Effects of Leaf Loss by Artificial Defoliation on the Growth of Different Poplar and Willow Varieties
Abstract
:1. Introduction
2. Materials and Methods
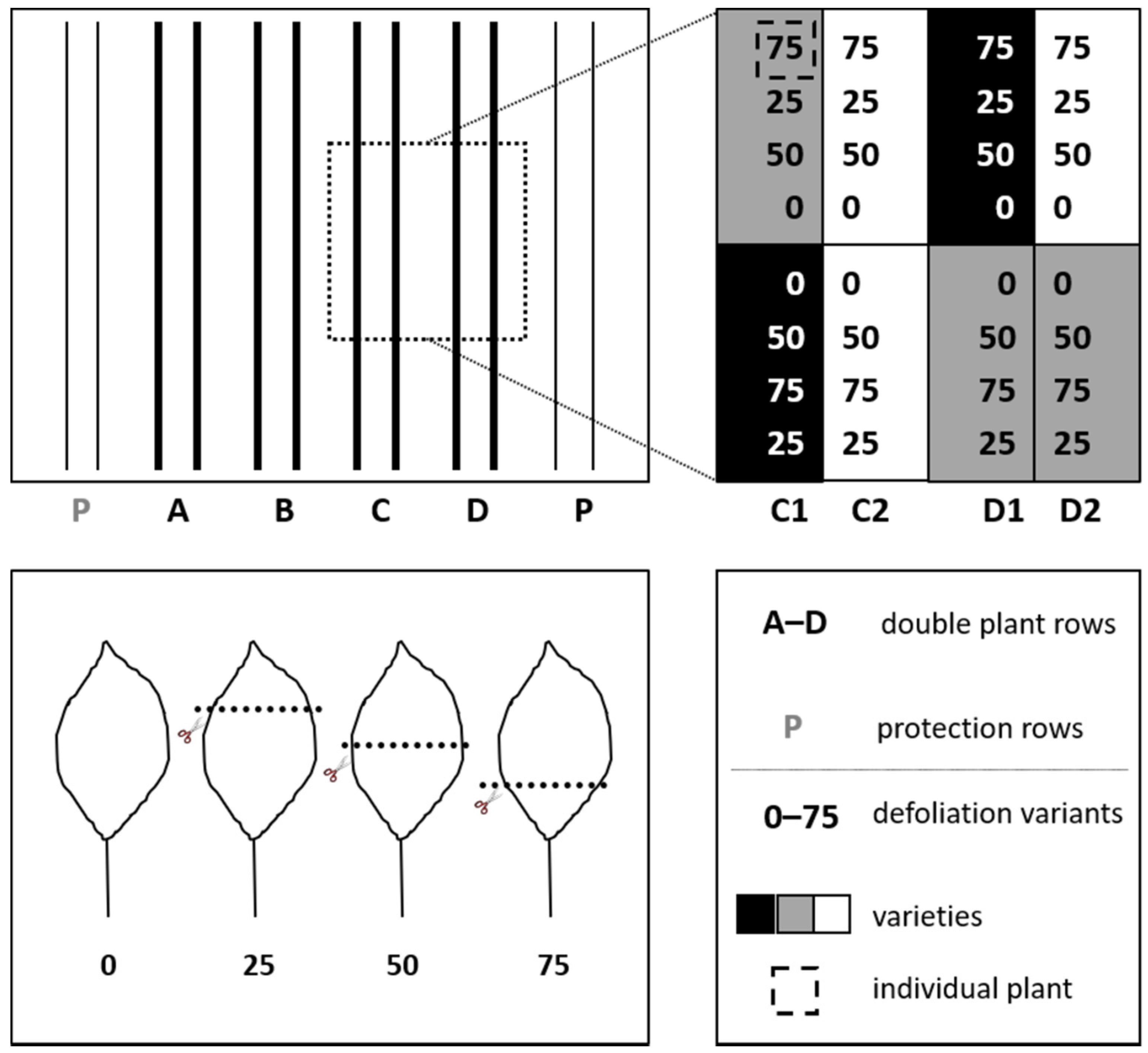
2.1. Sites, Plant Material, and Experimental Design
2.2. Experimental Process and Data Recording
2.3. Data Analysis
3. Results
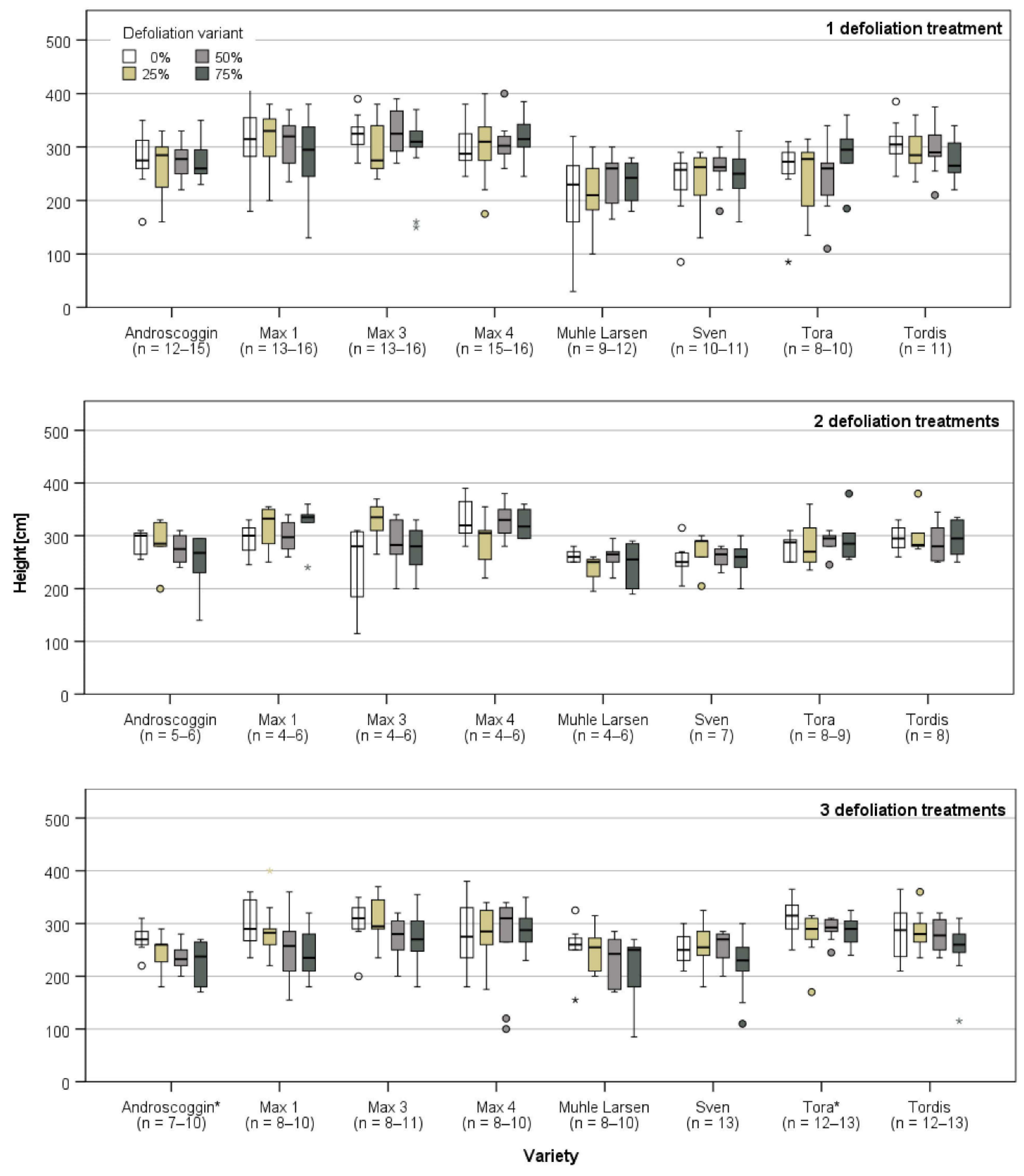
3.1. Plant Growth Directly Prior and after the First Defoliation Treatment
3.2. Plant Growth after the Last Defoliation
3.3. Plant Growth after the End of the Defoliation Treatments
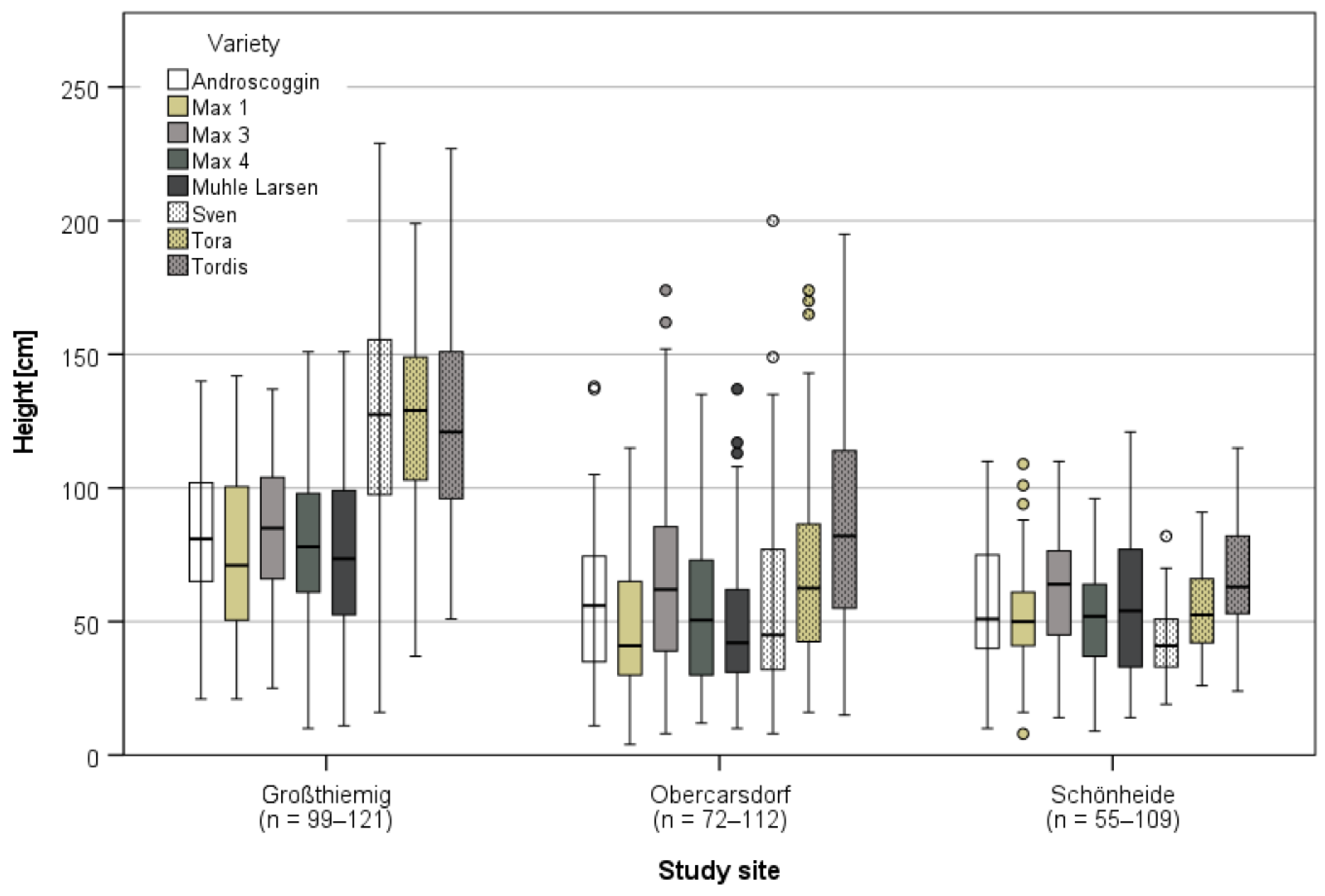
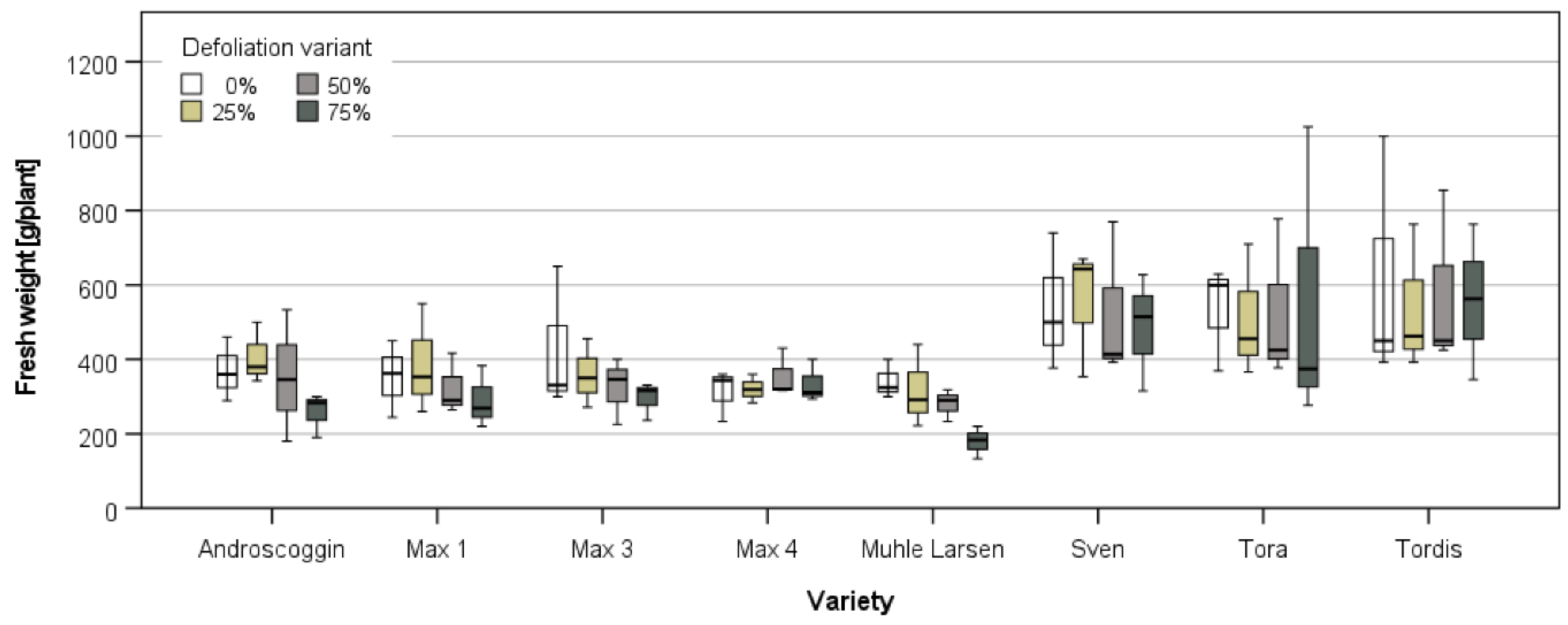
3.3.1. Harvest and Growth in Großthiemig
3.3.2. Growth in Obercarsdorf and Schönheide
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Study Site | ||||||
|---|---|---|---|---|---|---|
| Genus/Variety | Großthiemig | Obercarsdorf | Schönheide | |||
| Poplars | 93.3 | A | 82.0 | A | 81.4 | A |
| Androscoggin | 89.8 | a | 65.6 | a | 70.3 | a |
| Max 1 | 96.1 | ab | 87.5 | bc | 85.9 | bc |
| Max 3 | 94.5 | ab | 93.7 | c | 89.1 | c |
| Max 4 | 98.4 | b | 87.5 | bc | 88.3 | c |
| Muhle Larsen | 87.5 | a | 75.8 | ab | 73.4 | ab |
| Willows | 96.4 | B | 84.1 | A | 84.1 | A |
| Sven | 94.5 | a | 82.0 | a | 83.6 | a |
| Tora | 96.1 | a | 76.6 | a | 82.8 | a |
| Tordis | 98.4 | a | 93.7 | b | 85.9 | a |


References
- Landgraf, D.; Carl, C.; Neupert, M. Biomass yield of 37 different SRC poplar varieties grown on a typical site in north eastern Germany. Forests 2020, 11, 1048. [Google Scholar] [CrossRef]
- Bielefeldt, J.; Bolte, A.; Busch, G.; Dohrenbusch, A.; Kroiher, F.; Lamersdorf, N.; Schulz, U.; Stoll, B. Energieholzproduktion in der Landwirtschaft—Chancen und Risiken aus Sicht des Natur—und Umweltschutzes; Naturschutzbund Deutschland, e.V.: Berlin, Germany, 2008. [Google Scholar]
- Ball, J.P.; Carle, J.; del Lungo, A. Contribution of poplars and willows to sustainable forestry and rural development. Unasylva Engl. 2005, 56, 3–9. [Google Scholar]
- Dimitriou, I.; Aronsson, P. Willows for energy and phytoremediation in Sweden. Unasylva Engl. 2005, 56, 47–50. [Google Scholar]
- Hofmann, M. Energieholzproduktion in der Landwirtschaft; Fachagentur Nachwachsende Rohstoffe e.V.: Gülzow, Germany, 2010.
- Isebrands, J.G.; Richardson, J. (Eds.) Poplars and Willows: Trees for Society and the Environment; CABI: Wallingford, UK, 2014. Available online: http://www.fao.org/3/i2670e/i2670e.pdf (accessed on 23 August 2021).
- Wolf, H.; Schildbach, M.; Hartmann, K.-U. Plantagenbaumarten und deren Züchtung. In AGROWOOD: Kurzumtriebsplantagen in Deutschland und Europäische Perspektiven; Bemmann, A., Knust, C., Eds.; Weißensee-Verlag: Berlin, Germany, 2010; pp. 30–43. [Google Scholar]
- Schwerdtfeger, F. Pappelkrankheiten und Pappelschutz. In Das Pappelbuch; Hesmer, H., Ed.; Verlag des Deutschen Pappelvereins: Bonn, Germany, 1951; pp. 155–186. [Google Scholar]
- Prien, S.; Sann, H. Probleme des Forstschutzes in forstlichen Plantagen. In Probleme und Methodische Fragen der Plantagenwirtschaft mit Forstlichen Baumarten; Walter, F., Ed.; Agrarwissenschaftliche Gesellschaft der Deutschen Demokratischen Republik: Dresden, Germany, 1981; pp. 51–57. [Google Scholar]
- Andow, D.A. Vegetational Diversity and Arthropod Population Response. Annu. Rev. Entomol. 1991, 36, 561–586. [Google Scholar] [CrossRef]
- Führer, E.; Bacher, H. Biotische Schadrisiken in Energieholzplantagen. Anz. Schädlingskunde 1991, 64, 1–8. [Google Scholar] [CrossRef]
- Coyle, D.R.; Nebeker, T.E.; Hart, E.R.; Mattson, W.J. Biology and management of insect pests in North American intensively managed hardwood forest systems. Annu. Rev. Entomol. 2005, 50, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Splechtna, B.; Glatzel, G. Optionen der Bereitstellung von Biomasse aus Wäldern und Energieholzplantagen für die Energetische Nutzung: Szenarien, ökologische Auswirkungen, Forschungbedarf. In Materialien der Interdisziplinären Arbeitsgruppe Zukunftsorientierte Nutzung Länderlicher Räume—LandInnovation Nr. 1; Berlin-Brandenburgische Akademie der Wissenschaften: Berlin, Germany, 2005. [Google Scholar]
- Brändle, M.; Brandl, R. Species richness of insects and mites on trees: Expanding Southwood. J. Anim. Ecol. 2001, 70, 491–504. [Google Scholar] [CrossRef]
- Bußler, H. Schmetterlinge und Käfer an der Schwarzpappel. LWF Wissen 2006, 52, 35–38. [Google Scholar]
- Hacker, H. Die Insektenwelt der Weiden. LWF Wissen 2000, 24, 26–29. [Google Scholar]
- Kennedy, C.E.J.; Southwood, T.R.E. The number of species of insects associated with British trees: A reanalysis. J. Anim. Ecol. 1984, 53, 455–478. [Google Scholar] [CrossRef]
- Delplanque, A. Les Insectes Associés aux Peupliers; Editions Memor: Bruxelles, Belgium, 1998. [Google Scholar]
- Richter, D. Insektenschäden in Weidenkulturen und Maßnahmen zu Ihrer Verhütung; Merkblatt 30; Institut für Forstwissenschaften Eberswalde, Abteilung Forstschutz Gegen Tierische Schädlinge: Eberswalde, Germany, 1959.
- Helbig, C.; Müller, M.; Landgraf, D. Insect pests in short rotation coppice in Germany—An overview of their effects, risk potential and control measures. In Short Rotation Forestry and Agroforestry: An Exchange of Experience between CDM Countries and Europe; Benwood Project Consortium: Barolo, Italy, 2011; pp. 46–56. [Google Scholar]
- Jaskiewicz, B.; Gorska Drabik, E.; Golan, K. Szkodniki zagrazajace uprawom wierzby wiciowej [Salix viminalis]. Ochr. Rosl. 2004, 48, 24–25. [Google Scholar]
- Georgi, R.; Müller, M. Biotic risk factors in short rotation coppice in Germany: Current situation, new findings and future prospects. In Bioenergy from Dendromass for the Sustainable Development of Rural Areas; Butler Manning, D., Bemmann, A., Bredemier, M., Lamersdorf, N., Ammer, C., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp. 199–216. [Google Scholar]
- Helbig, C.; Müller, M. Naturale Risiken und Grundzüge des Schadensmanagements in Kurzumtriebsplantagen. In AGROWOOD: Kurzumtriebsplantagen in Deutschland und Europäische Perspektiven; Bemmann, A., Knust, C., Eds.; Weißensee-Verlag: Berlin, Germany, 2010; pp. 74–87. [Google Scholar]
- Bell, A.C.; Clawson, S.; Watson, S. The long-term effect of partial defoliation on the yield of short-rotation coppice willow. Ann. Appl. Biol. 2006, 148, 97–104. [Google Scholar] [CrossRef]
- Larsson, S. Effects of artificial defoliation on stem growth in Salix smithiana grown under intensive culture. Oecologica Appl. 1983, 4, 343–349. [Google Scholar]
- Crawley, M.J. Reduction of oak fecundity by low-density herbivore populations. Nature 1985, 314, 163–164. [Google Scholar] [CrossRef]
- Kosola, K.R.; Dickmann, D.I.; Paul, E.A.; Parry, D. Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 2001, 129, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gieger, T.; Thomas, F.M. Effects of defoliation and drought stress on biomass partitioning and water relations of Quercus robur and Quercus petraea. Basic Appl. Ecol. 2002, 3, 171–181. [Google Scholar] [CrossRef]
- Chen, Z.; Kolb, T.E.; Clancy, K.M. Effects of artificial and western spruce budworm (Lepidoptera: Tortricidae) defoliation on growth and biomass allocation of Douglas-fir seedlings. J. Econ. Entomol. 2002, 95, 587–594. [Google Scholar] [CrossRef]
- Baldwin, I.T. Herbivory simulations in ecological research. Trends Ecol. Evol. 1990, 5, 91–93. [Google Scholar] [CrossRef]
- Wright, S.L.; Hall, R.W.; Peacock, J.W. Effect of simulated insect damage on growth and survival of northern red oak (Quercus rubra L.) seedlings. Environ. Entomol. 1989, 18, 235–239. [Google Scholar] [CrossRef]
- Powers, S.J.; Peacock, L.; Yap, M.L.; Brain, P. Simulated beetle defoliation on willow genotypes in mixture and monotype plantations. Ann. Appl. Biol. 2006, 148, 27–38. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, S.; Chenault, J.; Augustin, S. Short time induction defense of a leuce poplar clone (P. tremula x P. tremuloides) against Chrysomela tremulae-propagation and evolution. Acta Hortic. 1994, 381, 540–543. [Google Scholar] [CrossRef]
- Coyle, D.R.; McMillin, J.D.; Hall, R.B.; Hart, E.R. Cottonwood leaf beetle (Coleoptera: Chrysomelidae) defoliation impact on Populus growth and above-ground volume in a short-rotation woody crop plantation. Agric. For. Entomol. 2002, 4, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Anttonen, S.; Piispanen, R.; Ovaska, J.; Mutikainen, P.; Saranpaa, P.; Vapaavuori, E. Effects of defoliation on growth, biomass allocation, and wood properties of Betula pendula clones grown at different nutrient levels. Can. J. For. Res. 2002, 32, 498–508. [Google Scholar] [CrossRef]
- Gao, R.T.; Qin, X.X.; Li, J.Z.; Hao, W.Q.; Wang, X.Z. A preliminary study on the relationship between artificial defoliation of poplar trees and their growth. Sci. Silvae Sin. 1985, 21, 199–205. [Google Scholar]
- Houle, G.; Simard, G. Additive effects of genotype, nutrient availability and type of tissue damage on the compensatory response of Salix planifolia ssp. planifolia to simulated herbivory. Oecologia 1996, 107, 373–378. [Google Scholar] [CrossRef]
- Kendall, D.A. Phenology and Population Dynamics of Willow Beetles (Coleoptera: Chrysomelidae) in Short-Rotation Coppiced Willows at Long Ashton; ETSU B/M4/00487/14/REP; Energy Technology Support Unit: Harwell, UK, 1996. [Google Scholar]
- Osier, T.L.; Lindroth, R.L. Long-term effects of defoliation on quaking aspen in relation to genotype and nutrient availability: Plant growth, phytochemistry and insect performance. Oecologia 2004, 139, 55–65. [Google Scholar] [CrossRef]
- Reichenbacker, R.R.; Schultz, R.C.; Hart, E.R. Artificial defoliation effect on Populus growth, biomass production, and total nonstructural carbohydrate concentration. Environ. Entomol. 1996, 25, 632–642. [Google Scholar] [CrossRef]
- Tucker, S.A.; Nebeker, T.E.; Warriner, M.D.; Jones, W.D.; Beatty, T.K. Effects of artificial defoliation on the growth of cottonwood: Simulation of cottonwood leaf beetle defoliation. In Proceedings of the 12th Biennial Southern Silvicultural Research Conference; Biloxi, MS, USA, 24–28 February 2003, General Technical Report Southern Research Station, USDA Forest Service, SRS-71; USDA Forest Service: Asheville, NC, USA, 2004; pp. 169–171. [Google Scholar]
- Deutscher Wetterdienst. Klimadaten zum Direkten Download. Vieljährige Mittelwerte für den Zeitraum 1981–2021. Available online: https://www.dwd.de/DE/leistungen/cdc/cdc_ueberblick-klimadaten.html?nn=17626&lsbId=344084 (accessed on 23 August 2021).
- Peacock, L.; Herrick, S.; Harris, J. Interactions between the willow beetle Phratora vulgatissima and different genotypes of Salix viminalis. Agric. For. Entomol. 2002, 4, 71–79. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Windows; Version 26.0.; IBM SPSS: Armonk, NY, USA, 2019.
- Bassman, J.; Myers, W.; Dickmann, D.; Wilson, L. Effects of simulated insect damage on early growth of nursery-grown hybrid poplars in northern Wisconsin. Can. J. For. Res. 1982, 12, 1–9. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Effects of pollution-induced habitat disturbance on the response of willows to simulated herbivory. J. Ecol. 2001, 89, 21–30. [Google Scholar] [CrossRef]
- Kendall, D.A.; Wiltshire, C.W.; Royle, D.J.; Paul, L.; Holdenrieder, O. Life-cycles and ecology of willow beetles on Salix viminalis in England. Eur. J. For. Pathol. 1998, 28, 281–288. [Google Scholar] [CrossRef]
- Björkman, C.; Hoglund, S.; Eklund, K.; Larsson, S. Effects of leaf beetle damage on stem wood production in coppicing willow. Agric. For. Entomol. 2000, 2, 131–139. [Google Scholar] [CrossRef]
- Maschinski, J.; Whitham, T.G. The continuum of plant responses to herbivory: The influence of plant association, nutrient availability, and timing. Am. Nat. 1989, 134, 1–19. [Google Scholar] [CrossRef]
- Boelcke, B. Schnellwachsende Baumarten auf Landwirtschaftlichen Flächen; Ministeriums für Ernährung, Landwirtschaft, Forsten und Fischerei. 2006. Available online: https://docplayer.org/52963448-Schnellwachsende-baumarten-auf-landwirtschaftlichen-flaechen-leitfaden-zur-erzeugung-von-energieholz.html (accessed on 23 August 2021).
- Begon, M.; Mortimer, M.; Thompson, D.J.; Müller, J. Populationsökologie; Spektrum-Lehrbuch; Spektrum Akademischer Verlag: Heidelberg, Germany, 1997. [Google Scholar]
- Christersson, L.; Ramstedt, M.; Forsberg, J. Pests, diseases and injuries in intensive short-rotation forestry. In Ecophysiology of Short Rotation Forest Crops; Mitchell, C.P., Ed.; Elsevier Science Publisher: London, UK, 1992; pp. 185–215. [Google Scholar]
- Jug, A. Ernährungs-und standortskundliche Untersuchungen. In Modellvorhaben “Schnellwachsende Baumarten”: Zusammenfassender Abschlussbericht; Schriftenreihe Nachwachsende Rohstoffe; Hofmann, M., Ed.; Landwirtschaftsverlag: Münster, Germany, 1999; Volume 13, pp. 369–396. [Google Scholar]
- Albrecht, J.; Böden, E. Zur Krankheitsanfälligkeit von zugelassenen Pappelklonen der Sektionen Aigeiros und Tacamahaca. Holzzucht 1988, 42, 25–29. [Google Scholar]
- Schildbach, M.; Grünewald, H.; Wolf, H.; Schneider, B.-U. Begründung von Kurzumtriebsplantagen: Baumartenwahl und Anlageverfahren. In Anbau und Nutzung von Bäumen auf Landwirtschaftlichen Flächen; Reeg, T., Bemmann, A., Konold, W., Murach, D., Spieker, H., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp. 57–71. [Google Scholar]
- Solomon, J.D.; Oliveria, F.L. Evaluation of Poplar Tentmaker Defoliation on Growth and Survival of Older Cottonwood Plantations; Research NoteSO-271; U.S. Department of Agriculture, Forest Service, Southern Forest Experiment Station: Asheville, NC, USA, 1993.
- Bogacheva, I.A. Compensation of foliage losses in willow in the forest-tundra during an outbreak of leaf-eating insects. Lesovedenie 1994, 6, 62–69. [Google Scholar]
- Boelcke, B. Ertragspotenzial und Ertragsaufbau von Weiden spec. in Kurzumtriebsplantagen. In Anbau und Nutzung von Bäumen auf Landwirtschaftlichen Flächen: 1. Fachtagung; Technische Universität Dresden: Tharandt, Germany, 2006; pp. 51–56. [Google Scholar]
- Bach, C.E. Effects of herbivory and genotype on growth and survivorship of sand-dune willow (Salix cordata). Ecol. Entomol. 1994, 19, 303–309. [Google Scholar] [CrossRef]



| Study Site | ||||||
|---|---|---|---|---|---|---|
| Großthiemig | Obercarsdorf | Schönheide | ||||
| Genus/Variety | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] |
| Poplars | −13.4 * | −10.7 | −5.8 | −8.3 | −13.0 * | −16.0 |
| Androscoggin | −14.6 | −10.3 | −20.7 | −24.8 | −34.3 | −38.2 |
| Max 1 | −1.2 | −1.1 | −7.0 | −10.8 | −0.8 | −1.2 |
| Max 3 | −15.9 | −12.3 | 10.3 | 13.9 | −17.1 | −19.1 |
| Max 4 | −11.2 | −9.1 | −6.4 | −9.4 | −9.8 | −12.5 |
| Muhle Larsen | −30.8 * | −24.2 | −15.2 | −23.6 | −23.3 | −28.9 |
| Willows | 16.5 | 11.7 | −1.4 | −1.7 | −20.8 | −22.4 |
| Sven | 6.2 | 4.2 | 5.0 | 7.5 | −3.5 | −12.1 |
| Tora | 16.7 | 11.8 | −26.5 | −30.1 | −18.8 | −18.8 |
| Tordis | 26.5 | 19.8 | 14.3 | 15.2 | −7.0 | −5.2 |
| Genus Level | Variety Level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Großthiemig (1 defoliation treatment) | |||||||||||
| Willows | |||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Poplars | 0% | — | 0.675 | 0.997 | 0.604 | Max 3 | 0% | — | |||
| 25% | 0.507 | — | 0.800 | 0.999 | 25% | 0.290 | — | ||||
| 50% | 1.000 | 0.513 | — | 0.736 | 50% | 0.574 | 0.024 | — | |||
| 75% | 0.305 | 0.987 | 0.315 | — | 75% | 0.876 | 0.774 | 0.229 | — | ||
| Großthiemig (2 defoliation treatments) | |||||||||||
| Willows | Tora | ||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Poplars | 0% | — | 0.964 | 0.998 | 0.547 | Muhle Larsen | 0% | — | 0.440 | 0.064 | 0.005 |
| 25% | 0.917 | — | 0.919 | 0.268 | 25% | 0.430 | — | 0.666 | 0.134 | ||
| 50% | 0.399 | 0.795 | — | 0.651 | 50% | 0.123 | 0.883 | — | 0.666 | ||
| 75% | 0.024 | 0.115 | 0.515 | — | 75% | 0.020 | 0.378 | 0.773 | — | ||
| Großthiemig (3 defoliation treatments) | |||||||||||
| Willows | Tora | ||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Poplars | 0% | — | 0.123 | 0.290 | 0.027 | Muhle Larsen | 0% | — | 0.013 | 0.035 | 0.004 |
| 25% | 0.592 | — | 0.969 | 0.943 | 25% | 0.107 | — | 0.981 | 0.978 | ||
| 50% | 0.012 | 0.276 | — | 0.733 | 50% | 0.195 | 0.982 | — | 0.857 | ||
| 75% | 0.001 | 0.062 | 0.893 | — | 75% | 0.018 | 0.892 | 0.683 | — | ||
| Genus/Variety Level | Variety Level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obercarsdorf (3 defoliation treatments) | |||||||||||
| Willows | |||||||||||
| DV | 0% | 25% | 50% | 75% | |||||||
| Poplars | 0% | — | 0.995 | 0.905 | 0.752 | no case of pairwise comparisons among defoliation variants with at least one significant value | |||||
| 25% | 0.827 | — | 0.981 | 0.914 | |||||||
| 50% | 0.043 | 0.261 | — | 0.992 | |||||||
| 75% | 0.057 | 0.317 | 0.999 | — | |||||||
| Schönheide (poplars: 3 defoliation treatments, willows: 2 defoliation treatments) | |||||||||||
| Willows | |||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Poplars | 0% | — | 0.989 | 0.747 | 0.644 | Andros-coggin | 0% | — | |||
| 25% | 0.261 | — | 0.905 | 0.832 | 25% | 0.154 | — | ||||
| 50% | 0.000 | 0.013 | — | 0.998 | 50% | 0.029 | 0.930 | — | |||
| 75% | 0.000 | 0.000 | 0.100 | — | 75% | 0.004 | 0.457 | 0.769 | — | ||
| Max 3 | Muhle Larsen | ||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Max 1 | 0% | — | 0.805 | 0.133 | 0.004 | Max 4 | 0% | — | 0.960 | 0.084 | 0.004 |
| 25% | 0.979 | — | 0.586 | 0.060 | 25% | 0.941 | — | 0.262 | 0.026 | ||
| 50% | 0.241 | 0.451 | — | 0.571 | 50% | 0.125 | 0.352 | — | 0.761 | ||
| 75% | 0.028 | 0.077 | 0.780 | — | 75% | 0.011 | 0.050 | 0.773 | — | ||
| Study Site (Number of Defoliation Treatments) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Großthiemig (1 Defoliation Treatment) | Großthiemig (2 Defoliation Treatments) | Großthiemig (3 Defoliation Treatments) | Obercarsdorf (3 Defoliation Treatments) | Schönheide (3/2 Defoliation Treatments) | Total | |||||||
| Genus/Variety | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] |
| Poplars | −11.4 | −6.3 | −26.9 * | −14.2 | −30.1 * | −16.6 | −21.5 * | −24.0 | −37.3 * | −28.3 | −26.7 * | −18.3 |
| Androscoggin | −7.3 | −3.7 | −21.8 | −10.3 | −36.1 | −19.0 | −38.3 * | −42.0 | −46.0 * | −34.2 | −33.3 * | −20.9 |
| Max 1 | −2.2 | −1.3 | −3.3 | −2.0 | −13.2 | −8.4 | −28.6 | −31.0 | −30.0 * | −22.6 | −15.2 | −11.2 |
| Max 3 | −9.9 | −5.5 | −38.3 | −20.4 | −31.0 | −16.7 | −3.0 | −3.3 | −41.1 * | −28.7 | −29.5 * | −19.4 |
| Max 4 | −9.4 | −5.3 | −22.8 | −13.0 | −15.0 | −9.0 | −20.1 | −23.1 | −35.2 * | −25.2 | −24.1 * | −16.7 |
| Muhle Larsen | −33.8 | −18.3 | −56.5 * | −26.4 | −54.7 * | −26.5 | −26.3 | −31.2 | −34.5 * | −32.1 | −34.9 * | −24.9 |
| Willows | −13.1 | −4.9 | −24.2 | −8.6 | −29.6 | −11.5 | −15.3 | −10.2 | −7.2 | −6.7 | −16.6 | −8.9 |
| Sven | −20.2 | −7.5 | −44.3 | −16.3 | −15.6 | −6.3 | 5.2 | 4.1 | −4.4 | −4.2 | −10.2 | −5.6 |
| Tora | 12.0 | 5.2 | −58.3 * | −19.5 | −62.0 * | −21.9 | −39.5 | −25.7 | −18.5 | −16.4 | −33.0 * | −17.6 |
| Tordis | −28.8 | −9.7 | 39.0 | 14.4 | −13.6 | −5.6 | −10.4 | −6.5 | 1.6 | 1.5 | −6.7 | −3.5 |
| Poplars | Willows | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of Defoliation Treatments | Number of Defoliation Treatments | |||||||
| DV | 1 | 2 | 3 | Total | 1 | 2 | 3 | Total |
| 0% | 344.5 | 438.7 | 298.7 | 360.6 (a) | 790.0 | 516.7 | 379.5 | 562.1 (a) |
| 25% | 323.0 | 424.7 | 328.1 | 358.6 (ab) | 714.5 | 520.3 | 370.9 | 535.3 (a) |
| 50% | 319.1 | 380.0 | 283.0 | 327.4 (ab) | 800.8 | 414.0 | 414.1 | 543.0 (a) |
| 75% | 265.3 | 313.3 | 235.5 | 271.4 (b) | 805.3 | 483.9 | 312.8 | 534.0 (a) |
| Total | 313.0 (a) | 389.2 (b) | 286.3 (a) | 329.5 | 777.7 (a) | 483.7 (b) | 369.3 (c) | 543.6 |
| Number of Defoliation Treatments | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Total | |||||||||||||
| Defoliation Variant [%] | ||||||||||||||||
| Genus/Variety | 0 | 25 | 50 | 75 | 0 | 25 | 50 | 75 | 0 | 25 | 50 | 75 | 0 | 25 | 50 | 75 |
| Poplars | 8 | 8 | 8 | 7 | 11 | 7 | 6 | 7 | 7 | 6 | 5 | 5 | 8 | 7 | 7 | 6 |
| Androscoggin | 7 | 8 | 7 | 7 | 16 | 7 | 7 | 6 | 7 | 7 | 6 | 4 | 9 | 7 | 6 | 6 |
| Max 1 | 8 | 6 | 8 | 7 | 9 | 7 | 6 | 5 | 5 | 6 | 4 | 5 | 8 | 6 | 6 | 6 |
| Max 3 | 11 | 10 | 10 | 7 | 13 | 9 | 7 | 9 | 9 | 8 | 6 | 7 | 11 | 9 | 8 | 7 |
| Max 4 | 6 | 8 | 7 | 8 | 6 | 6 | 5 | 7 | 7 | 6 | 6 | 5 | 7 | 7 | 6 | 7 |
| Muhle Larsen | 6 | 7 | 6 | 6 | 8 | 7 | 4 | 4 | 5 | 5 | 5 | 5 | 6 | 6 | 5 | 5 |
| Willows | 19 | 16 | 15 | 16 | 17 | 17 | 17 | 18 | 16 | 16 | 17 | 15 | 17 | 17 | 16 | 17 |
| Sven | 22 | 18 | 16 | 17 | 18 | 18 | 20 | 18 | 17 | 18 | 20 | 19 | 19 | 18 | 19 | 18 |
| Tora | 16 | 15 | 12 | 19 | 15 | 16 | 14 | 19 | 17 | 15 | 15 | 12 | 16 | 15 | 14 | 17 |
| Tordis | 18 | 16 | 16 | 13 | 16 | 18 | 15 | 18 | 16 | 16 | 15 | 14 | 17 | 16 | 16 | 15 |
| June 2009 | September 2009 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Großthiemig (1 defoliation treatment) | |||||||||||
| Willows | Willows | ||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Poplars | 0% | — | 0.931 | 0.992 | 0.991 | Poplars | 0% | — | 0.988 | 1.000 | 0.996 |
| 25% | 1.000 | — | 0.989 | 0.810 | 25% | 0.670 | — | 0.972 | 0.945 | ||
| 50% | 0.787 | 0.794 | — | 0.940 | 50% | 0.983 | 0.459 | — | 0.999 | ||
| 75% | 1.000 | 1.000 | 0.784 | — | 75% | 0.948 | 0.937 | 0.808 | — | ||
| Großthiemig (2 defoliation treatments) | |||||||||||
| Willows | Willows | ||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Poplars | 0% | — | 0.979 | 0.973 | 0.960 | Poplars | 0% | — | 0.854 | 0.995 | 0.960 |
| 25% | 0.992 | — | 1.000 | 1.000 | 25% | 0.953 | — | 0.942 | 0.990 | ||
| 50% | 0.890 | 0.972 | — | 1.000 | 50% | 0.998 | 0.985 | — | 0.994 | ||
| 75% | 0.865 | 0.960 | 1.000 | — | 75% | 0.959 | 0.717 | 0.894 | — | ||
| DV | 0% | 25% | 50% | 75% | |||||||
| Max 1 | 0% | — | no case of pairwise comparisons among defoliation variants with at least one significant value | ||||||||
| 25% | 0.100 | — | |||||||||
| 50% | 0.904 | 0.030 | — | ||||||||
| 75% | 0.843 | 0.338 | 0.457 | — | |||||||
| Großthiemig (3 defoliation treatments) | |||||||||||
| Willows | Willows | ||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Poplars | 0% | — | 0.518 | 0.956 | 0.147 | Poplars | 0% | — | 0.746 | 0.794 | 0.014 |
| 25% | 0.998 | — | 0.832 | 0.878 | 25% | 0.946 | — | 1.000 | 0.179 | ||
| 50% | 0.092 | 0.149 | — | 0.385 | 50% | 0.040 | 0.156 | — | 0.156 | ||
| 75% | 0.018 | 0.034 | 0.930 | — | 75% | 0.013 | 0.064 | 0.983 | — | ||
| Tora | Tora | ||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Max 3 | 0% | — | 0.023 | 0.798 | 0.179 | Andros-coggin in | 0% | — | 0.057 | 0.315 | 0.083 |
| 25% | 0.507 | — | 0.194 | 0.777 | 25% | 0.351 | — | 0.831 | 0.997 | ||
| 50% | 0.223 | 0.014 | — | 0.683 | 50% | 0.099 | 0.955 | — | 0.913 | ||
| 75% | 0.077 | 0.003 | 0.984 | — | 75% | 0.019 | 0.642 | 0.882 | — | ||
| Number of Defoliation Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Total | |||||
| Genus/Variety | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] |
| Poplars | −0.1 | −0.1 | −4.2 | −3.6 | −12.5* | −10.8 | −4.9* | −4.3 |
| Androscoggin | −10.5 | −8.8 | −8.9 | −7.5 | −13.5 | −11.9 | −11.4* | −9.7 |
| Max 1 | −2.5 | −2.2 | 6.7 | 5.8 | −20.1* | −17.9 | −6.3 | −5.6 |
| Max 3 | 5.6 | 4.8 | −3.8 | −3.2 | −17.7* | −14.4 | −4.5 | −3.8 |
| Max 4 | 3.3 | 2.9 | 4.1 | 3.3 | −1.1 | −1.0 | 1.9 | 1.6 |
| Muhle Larsen | 6.0 | 6.1 | −16.2 | −14.1 | −8.3 | −7.4 | −3.4 | −3.1 |
| Willows | 2.7 | 1.5 | 3.3 | 1.7 | −16.9 | −8.5 | −5.4 | −2.8 |
| Sven | −2.2 | −1.3 | −8.6 | −4.6 | −23.8 | −12.7 | −13.0 | −7.2 |
| Tora | 33.1 | 18.5 | 6.3 | 3.1 | −23.5* | −10.4 | 2.1 | 1.0 |
| Tordis | −14.4 | −7.1 | 10.8 | 5.6 | −3.5 | −1.9 | −3.7 | −1.9 |
| Number of Defoliation Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Total | |||||
| Genus/Variety | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] |
| Poplars | −5.3 | −1.8 | −6.9 | −2.4 | −33.6* | −11.9 | −15.3 * | −5.3 |
| Androscoggin | −7.1 | −2.6 | −37.8 | −13.2 | −45.6* | −16.8 | −26.1 * | −9.4 |
| Max 1 | −24.1 | −7.6 | 28.8 | 9.8 | −57.0* | −19.0 | −25.8 | −8.4 |
| Max 3 | −24.5 | −7.6 | 27.9 | 11.3 | −28.7 | −9.5 | −21.1 | −6.9 |
| Max 4 | 15.6 | 5.2 | −7.5 | −2.3 | 5.1 | 1.8 | 8.4 | 2.8 |
| Muhle Larsen | 34.6 | 17.0 | −16.2 | −6.2 | −35.6 | −13.9 | −2.8 | −1.2 |
| Willows | 3.2 | 1.2 | 5.0 | 1.8 | −28.9* | −10.2 | −9.8 | −3.5 |
| Sven | 14.6 | 6.2 | 0.0 | 0.0 | −26.5 | −10.5 | −6.4 | −2.6 |
| Tora | 34.1 | 13.4 | 14.4 | 5.2 | −30.0* | −9.6 | 2.1 | 0.7 |
| Tordis | −30.0 | −9.8 | 0.0 | 0.0 | −30.2 | −10.7 | −23.0* | −7.8 |
| Genus Level | Variety Level | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obercarsdorf | |||||||||||
| Willows | |||||||||||
| DV | 0% | 25% | 50% | 75% | |||||||
| Poplars | 0% | — | 0.124 | 0.032 | 0.165 | no case of pairwise comparisons among defoliation variants with at least one significant value | |||||
| 25% | 1.000 | — | 0.970 | 0.997 | |||||||
| 50% | 0.335 | 0.282 | — | 0.909 | |||||||
| 75% | 0.155 | 0.123 | 0.978 | — | |||||||
| Schönheide | |||||||||||
| Willows | Max 3 | ||||||||||
| DV | 0% | 25% | 50% | 75% | DV | 0% | 25% | 50% | 75% | ||
| Poplars | 0% | — | 0.988 | 0.999 | 0.088 | Max 1 | 0% | — | 0.725 | 0.114 | 0.006 |
| 25% | 0.632 | — | 0.967 | 0.041 | 25% | 0.496 | — | 0.614 | 0.097 | ||
| 50% | 0.000 | 0.026 | — | 0.116 | 50% | 0.153 | 0.900 | — | 0.683 | ||
| 75% | 0.000 | 0.000 | 0.376 | — | 75% | 0.028 | 0.501 | 0.890 | — | ||
| Study Site | ||||||
|---|---|---|---|---|---|---|
| Obercarsdorf | Schönheide | Total | ||||
| Genus/Variety | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] | Δ 75-0 [cm] | Δ 75-0 [%] |
| Poplars | −23.0 * | −13.9 | −55.2 * | −18.5 | −41.8 * | −17.0 |
| Androscoggin | −33.8 | −21.1 | −42.9 * | −13.4 | −43.3 | −16.5 |
| Max 1 | −33.4 | −21.4 | −63.7 * | −21.2 | −33.2 | −14.2 |
| Max 3 | −17.4 | −9.9 | −71.6 * | −23.0 | −56.7 * | −21.9 |
| Max 4 | −40.0 | −21.7 | −36.2 | −12.3 | −43.3 * | −17.3 |
| Muhle Larsen | 7.3 | 5.2 | −55.9 * | −20.5 | −31.0 | −13.6 |
| Willows | −41.2 * | −15.7 | −22.3 * | −8.8 | −29.3 * | −11.5 |
| Sven | −65.4 * | −28.1 | −28.4 | −12.0 | −40.7 * | −17.3 |
| Tora | −81.3 * | −30.4 | −27.6 | −10.5 | −46.2 * | −17.4 |
| Tordis | 4.9 | 1.7 | −12.5 | −4.9 | −3.5 | −1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helbig, C.E.; Müller, M.G.; Landgraf, D. Effects of Leaf Loss by Artificial Defoliation on the Growth of Different Poplar and Willow Varieties. Forests 2021, 12, 1224. https://doi.org/10.3390/f12091224
Helbig CE, Müller MG, Landgraf D. Effects of Leaf Loss by Artificial Defoliation on the Growth of Different Poplar and Willow Varieties. Forests. 2021; 12(9):1224. https://doi.org/10.3390/f12091224
Chicago/Turabian StyleHelbig, Christiane E., Michael G. Müller, and Dirk Landgraf. 2021. "Effects of Leaf Loss by Artificial Defoliation on the Growth of Different Poplar and Willow Varieties" Forests 12, no. 9: 1224. https://doi.org/10.3390/f12091224






