica de bosques tropicales en Costa Rica: Efecto de varios modelos en la producción, acumulación y descomposición de hojarasca. Rev. Biol. Trop. 2011, 59, 1323–1336. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.F.; He, Z.B.; Wu, X.R.; Du, J.; Zhu, X.; Lin, P.F.; Tian, Q.Y.; Kong, J.Q. Linkages between soil respiration and microbial communities following afforestation of alpine grasslands in the northeastern Tibetan Plateau. Appl. Soil Ecol. 2021, 161, 103882. [Google Scholar] [CrossRef]
- Mayer, P.M. Ecosystem and decomposer effects on litter dynamics along an old field to old-growth forest successional gradient. Acta Oecol. 2008, 33, 222–230. [Google Scholar] [CrossRef]
- Muller, R. Nutrient relations of the herbaceous layer in deciduous forest ecosystems. In The Herbaceous Layer in Forests of Eastern North America; Gilliam, F., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 12–34. [Google Scholar]
- Halabuk, A.; Gerhátová, K. Comparative study of leaf litter decomposition of exotic and native species in an ecotop of the hornbeam-oak forest near Báb village, SW Slovakia. Folia Oecol. 2011, 38, 17–27. [Google Scholar]
- Wang, Q.; Wang, S.; He, T.; Liu, L.; Wu, J. Response of organic carbon mineralization and microbial community to leaf litter and nutrient additions in subtropical forest soils. Soil Biol. Biochem. 2014, 71, 13–20. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Zhu, B.; Bing, Y.; Luc, N.T.; Du, L.; Zhu, Z. Impacts of mixed litter decomposition from Robinia pseudoacacia and other tree species on C loss and nutrient release in the Loess Plateau of China. J. For. Res. 2016, 27, 525–532. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Garcia-Palacios, P.; Milla, R.; Gallardo, A.; Maestre, F.T. Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biol. Biochem. 2015, 81, 134–142. [Google Scholar] [CrossRef]
- Hansson, K.; Olsson, B.A.; Olsson, M.; Johansson, U.; Kleja, D.B. Differences in soil properties in adjacent stands of Scots pine, Norway spruce and silver birch in SW Sweden. For. Ecol. Manag. 2011, 262, 522–530. [Google Scholar] [CrossRef]
- Zhu, L.; Deng, Z.; Xie, Y.; Li, X.; Li, F.; Chen, X.; Zou, Y.; Zhang, C.; Wang, W. Factors controlling Carex brevicuspis leaf litter decomposition and its contribution to surface soil organic carbon pool at different water levels. Biogeosciences 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Bani, A.; Pioli, S.; Ventura, M.; Panzacchi, P.; Borruso, L.; Tognetti, R.; Tonon, G.; Brusetti, L. The role of microbial community in the decomposition of leaf litter and deadwood. Appl. Soil Ecol. 2018, 126, 75–84. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, J.; Qiu, X.; Wei, F.; Xu, X. Decomposing litter and associated microbial activity responses to nitrogen deposition in two subtropical forests containing nitrogen-fixing or non-nitrogen-fixing tree species. Sci. Rep. 2018, 8, 12934. [Google Scholar] [CrossRef]
- Li, Q.; Moorhead, D.L.; DeForest, J.L.; Henderson, R.; Chen, J.; Jensen, R. Mixed litter decomposition in a managed Missouri Ozark forest ecosystem. For. Ecol. Manag. 2009, 257, 688–694. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Zhang, W.; Shao, Y.; Duan, H.; Chen, B.; Wei, X.; Fan, H. Long-term nitrogen addition changes soil microbial community and litter decomposition rate in a subtropical forest. Appl. Soil Ecol. 2019, 142, 43–51. [Google Scholar] [CrossRef]
- Mariano, E.; Jones, D.; Hill, P.W.; Trivelin, P.C. Mineralisation and sorption of dissolved organic nitrogen compounds in litter and soil from sugarcane fields. Soil Biol. Biochem. 2016, 103, 522–532. [Google Scholar] [CrossRef]
- Lajtha, K.; Bowden, R.D.; Nadelhoffer, K. Litter and root manipulations provide insights into soil organic matter dynamics and stability. Soil Sci. Soc. Am. J. 2014, 78, S261–S269. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Zhao, L.; Zhou, G.; Huang, W.; Liu, J. Increased litter input increases litter decomposition and soil respiration but has minor effects on soil organic carbon in subtropical forests. Plant Soil 2015, 392, 139–153. [Google Scholar] [CrossRef]
- Sayer, E.J.; Wright, S.J.; Tanner, E.V.J.; Yavitt, J.B.; Harms, K.E.; Powers, J.S.; Kaspari, M.; Garcia, M.N.; Turner, B. Variable responses of lowland tropical forest nutrient status to fertilization and litter manipulation. Ecosystems 2012, 15, 387–400. [Google Scholar] [CrossRef]
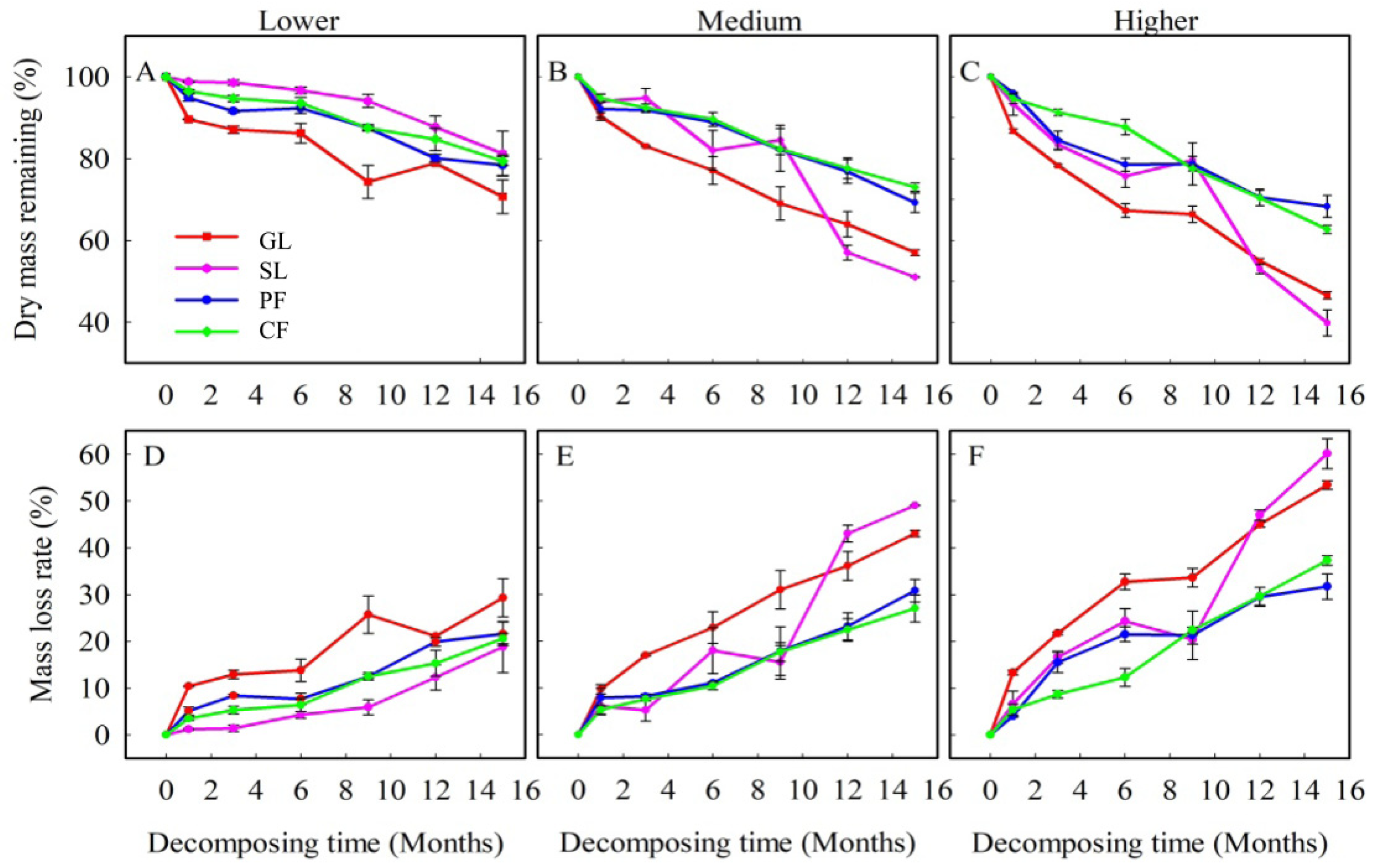



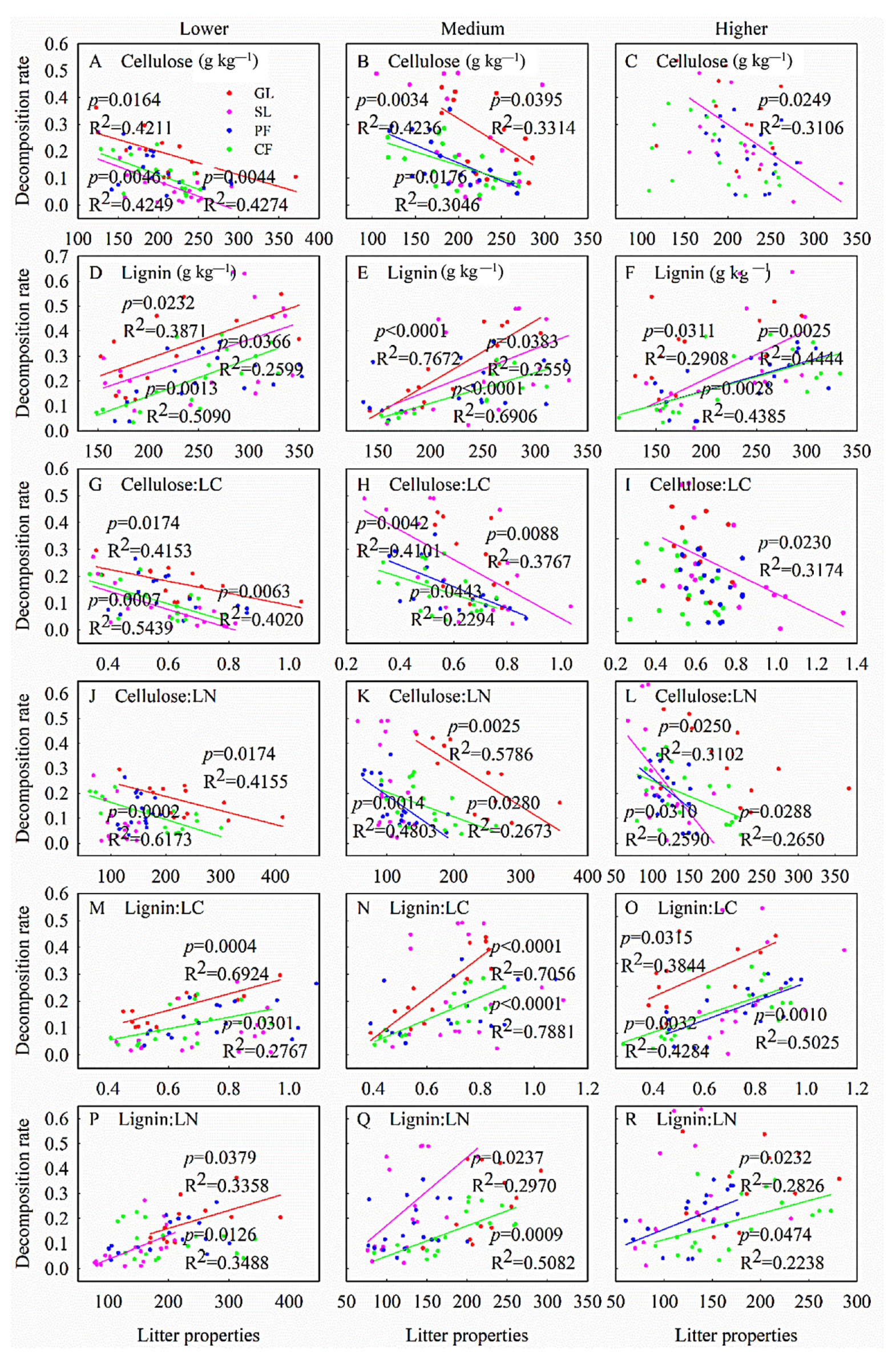
| Vegetation Type | Equation | R2 | F |
|---|---|---|---|
| GL | LC = 334.108 + 9.0460X − 0.6632X2 | 0.3189 *** | 11.9390 |
| SL | LC = 264.2299 + 13.4820X − 0.4378X2 | 0.3877 *** | 16.1432 |
| PF | LC = 326.8054 + 2.515X − 0.1620X2 | 0.02 | 0.5216 |
| CF | LC = 382.8769 − 6.7872X + 0.3938X2 | 0.1646 * | 5.0227 |
| GL | LN = 1.0444 − 0.0473X + 0.004X2 | 0.4652 *** | 22.1829 |
| SL | LN = 2.1413 − 0.0542X + 0.0027X2 | 0.0747 | 2.0582 |
| PF | LN = 1.6307 + 0.0109X | 0.0565 | 3.1158 |
| CF | LN = 1.1645 + 0.0123X | 0.0936 * | 5.3700 |
| GL | LC:LN = 319.8364 + 27.9883X − 2.1552X2 | 0.4298 *** | 19.2200 |
| SL | LC:LN = 140.6932 + 4.4725X | 0.2343 *** | 15.9100 |
| PF | LC:LN = 209.1411 − 1.5415X | 0.0667 | 3.7163 |
| CF | LC:LN = 366.5378 − 19.9371X + 0.9920X2 | 0.3207 *** | 12.0394 |
| GL | Cellulose = 246.1229 − 1.5756X − 0.1910X2 | 0.2342 *** | 5.3508 |
| SL | Cellulose = 253.3808 − 5.4886X | 0.3415 *** | 24.8911 |
| PF | Cellulose = 252.1429 − 7.7153X + 0.1690X2 | 0.3371 *** | 12.7149 |
| CF | Cellulose = 252.1429 − 3.6091X − 0.0903X2 | 0.2906 *** | 10.2428 |
| GL | Lignin = 159.9363 + 7.3713X | 0.4216 *** | 26.2382 |
| SL | Lignin = 159.7506 + 17.1866X − 0.5830X2 | 0.4489 *** | 19.1421 |
| PF | Lignin = 130.5952 + 26.8986X − 1.2207X2 | 0.5156 *** | 26.6058 |
| CF | Lignin = 116.9031 + 26.8906X − 1.0978X2 | 0.6808 *** | 53.3160 |
| Treatments | df | LC | LN | LC:LN | Cellulose: LC | Lignin: LC | Cellulose: LN | Lignin: LN | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Square | F | Mean Square | F | Mean Square | F | Mean Square | F | Mean Square | F | Mean Square | F | Mean Square | F | ||
| Vegetation type | 3 | 28,929.99 | 30.20 *** | 17.04 | 397.24 *** | 747,116.66 | 266.27 *** | 0.14 | 5.74 *** | 0.16 | 5.12 ** | 108,014.26 | 48.98 *** | 62,473.48 | 24.25 *** |
| Soil moisture | 2 | 9067.13 | 9.47 *** | 0.56 | 12.96 *** | 30,009.85 | 10.70 *** | 0.03 | 1.11 | 0.01 | 0.16 | 8775.18 | 3.98 * | 27,263.86 | 10.58 *** |
| Vegetation type × Soil moisture | 6 | 6077.91 | 6.35 *** | 0.01 | 0.30 | 1210.64 | 0.43 | 0.05 | 2.02 | 0.03 | 0.81 | 1967.88 | 0.89 | 3938.45 | 1.53 |
| Soil Moisture | Vegetation Types | Soil Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOC | TN | NH4+ | NO3− | pH | MBC | MBN | DOC | DON | ||
| Lower | GL | 0.338 | −0.153 | 0.527 * | 0.684 ** | 0.453 | 0.082 | 0.014 | 0.714 ** | 0.324 |
| SL | −0.304 | −0.205 | 0.589 * | 0.828 ** | 0.099 | −0.026 | 0.224 | 0.411 | 0.563 * | |
| PF | 0.214 | −0.297 | 0.197 | 0.913 ** | 0.521 * | 0.789 ** | 0.661 ** | −0.070 | 0.374 | |
| CF | 0.040 | −0.275 | −0.204 | 0.872 ** | 0.072 | 0.315 | 0.405 | 0.575 * | 0.784 ** | |
| Medium | GL | −0.265 | −0.303 | 0.681 ** | 0.860 ** | 0.431 | −0.291 | 0.439 | 0.615 ** | 0.795 ** |
| SL | −0.461 | −0.494 * | 0.007 | 0.274 | −0.232 | −0.284 | 0.621 ** | −0.118 | 0.312 | |
| PF | −0.170 | −0.050 | 0.281 | 0.713 ** | 0.429 | 0.614 ** | 0.644 ** | 0.580 * | 0.763 ** | |
| CF | 0.155 | 0.078 | −0.341 | 0.662 ** | 0.456 | 0.405 | 0.686 ** | 0.245 | 0.353 | |
| Higher | GL | −0.783 ** | −0.603 ** | 0.667 ** | 0.797 ** | 0.361 | −0.386 | 0.467 | 0.730 ** | 0.767 ** |
| SL | −0.477 * | −0.088 | 0.121 | 0.449 | −0.293 | −0.180 | 0.633 ** | −0.056 | 0.475 * | |
| PF | 0.172 | −0.651 ** | 0.460 | 0.644 ** | 0.388 | 0.179 | 0.560 * | 0.180 | 0.743 ** | |
| CF | −0.234 | 0.286 | −0.402 | 0.489 * | 0.291 | 0.101 | 0.343 | 0.299 | 0.660 ** | |
| Soil Moisture | Vegetation Type | Equation | R2 |
|---|---|---|---|
| Lower | GL | Mass loss = 0.856Cellulose:LC − 0.041 | 0.733 *** |
| SL | Mass loss = 0.819NO3− − 0.041 | 0.631 *** | |
| PF | Mass loss = 0.907NO3− + 0.057 | 0.827 *** | |
| CF | Mass loss = 0.817MBC + 0.923DON − 0.356pH + 0.471 | 0.958 *** | |
| Medium | GL | Mass loss = −0.305Cellulose + 0.774NO3− + 0.302 | 0.927 *** |
| SL | Mass loss = −0.332SOC + 0.744DON + 0.653 | 0.738 *** | |
| PF | Mass loss = 0.744DON + 0.345Lignin:LC + 0.343pH − 2.152 | 0.870 *** | |
| CF | Mass loss = 0.306MBN − 0.334NH4+ + 0.736Cellulose:LC − 0.142 | 0.942 *** | |
| Higher | GL | Mass loss = −0.254SOC + 0.415DON + 0.562NO3− + 0.575 | 0.967 *** |
| SL | Mass loss = −0.18SOC + 0.277MBN − 0.246TN + 0.773DON + 1.884 | 0.948 *** | |
| PF | Mass loss = 0.645DON − 0.532TN + 0.802 | 0.826 *** | |
| CF | Mass loss = 0.508DON + 0.511Lignin − 0.06 | 0.674 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Shangguan, Z.; Deng, L. Vegetation Type and Soil Moisture Drive Variations in Leaf Litter Decomposition Following Secondary Forest Succession. Forests 2021, 12, 1195. https://doi.org/10.3390/f12091195
Liu Y, Shangguan Z, Deng L. Vegetation Type and Soil Moisture Drive Variations in Leaf Litter Decomposition Following Secondary Forest Succession. Forests. 2021; 12(9):1195. https://doi.org/10.3390/f12091195
Chicago/Turabian StyleLiu, Yulin, Zhouping Shangguan, and Lei Deng. 2021. "Vegetation Type and Soil Moisture Drive Variations in Leaf Litter Decomposition Following Secondary Forest Succession" Forests 12, no. 9: 1195. https://doi.org/10.3390/f12091195





