Predictive Models to Estimate Carbon Stocks in Agroforestry Systems
Abstract
:1. Introduction
2. Materials and Methods
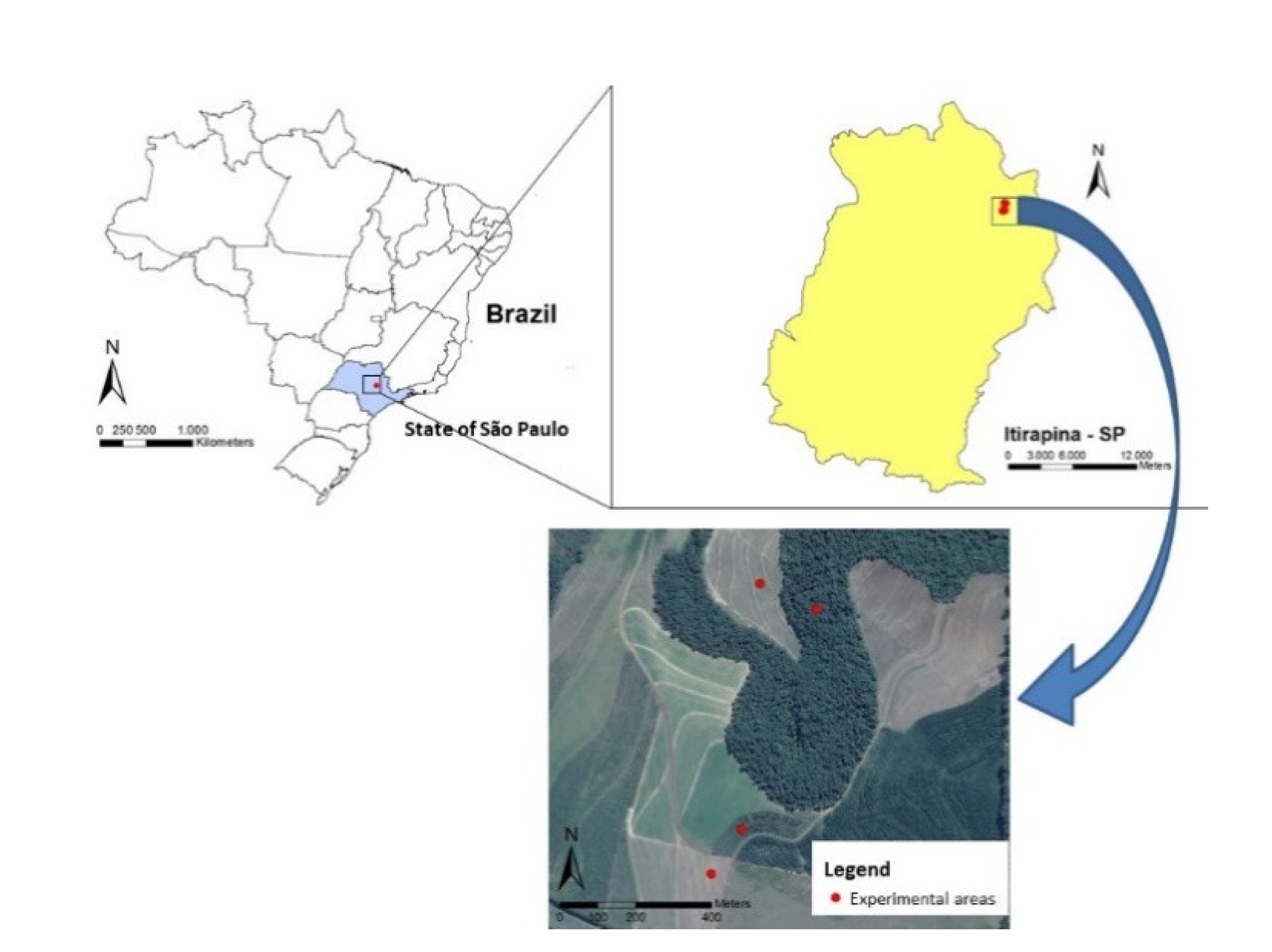
2.1. Description of the Study Area and History of the Areas
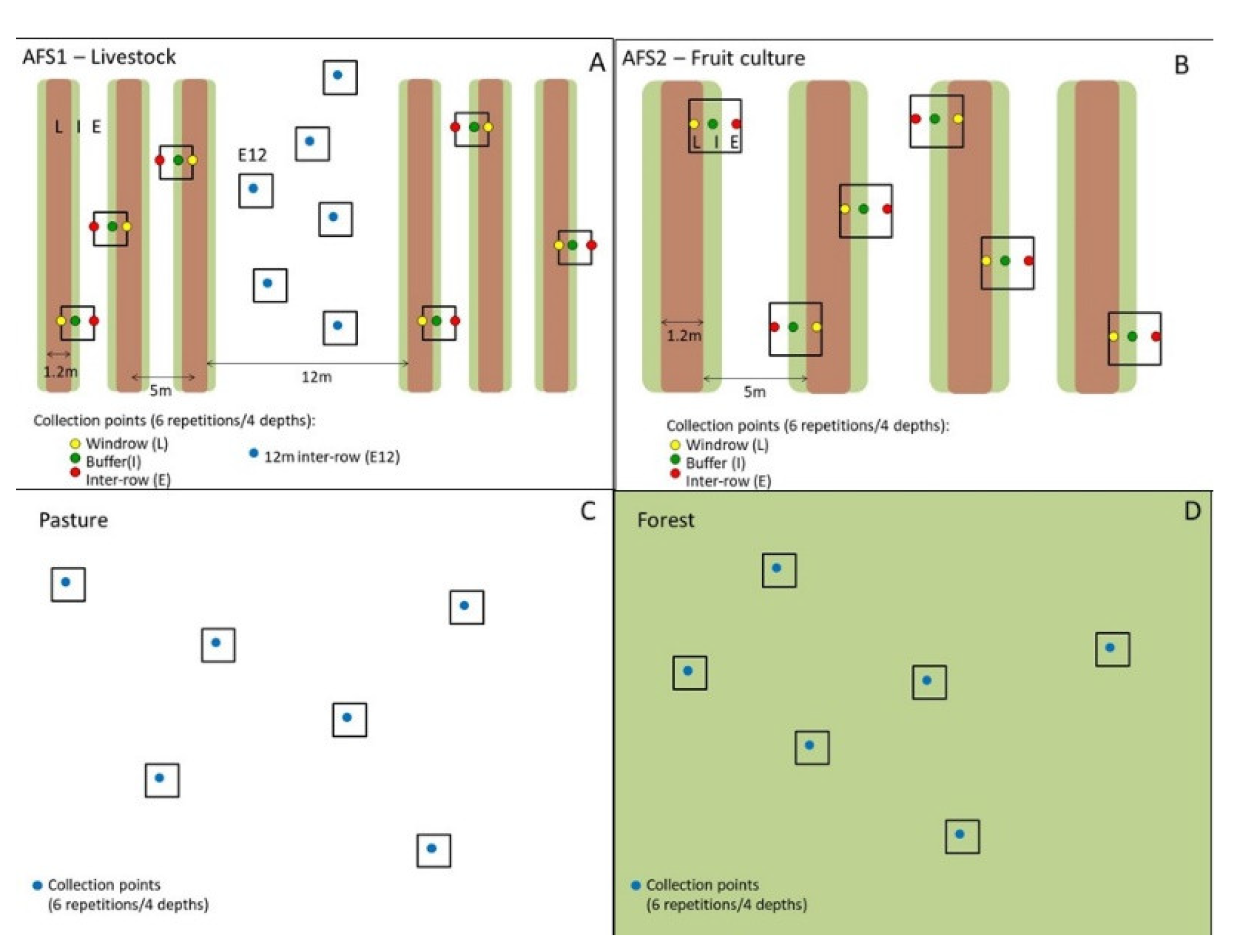
2.2. Experimental Design, Soil Collection and Analyzed Physical and Chemical Properties
2.3. Predictive Modeling
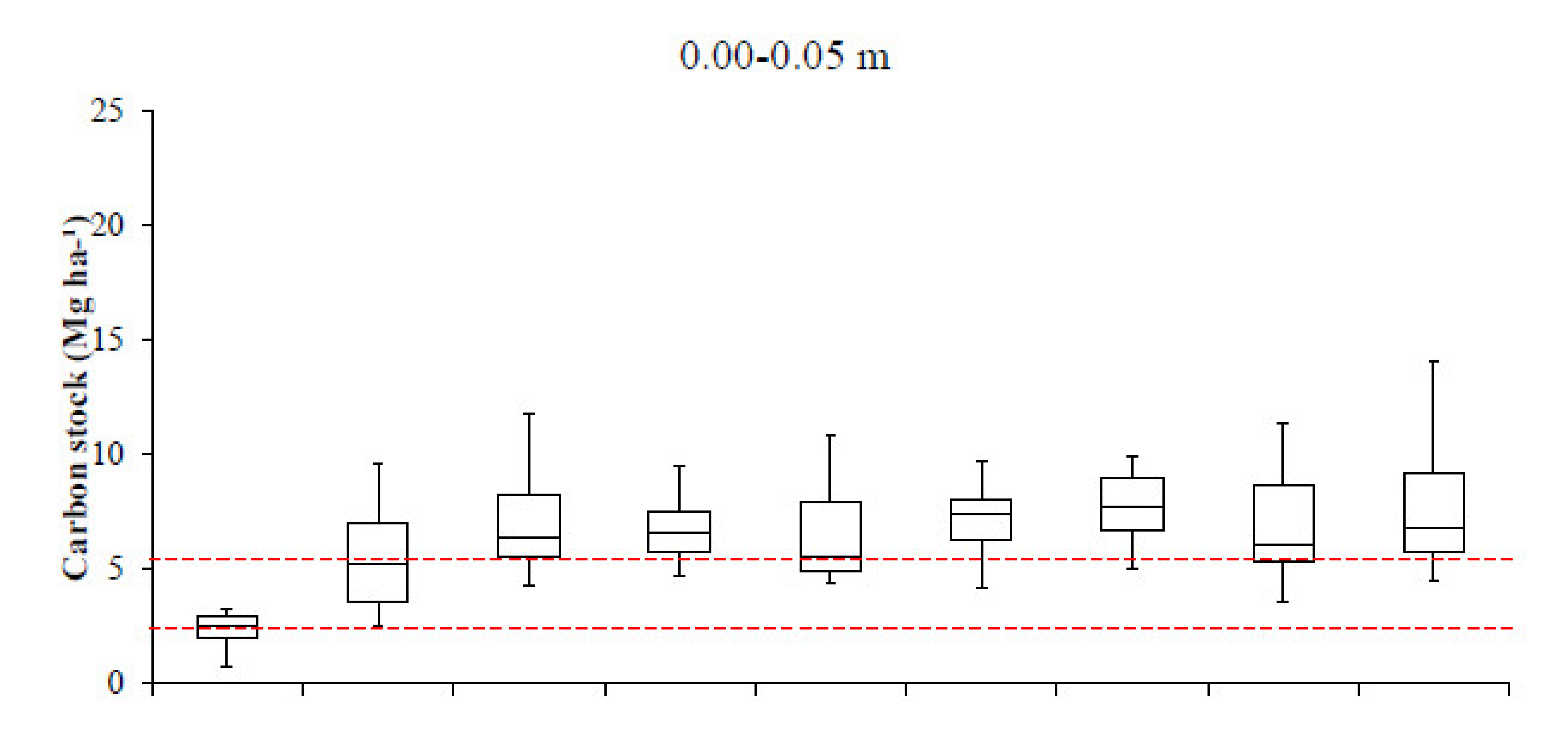
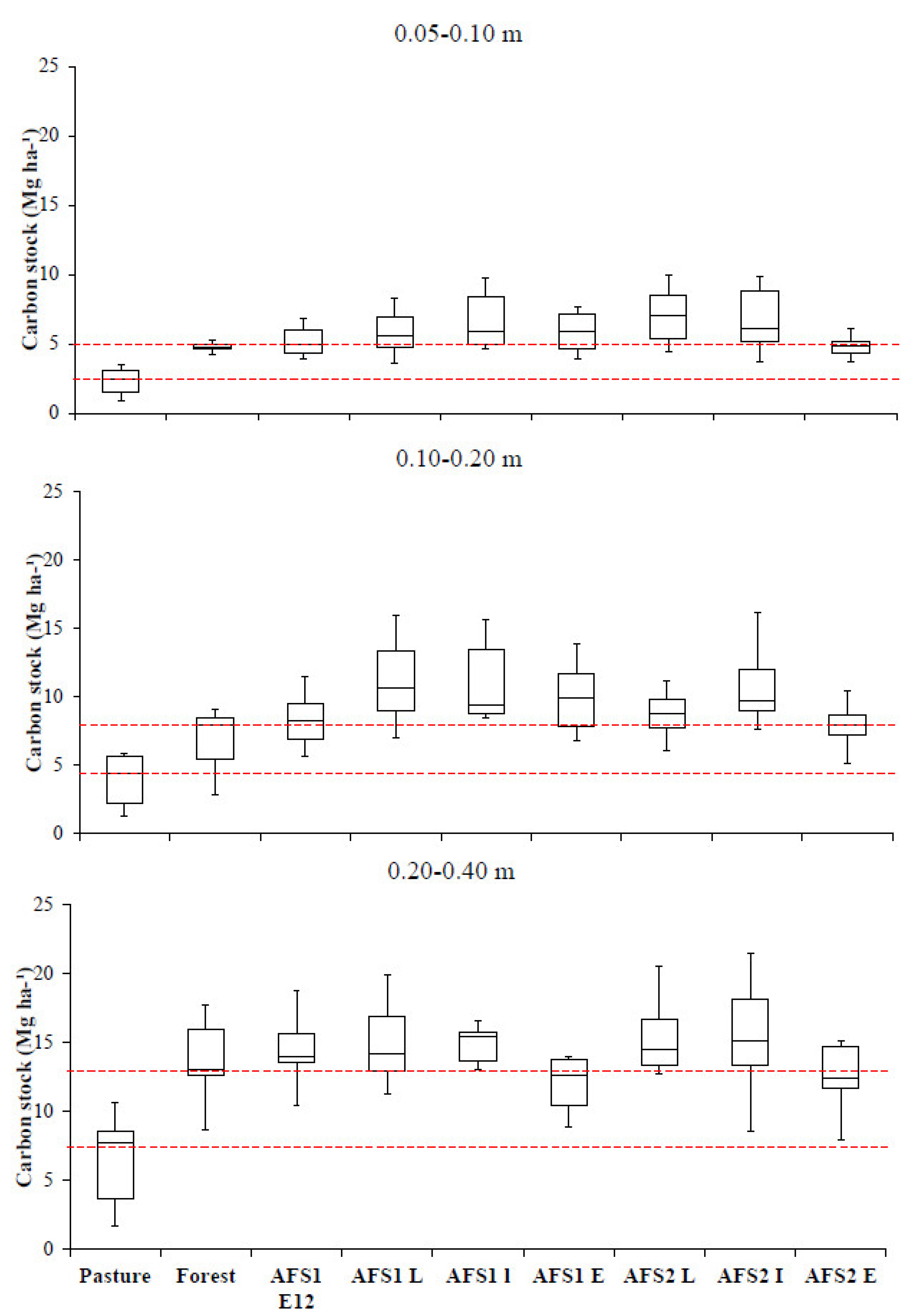
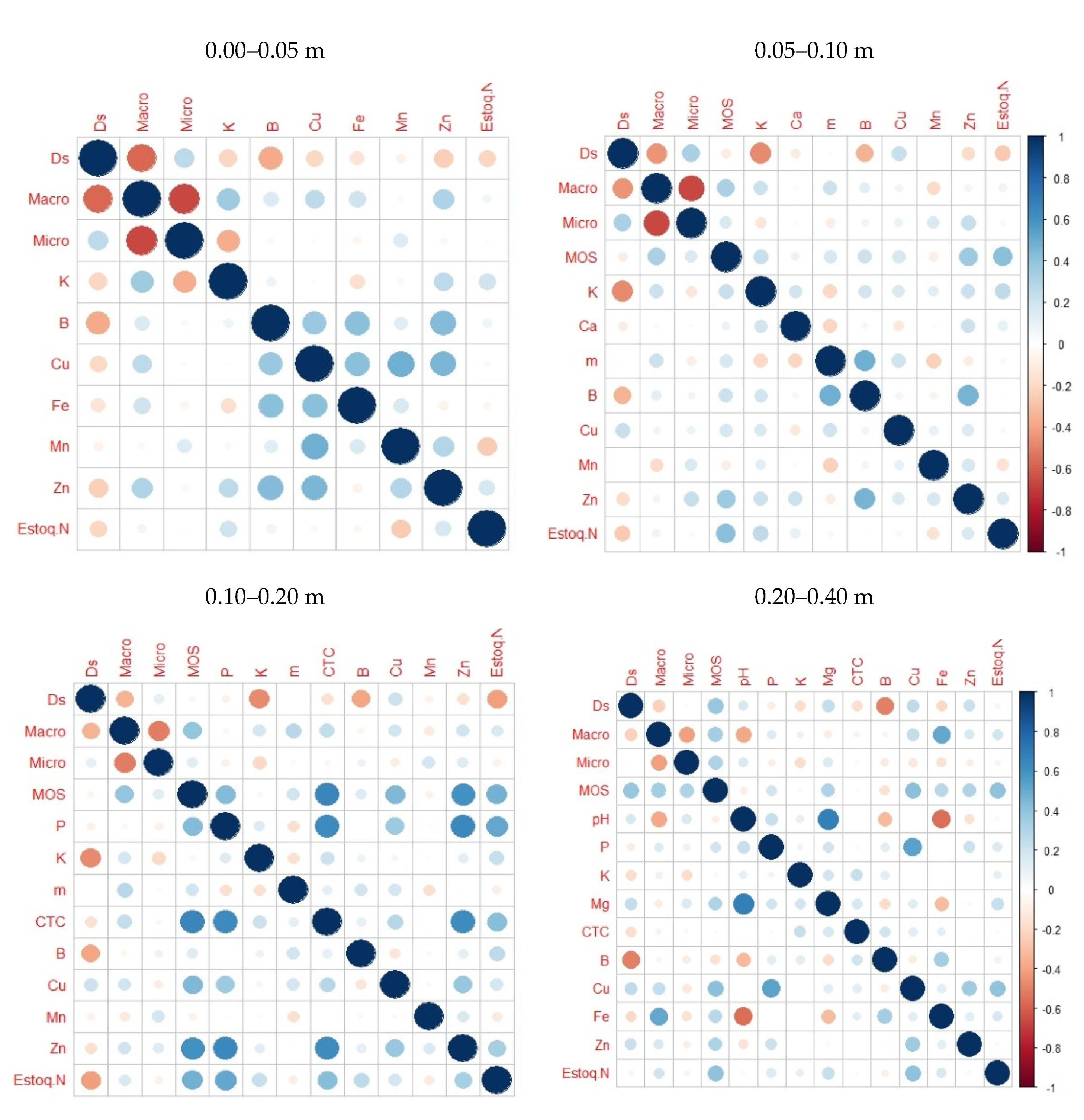
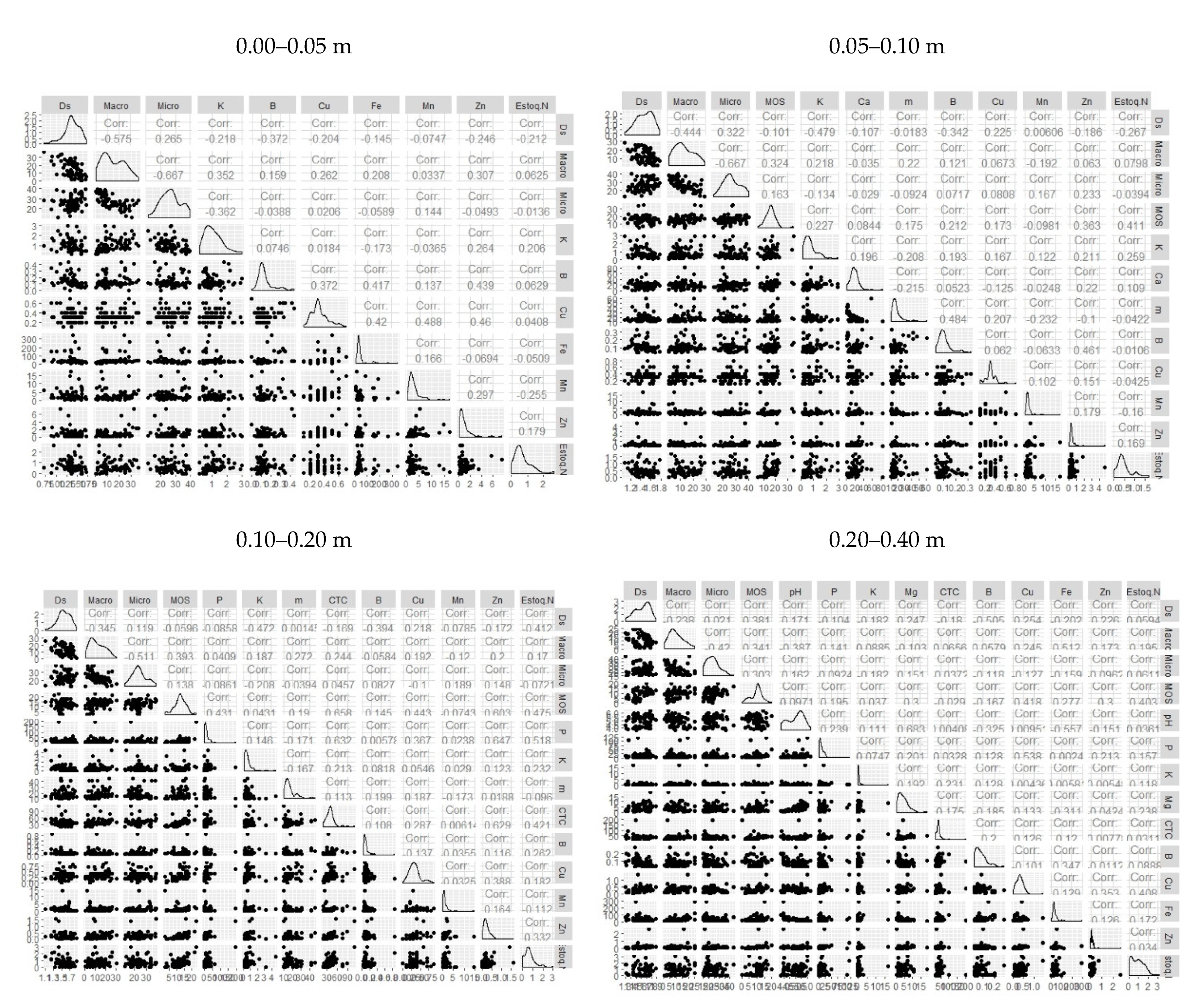
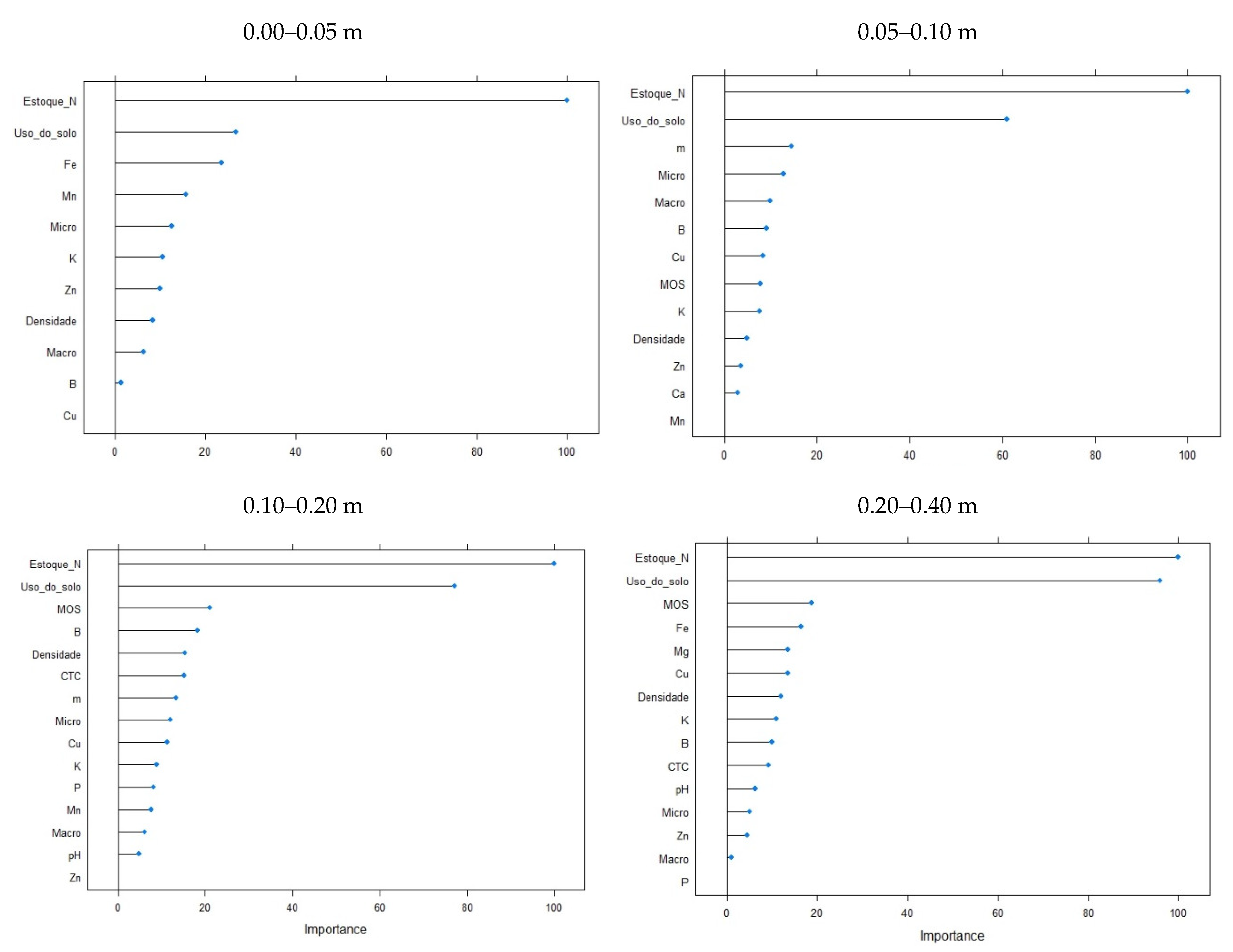
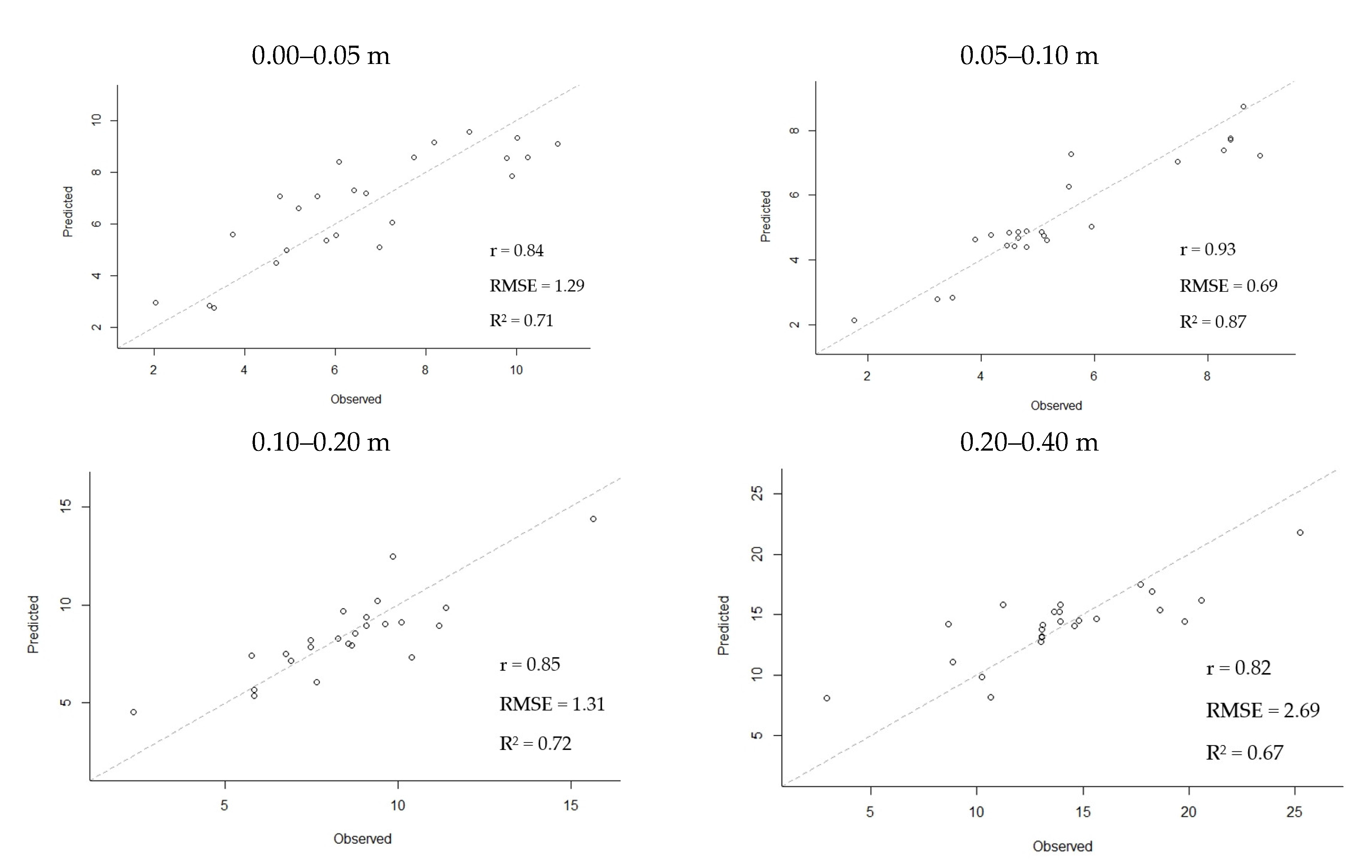
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Da Conceição, M.C.G.; Matos, E.S.; Bidone, E.D.; Rodrigues, R.D.A.R.; Cordeiro, R.C. Changes in Soil Carbon Stocks under Integrated Crop-Livestock-Forest System in the Brazilian Amazon Region. Agric. Sci. 2017, 8, 904–913. [Google Scholar] [CrossRef] [Green Version]
- Bayer, C.; Amado, T.J.C.; Tornquist, C.G.; Cerri, C.E.C.; Dieckow, J.; Zanatta, J.A.Z.; Nicoloso, R.S. Estabilização do carbono no solo e mitigação das emissões de gases de efeito estufa na agricultura conservacionista. In Tópicos em Ciência do Solo, 1st ed.; Klauberg Filho, O., Mafra, A.L., Gatiboni, L.C., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2011; Volume 7, pp. 55–118. [Google Scholar]
- Stockmann, U.; Adams, M.; Crawford, J.; Field, D.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.; Courcelles, V.D.R.D.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon sequestration in soil. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar] [CrossRef]
- Paustian, L.; Babcock, B.; Hatfield, J.L.; Lal, R.; Mccarl, B.A.; Mclaughlin, S.; Mosier, A.; Rice, C.; Robertson, G.P.; Rosenberg, N.J.; et al. Agricultural mitigation of greenhouse gases: Science and policy options. In Proceedings of the Anais Conference Proceedings, First National Conference on Carbon Sequestration, Washington, DC, USA, 14–17 May 2001. [Google Scholar]
- Chenu, C.; Angers, D.A.; Barre, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Lal, R.; Kundu, S.; Thakur, P.B. Conservation Agriculture and Soil Carbon Sequestration. In Conservation Agriculture; Springer: Cham, Switzerland, 2014; pp. 479–524. [Google Scholar]
- Kim, D.-G.; Kirschbaum, M.U.; Beedy, T.L. Carbon sequestration and net emissions of CH4 and N2O under agroforestry: Synthesizing available data and suggestions for future studies. Agric. Ecosyst. Environ. 2016, 226, 65–78. [Google Scholar] [CrossRef]
- Behrens, T.; Scholten, T. Chapter 25 A Comparison of Data-Mining Techniques in Predictive Soil Mapping. Vital Soil Funct. Value Prop. 2006, 31, 353–617. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Zhu, X.; Dong, Z.; Guo, W. Estimation of biomass in wheat using random forest regression algorithm and remote sensing data. Crop J. 2016, 4, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Everingham, Y.; Sexton, J.; Skocaj, D.; Inman-Bamber, G. Accurate prediction of sugarcane yield using a random forest algorithm. Agron. Sustain. Dev. 2016, 36, 27. [Google Scholar] [CrossRef] [Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; p. 353. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solos, 3rd ed.; Revista e Ampliada; Embrapa: Brasília, Brazil, 2017; p. 573. [Google Scholar]
- Raij, B.V.; Andrade, J.C.; Cantarella, H.; Quaggio, J.A. Análise Química Para Avaliação da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 3; Black, C.A., Ed.; Soil Science of America/American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 18 July 2020).
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference and Prediction, 2nd ed.; Springer: Cham, Switzerland, 2009. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Were, K.; Bui, D.T.; Dick, Ø.B.; Singh, B.R. A comparative assessment of support vector regression, artificial neural networks, and random forests for predicting and mapping soil organic carbon stocks across an Afromontane landscape. Ecol. Indic. 2015, 52, 394–403. [Google Scholar] [CrossRef]
- Ostle, N.; Levy, P.; Evans, C.; Smith, P. UK land use and soil carbon sequestration. Land Use Policy 2009, 26, S274–S283. [Google Scholar] [CrossRef]
- Scott, N.; Tate, K.; Giltrap, D.; Smith, C.T.; Wilde, H.; Newsome, P.; Davis, M. Monitoring land-use change effects on soil carbon in New Zealand: Quantifying baseline soil carbon stocks. Environ. Pollut. 2002, 116, S167–S186. [Google Scholar] [CrossRef]
- Cardinael, R.; Chevallier, T.; Cambou, A.; Béral, C.; Barthès, B.G.; Dupraz, C.; Durand, C.; Kouakoua, E.; Chenu, C. Increased soil organic carbon stocks under agroforestry: A survey of six different sites in France. Agric. Ecosyst. Environ. 2017, 236, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Monroe, P.; Gama-Rodrigues, E.F.; Gama-Rodrigues, A.; Marques, J.R.B. Soil carbon stocks and origin under different cacao agroforestry systems in Southern Bahia, Brazil. Agric. Ecosyst. Environ. 2016, 221, 99–108. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Ramesh, T.; Manjaiah, K.M.; Mohopatra, K.P.; Rajasekar, K.; Ngachan, S.V. Assessment of soil organic carbon stocks and fractions under different agroforestry systems in subtropical hill agroecosystems of north-east India. Agrofor. Syst. 2015, 89, 677–690. [Google Scholar] [CrossRef]
- Lim, S.-S.; Baah-Acheamfour, M.; Choi, W.-J.; Arshad, M.A.; Fatemi, F.; Banerjee, S.; Carlyle, C.; Bork, E.W.; Park, H.-J.; Chang, S.X. Soil organic carbon stocks in three Canadian agroforestry systems: From surface organic to deeper mineral soils. For. Ecol. Manag. 2018, 417, 103–109. [Google Scholar] [CrossRef]

| Land Use Systems | Sand | Clay | Silt |
|---|---|---|---|
| g kg−1 | |||
| AFS1 | 897 | 75 | 28 |
| AFS2 | 888 | 85 | 27 |
| Pasture | 953 | 37 | 10 |
| Forest | 937 | 50 | 13 |
| Variable | Description | Abbreviation | Unit | Type |
|---|---|---|---|---|
| Land use | AFS1, AFS2, Pasture and Forest | – | – | Predictive |
| Physical | Bulk density | Bd | kg dm−3 | Predictive |
| Macroporosity | Macro | m3 m−3 | Predictive | |
| Microporosity | Micro | m3 m−3 | Predictive | |
| Chemical | pH | – | – | Predictive |
| Phosphorus | P | mg dm−3 | Predictive | |
| Potassium | K | mmolc dm−3 | Predictive | |
| Calcium | Ca | mmolc dm−3 | Predictive | |
| Magnesium | Mg | mmolc dm−3 | Predictive | |
| Saturation by aluminum | m | mmolc dm−3 | Predictive | |
| Sum of bases | SB | mmolc dm−3 | Predictive | |
| Cation-exchange capacity | CEC | mmolc dm−3 | Predictive | |
| Bases saturation | V | % | Predictive | |
| Boron | B | mg dm−3 | Predictive | |
| Copper | Cu | mg dm−3 | Predictive | |
| Iron | Fe | mg dm−3 | Predictive | |
| Manganese | Mn | mg dm−3 | Predictive | |
| Zinc | Zn | mg dm−3 | Predictive | |
| Soil organic matter | SOM | g dm−3 | Predictive | |
| Soil nitrogen stock | N stock | Mg ha−1 | Predictive | |
| Soil carbon stock | C stock | Mg ha−1 | Response |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marçal, M.F.M.; Souza, Z.M.d.; Tavares, R.L.M.; Farhate, C.V.V.; Oliveira, S.R.M.; Galindo, F.S. Predictive Models to Estimate Carbon Stocks in Agroforestry Systems. Forests 2021, 12, 1240. https://doi.org/10.3390/f12091240
Marçal MFM, Souza ZMd, Tavares RLM, Farhate CVV, Oliveira SRM, Galindo FS. Predictive Models to Estimate Carbon Stocks in Agroforestry Systems. Forests. 2021; 12(9):1240. https://doi.org/10.3390/f12091240
Chicago/Turabian StyleMarçal, Maria Fernanda Magioni, Zigomar Menezes de Souza, Rose Luiza Moraes Tavares, Camila Viana Vieira Farhate, Stanley Robson Medeiros Oliveira, and Fernando Shintate Galindo. 2021. "Predictive Models to Estimate Carbon Stocks in Agroforestry Systems" Forests 12, no. 9: 1240. https://doi.org/10.3390/f12091240
APA StyleMarçal, M. F. M., Souza, Z. M. d., Tavares, R. L. M., Farhate, C. V. V., Oliveira, S. R. M., & Galindo, F. S. (2021). Predictive Models to Estimate Carbon Stocks in Agroforestry Systems. Forests, 12(9), 1240. https://doi.org/10.3390/f12091240








