Effect of Acacia mangium Canopy on Physicochemical Characteristics and Nutrient Concentrations of the Soil at Ayer Hitam Forest Reserve, Malaysia
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Site Description
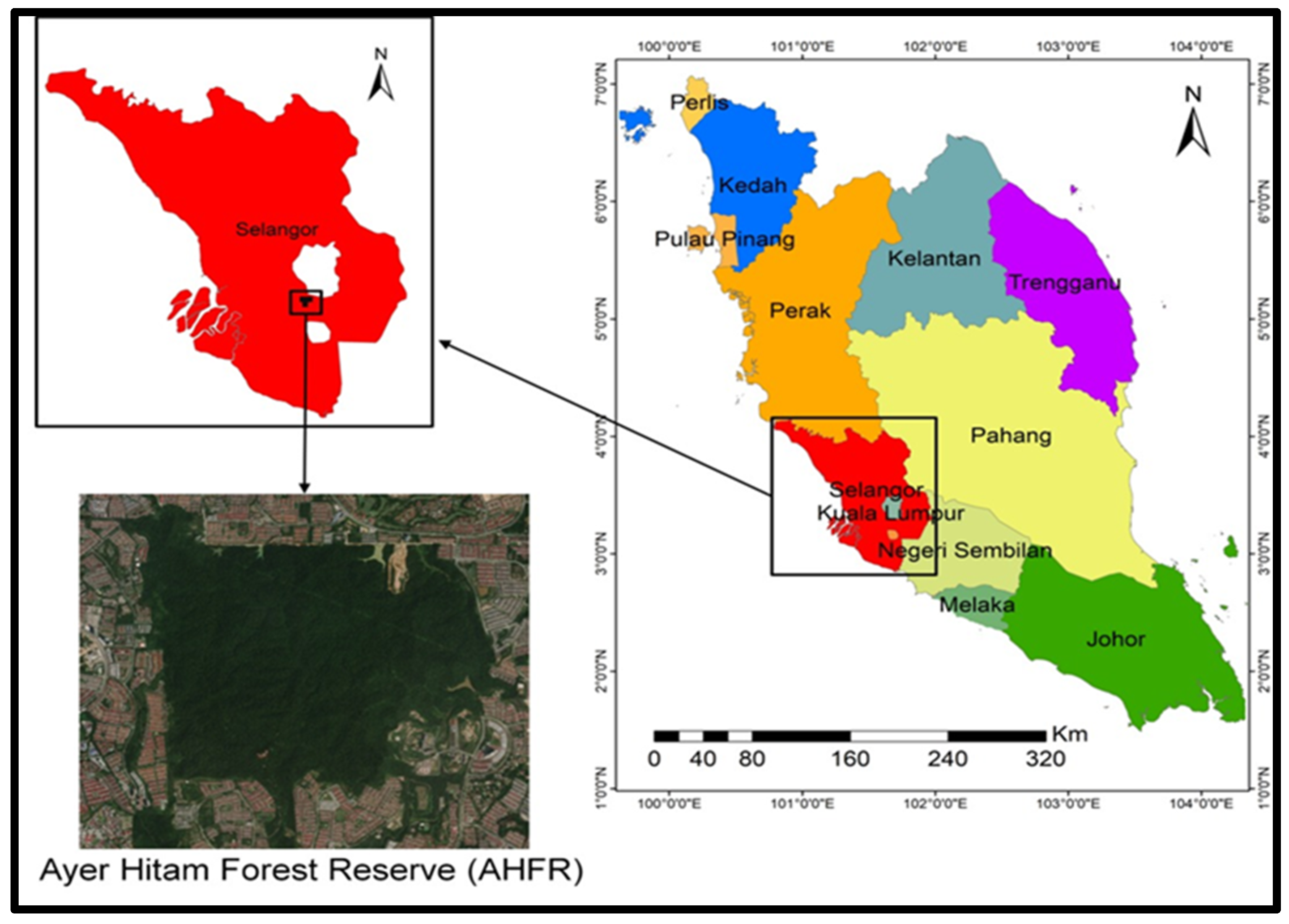
2.1.1. Geographical Distribution of the Study Site
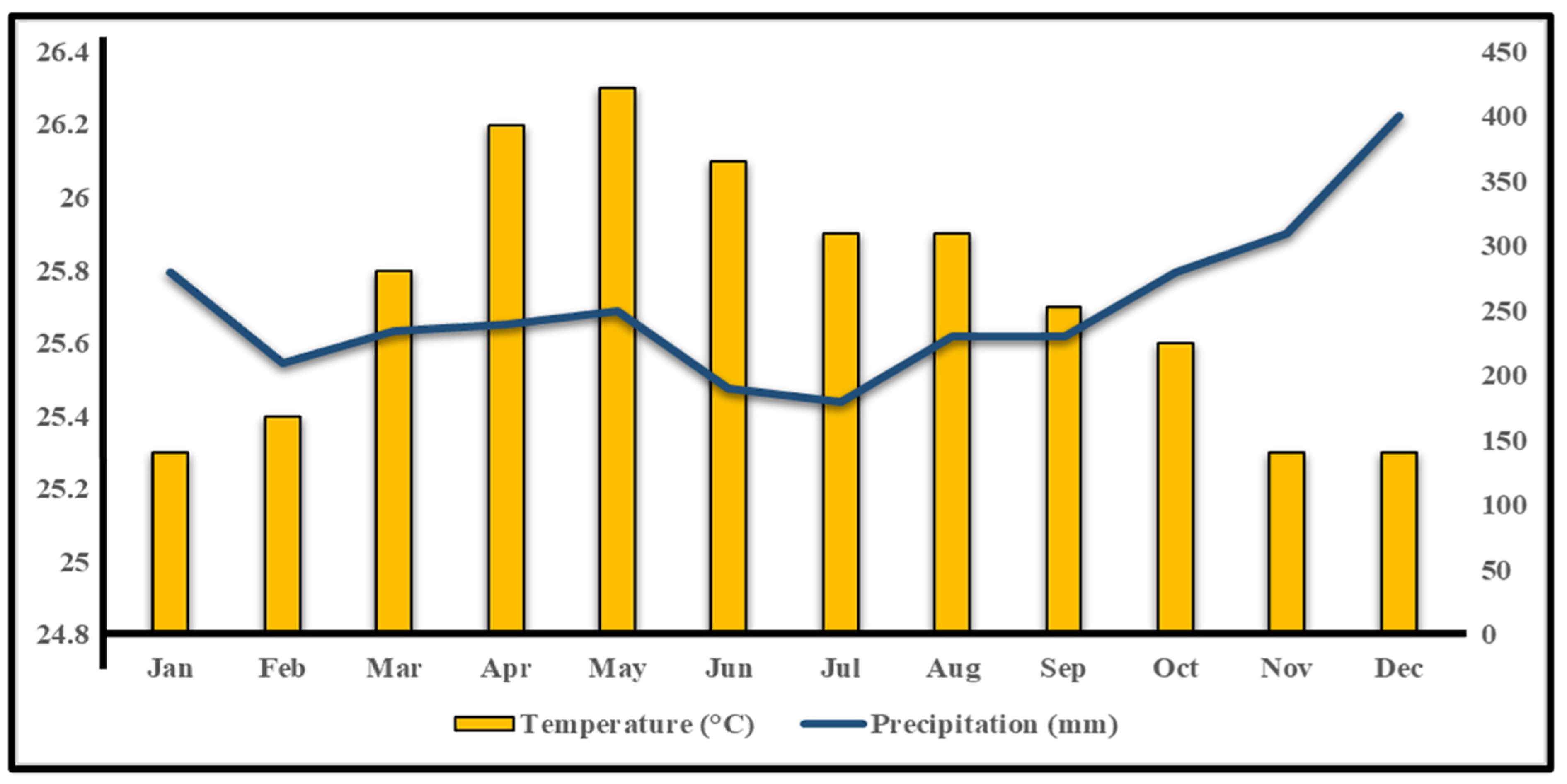
2.1.2. Climate Condition of the Study Site
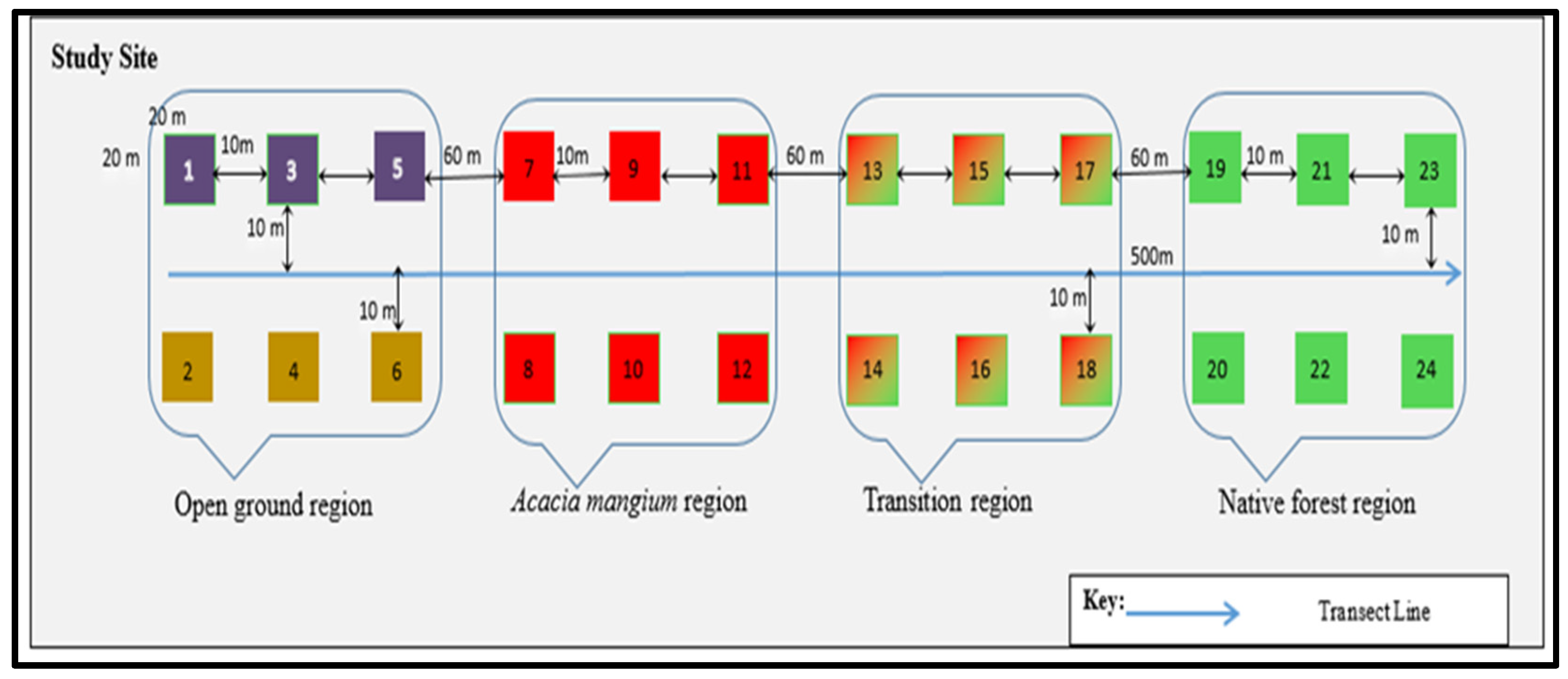
2.1.3. Physiographical and Vegetation of the Study Site
2.2. Data Collection
2.2.1. Soil Sampling, Preparation, and Analysis
2.2.2. Physical Properties of Soil
2.2.3. Chemical Properties of Soil
2.3. Statistical Analysis
3. Results and Discussion
3.1. The Physicochemical Properties of Soils
3.1.1. Physical Properties of Soil
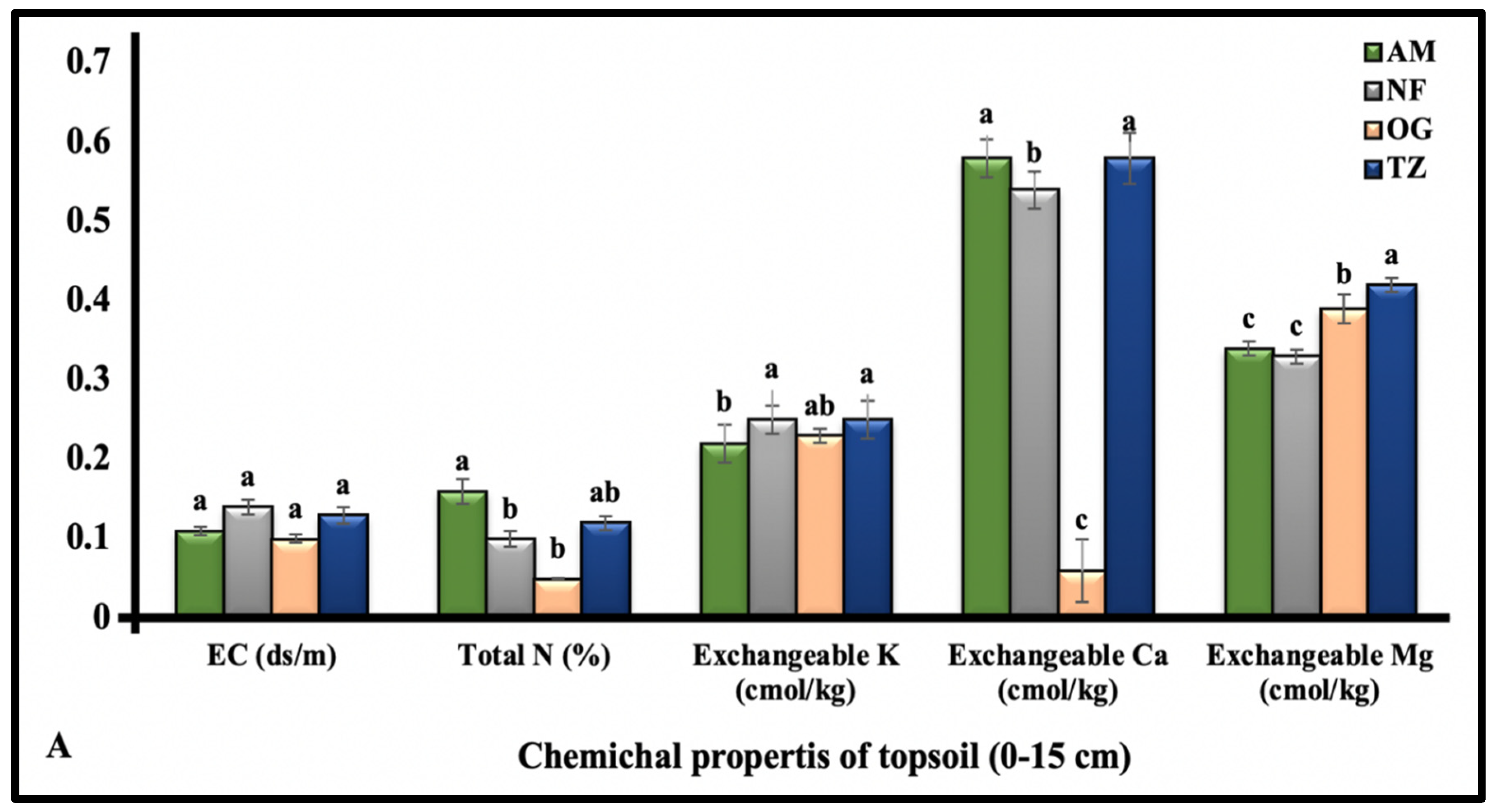
3.1.2. The Chemical Properties of Topsoil (0–15 cm Depth)
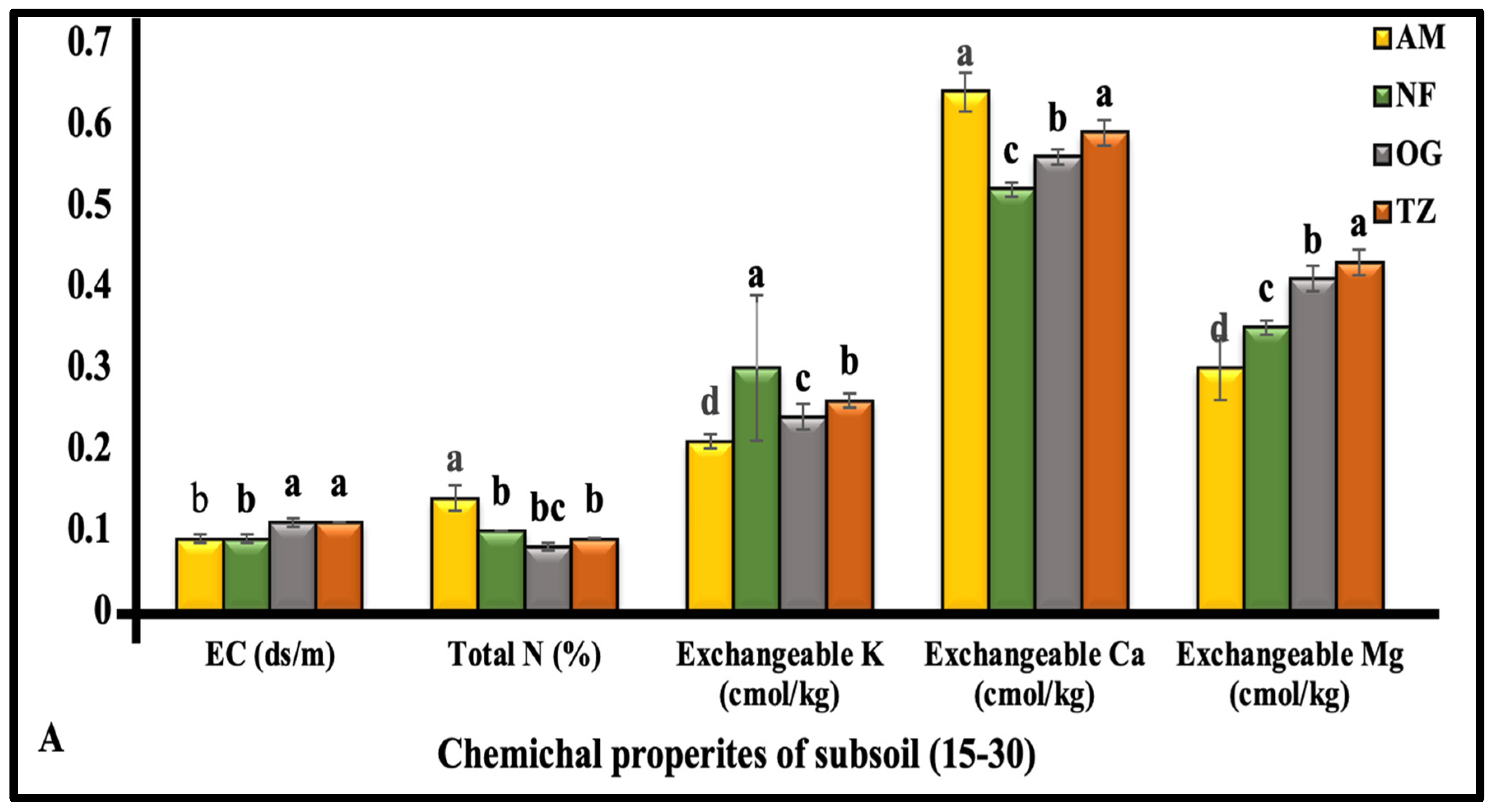
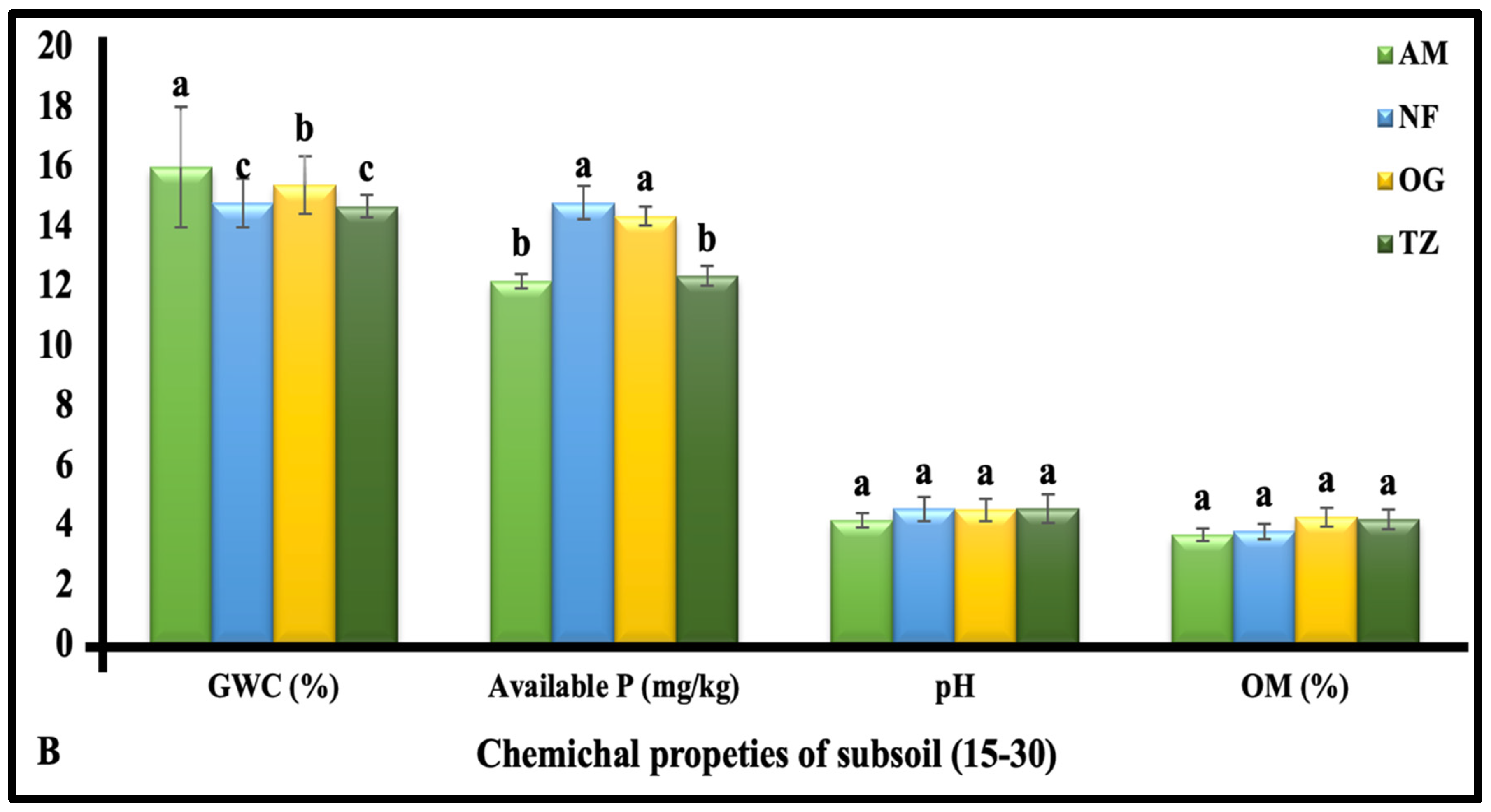
3.1.3. The Chemical Properties of Subsoil (15–30 cm Depth)
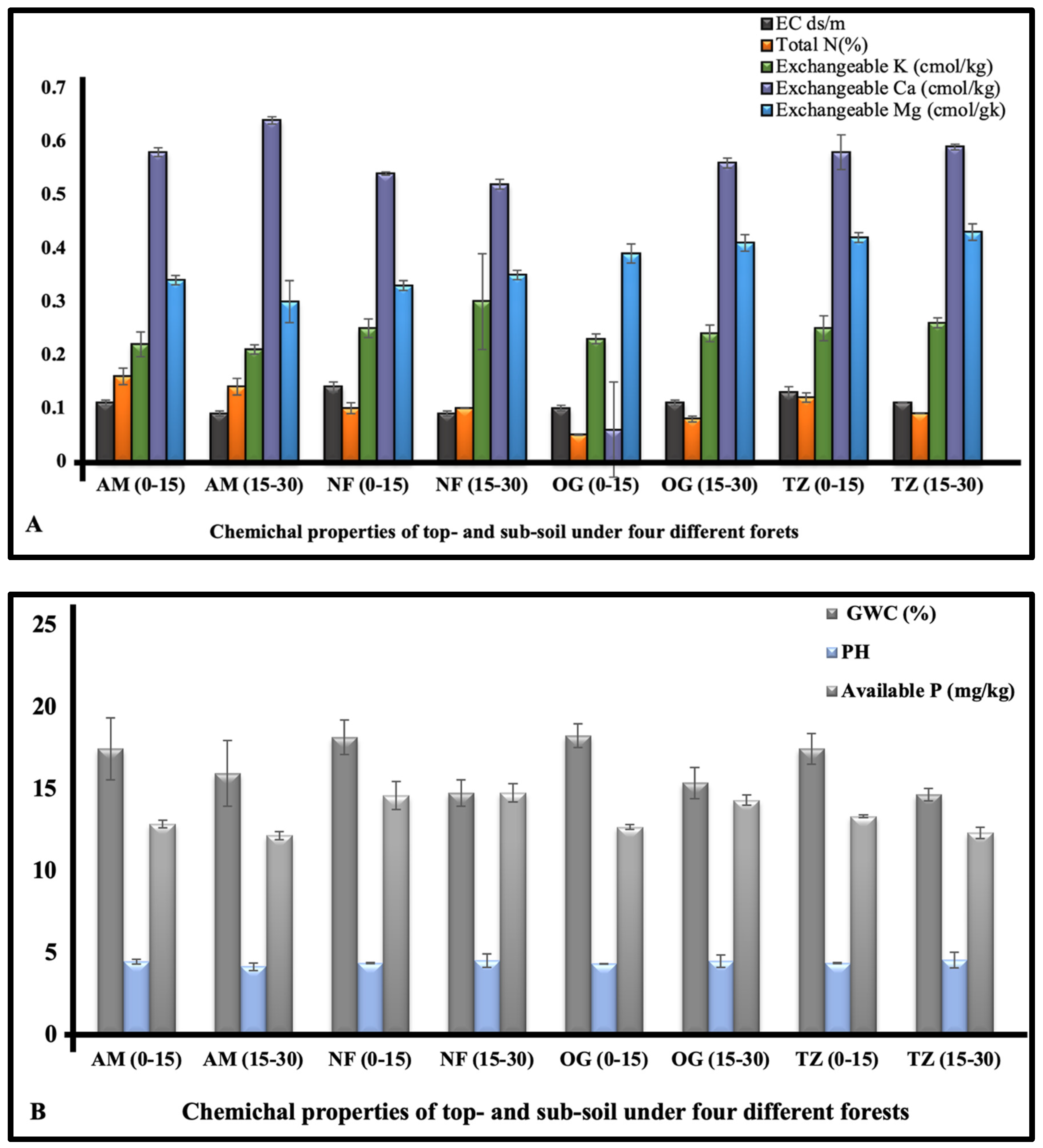
3.1.4. Comparison of the Physicochemical Properties of Top- and Sub-Soil in the Four Regions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamzah, M.; Arifin, A.; Zaidey, A.; Azirim, A.; Zahari, I.; Hazandy, A.; Affendy, H.; Wasli, M.; Shamshuddin, J.; Nik Muhamad, M. Characterizing soil nutrient status and growth performance of planted dipterocap and non-dipterocarp species on degraded forest land in Peninsular Malaysia. Res. J. Appl. Sci. 2009, 9, 4215–4223. [Google Scholar] [CrossRef] [Green Version]
- Abdu, A.; Zaidey, M.; Kadir, A.; Ibrahim, Z.; Hamzah, M.; Hamid, H.; Hassan, A.; Wasli, M.; Yusof, K.; Jusop, S. Properties of soils in the rehabilitated degraded tropical lowland and hill dipterocarp forest in Peninsular Malaysia. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Daisuke, H.; Tanaka, K.; Joseph Jawa, K.; Ikuo, N.; Katsutoshi, S. Rehabilitation of degraded tropical rainforest using dipterocarp trees in Sarawak, Malaysia. Int. J. For. Res. 2013, 2013, 683017. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, C.; Lejeune, P.; Michez, A.; Fayolle, A. How Can Remote Sensing Help Monitor Tropical Moist Forest Degradation?—A Systematic Review. Remote Sens. 2020, 12, 1087. [Google Scholar] [CrossRef] [Green Version]
- Stanturf, J.A. Landscape degradation and restoration. In Soils and Landscape Restoration; Elsevier: Amsterdam, The Netherlands, 2021; pp. 125–159. [Google Scholar]
- Bonfante, A.; Terribile, F.; Bouma, J. Refining physical aspects of soil quality and soil health when exploring the effects of soil degradation and climate change on biomass production: An Italian case study. Soil 2019, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yirdaw, E.; Tigabu, M.; Monge Monge, A.A. Rehabilitation of degraded dryland ecosystems–review. Silva Fenn. 2017, 51, 1673. [Google Scholar] [CrossRef] [Green Version]
- Kenzo, T.; Yoneda, R.; Matsumoto, Y.; Azani, M.A.; Majid, N.M. Leaf photosynthetic and growth responses on four tropical tree species to different light conditions in degraded tropical secondary forest, Peninsular Malaysia. Jpn. Agric. Res. Q. JARQ 2008, 42, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.R.; Moeliono, M.; Mulyana, A.; Yuliani, E.L.; Adriadi, A.; Judda, J.; Sahide, M.A.K. Assessing the new social forestry project in Indonesia: Recognition, livelihood and conservation? Int. For. Rev. 2018, 20, 346–361. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, J. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 106020. [Google Scholar]
- Bongaarts, J. IPBES 2019: Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Wiley Online Library: Bonn, Germany, 2019. [Google Scholar]
- Xiong, Y.; Xia, H.; Cai, X.a.; Fu, S. Impacts of litter and understory removal on soil properties in a subtropical Acacia mangium plantation in China. Plant Soil 2008, 304, 179–188. [Google Scholar] [CrossRef]
- Le Maitre, D.C.; Gaertner, M.; Marchante, E.; Ens, E.J.; Holmes, P.M.; Pauchard, A.; O’Farrell, P.J.; Rogers, A.M.; Blanchard, R.; Blignaut, J. Impacts of invasive Australian acacias: Implications for management and restoration. Divers. Distrib. 2011, 17, 1015–1029. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Perrett, C. Lantana camara L.(Verbenaceae) invasion effects on soil physicochemical properties. Biol. Fertil. Soils 2011, 47, 349–355. [Google Scholar] [CrossRef]
- Jeddi, K.; Chaieb, M. Restoring degraded arid Mediterranean areas with exotic tree species: Influence of an age sequence of Acacia salicina on soil and vegetation dynamics. Flora-Morphol. Distrib. Funct. Ecol. Plants 2012, 207, 693–700. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, Y. Effect of forest ecosystems on soil properties—A review. Agric. Rev. 2004, 25, 16–28. [Google Scholar]
- Islam, S.N.; Mohamad, S.M.B.H.; Azad, A.K. Acacia spp.: Invasive trees along the Brunei Coast, Borneo. In Impacts of Invasive Species on Coastal Environments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 455–476. [Google Scholar]
- Rachid, C.; Balieiro, F.; Peixoto, R.; Pinheiro, Y.; Piccolo, M.; Chaer, G.; Rosado, A. Mixed plantations can promote microbial integration and soil nitrate increases with changes in the N cycling genes. Soil Biol. Biochem. 2013, 66, 146–153. [Google Scholar] [CrossRef]
- Santos, F.M.; Chaer, G.M.; Diniz, A.R.; de Carvalho Balieiro, F. Nutrient cycling over five years of mixed-species plantations of Eucalyptus and Acacia on a sandy tropical soil. For. Ecol. Manag. 2017, 384, 110–121. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Fierro-Brunnenmeister, N.; González-Muñoz, N.; Gallardo, A. Effects of exotic and native tree leaf litter on soil properties of two contrasting sites in the Iberian Peninsula. Plant Soil 2012, 350, 179–191. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pereira, C.S.; Rodríguez-Echeverría, S. Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata Link. Soil Biol. Biochem. 2013, 57, 156–163. [Google Scholar] [CrossRef]
- Lorenzo, P.; Rodríguez-Echeverría, S. Influence of soil microorganisms, allelopathy and soil origin on the establishment of the invasive Acacia dealbata. Plant Ecol. Divers. 2012, 5, 67–73. [Google Scholar] [CrossRef]
- Le Roux, J.J. Molecular Ecology of Plant–-Microbial Interactions During Invasions: Progress and Challenges. In Plant Invasions: The Role of Biotic Interactions; CABI International: Wallingford, UK, 2020; pp. 65–93. [Google Scholar]
- Nsikani, M.M.; Novoa, A.; van Wilgen, B.W.; Keet, J.H.; Gaertner, M. Acacia saligna’s soil legacy effects persist up to 10 years after clearing: Implications for ecological restoration. Austral Ecol. 2017, 42, 880–889. [Google Scholar] [CrossRef] [Green Version]
- Kull, C.A.; Shackleton, C.M.; Cunningham, P.J.; Ducatillon, C.; Dufour-Dror, J.M.; Esler, K.J.; Friday, J.B.; Gouveia, A.C.; Griffin, A.; Marchante, E. Adoption, use and perception of Australian acacias around the world. Divers. Distrib. 2011, 17, 822–836. [Google Scholar] [CrossRef] [Green Version]
- Epron, D.; Nouvellon, Y.; Mareschal, L.; e Moreira, R.M.; Koutika, L.-S.; Geneste, B.; Delgado-Rojas, J.S.; Laclau, J.-P.; Sola, G.; de Moraes Goncalves, J.L. Partitioning of net primary production in Eucalyptus and Acacia stands and in mixed-species plantations: Two case-studies in contrasting tropical environments. For. Ecol. Manag. 2013, 301, 102–111. [Google Scholar] [CrossRef]
- Machado, M.R.; Camara, R.; Sampaio, P.d.T.B.; Pereira, M.G.; Ferraz, J.B.S. Land cover changes affect soil chemical attributes in the Brazilian Amazon. Acta Scientiarum. Agron. 2017, 39, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Tchichelle, S.V.; Epron, D.; Mialoundama, F.; Koutika, L.S.; Harmand, J.-M.; Bouillet, J.-P.; Mareschal, L. Differences in nitrogen cycling and soil mineralisation between a eucalypt plantation and a mixed eucalypt and Acacia mangium plantation on a sandy tropical soil. South. For. J. For. Sci. 2017, 79, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bouillet, J.-P.; Laclau, J.-P.; de Moraes Gonçalves, J.L.; Voigtlaender, M.; Gava, J.L.; Leite, F.P.; Hakamada, R.; Mareschal, L.; Mabiala, A.; Tardy, F. Eucalyptus and Acacia tree growth over entire rotation in single-and mixed-species plantations across five sites in Brazil and Congo. For. Ecol. Manag. 2013, 301, 89–101. [Google Scholar] [CrossRef]
- Paula, R.R.; Bouillet, J.-P.; Trivelin, P.C.O.; Zeller, B.; de Moraes Gonçalves, J.L.; Nouvellon, Y.; Bouvet, J.-M.; Plassard, C.; Laclau, J.-P. Evidence of short-term belowground transfer of nitrogen from Acacia mangium to Eucalyptus grandis trees in a tropical planted forest. Soil Biol. Biochem. 2015, 91, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, S.; Wang, H.; Hu, Z.; Li, Z.; You, Y. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48. [Google Scholar] [CrossRef]
- Pereira, A.P.d.A.; Andrade, P.A.M.d.; Bini, D.; Durrer, A.; Robin, A.; Bouillet, J.P.; Andreote, F.D.; Cardoso, E.J.B.N. Shifts in the bacterial community composition along deep soil profiles in monospecific and mixed stands of Eucalyptus grandis and Acacia mangium. PLoS ONE 2017, 12, e0180371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, N.; Ohta, S.; Hardjono, A. Soil changes induced by Acacia mangium plantation establishment: Comparison with secondary forest and Imperata cylindrica grassland soils in South Sumatra, Indonesia. For. Ecol. Manag. 2008, 254, 362–370. [Google Scholar] [CrossRef]
- Ludwig, F.; De Kroon, H.; Berendse, F.; Prins, H.H. The influence of savanna trees on nutrient, water and light availability and the understorey vegetation. Plant Ecol. 2004, 170, 93–105. [Google Scholar] [CrossRef]
- Dubiez, E.; Freycon, V.; Marien, J.-N.; Peltier, R.; Harmand, J.-M. Long term impact of Acacia auriculiformis woodlots growing in rotation with cassava and maize on the carbon and nutrient contents of savannah sandy soils in the humid tropics (Democratic Republic of Congo). Agrofor. Syst. 2019, 93, 1167–1178. [Google Scholar] [CrossRef]
- Fonge, B.; Tchetcha, D.; Nkembi, L. Diversity, distribution, and abundance of plants in Lewoh-Lebang in the Lebialem Highlands of southwestern Cameroon. Int. J. Biodivers. 2013, 2013, 642579. [Google Scholar] [CrossRef]
- Lee, K.L.; Ong, K.H.; King, P.J.H.; Chubo, J.K.; Su, D.S.A. Stand productivity, carbon content, and soil nutrients in different stand ages of Acacia mangium in Sarawak, Malaysia. Turk. J. Agric. For. 2015, 39, 154–161. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Guisande-Collazo, A.; González, L. Gradualism in Acacia dealbata Link invasion: Impact on soil chemistry and microbial community over a chronological sequence. Soil Biol. Biochem. 2015, 80, 315–323. [Google Scholar] [CrossRef]
- Karam, D.S.; Arifin, A.; Radziah, O.; Shamshuddin, J.; Hazandy, A.-H.; Majid, N.M.; Mohanaselvi, P.; Nor, H. Assessing soil biological properties of natural and planted forests in the Malaysian tropical lowland dipterocarp forest. Am. J. Appl. Sci. 2011, 8, 854–859. [Google Scholar] [CrossRef] [Green Version]
- Karam, D.S.; Abdu, A.; Othman, R.; Jusop, S.; Hanif, A.H.M. Impact of enrichment planting activity on soil physico-chemical properties of degraded forestland. Int. J. Environ. Sci. 2014, 5, 408. [Google Scholar]
- Lelago, A.; Buraka, T. Determination of physico-chemical properties and agricultural potentials of soils in Tembaro District, KembataTembaro Zone, Southern Ethiopia. Eurasian J. Soil Sci. 2019, 8, 118–130. [Google Scholar] [CrossRef]
- Arifin, A.; Karam, D.; Shamshuddin, J.; Majid, N.; Radziah, O.; Hazandy, A.; Zahari, I. Proposing a suitable soil quality index for natural, secondary and rehabilitated tropical forests in Malaysia. Afr. J. Biotechnol. 2012, 11, 3297–3309. [Google Scholar] [CrossRef]
- Selvaraj, P.; Muhammad, A. A Checklist of Plantation Trials in Peninsular Malaysia; Forest Research Institute, Peninsular Malaysia: Kuala Lumpur, Malaysia, 1980; 100p.
- Udarbe, M.; Hepburn, A. Development of Acacia mangium as a plantation species in Sabah. ACIAR Proc. Ed. JW Turnbull 1987, 16, 157–159. [Google Scholar]
- Lee, S. Observations on the successes and failures of acacia plantations in Sabah and Sarawak and the way forward. J. Trop. For. Sci. 2018, 30, 468–475. [Google Scholar] [CrossRef]
- Faridah-Hanum, I.; Philip, L.; Awang Noor, A. Sampling species diversity in a Malaysian rain forest: The case of a logged-over forest. Pak. J. Bot 2008, 40, 1729–1733. [Google Scholar]
- Abdul-Hamid, H.; Abdu, A.; Ismail, M.-K.; Rahim, M.-K.-A.; Senin, A.-L.; Wan-Abd-Rahman, W.-M.-N. Gas exchange of three dipterocarp species in a reciprocal planting. Asian J. Plant Sci. 2011, 10, 408–413. [Google Scholar] [CrossRef]
- Lai, F.; Halis, R.; Bakar, S.; Ramachandran, S.; Puan, C. In Proceedings of the International Forestry Graduate Students’ Conference 2013; Universiti Putra Malaysia: Seri Kembangan, Malaysia, 2013.
- Mohd Zaki, N.A.; Latif, Z.A.; Suratman, M.N. Modelling above-ground live trees biomass and carbon stock estimation of tropical lowland Dipterocarp forest: Integration of field-based and remotely sensed estimates. Int. J. Remote Sens. 2018, 39, 2312–2340. [Google Scholar] [CrossRef]
- Serreze, M.C.; Barry, R.G. Processes and impacts of Arctic amplification: A research synthesis. Glob. Planet. Chang. 2011, 77, 85–96. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Billa, L.; Singh, A. Effect of climate change on seasonal monsoon in Asia and its impact on the variability of monsoon rainfall in Southeast Asia. Geosci. Front. 2015, 6, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Cruz, A.G.; Castro, W.F.; Faria, J.A.; Bogusz, S., Jr.; Granato, D.; Celeguini, R.M.; Lima-Pallone, J.; Godoy, H.T. Glucose oxidase: A potential option to decrease the oxidative stress in stirred probiotic yogurt. LWT 2012, 47, 512–515. [Google Scholar] [CrossRef]
- Ariffin, E.H.; Sedrati, M.; Akhir, M.F.; Norzilah, M.N.M.; Yaacob, R.; Husain, M.L. Short-term observations of beach Morphodynamics during seasonal monsoons: Two examples from Kuala Terengganu coast (Malaysia). J. Coast. Conserv. 2019, 23, 985–994. [Google Scholar] [CrossRef]
- Alaswad, F.; Mohamat-Yusuff, F.; Khairiah, J.; Kusin, F.M.; Ismail, R.; Asha-Ari, Z.H. Effects of depth and land cover on soil properties as indicated by carbon and nitrogen-stable isotope analysis. Pol. J. Environ. Stud. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- Rosli, Z.; Zakaria, M.; Rajpar, M. Edge effects on foraging guilds of upperstory birds in an isolated tropical rainforest of Malaysia. J. Anim. Plant Sci. 2018, 28, 307–320. [Google Scholar]
- Sanei, A.; Zakaria, M.; Yusof, E.; Roslan, M. Estimation of leopard population size in a secondary forest within Malaysia’s capital agglomeration using unsupervised classification of pugmarks. Trop. Ecol. 2011, 52, 209–217. [Google Scholar]
- Shadbolt, A. Small Mammals of the Planted Forest Zone of Sarawak, East Malaysia; an Assessment of Dispersal Ability and Response to Habitat Fragmentation. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2014. [Google Scholar]
- Baioumy, H.; Anuar, M.N.A.B.; Nordin, M.N.M.; Arifin, M.H.; Al-Kahtany, K. Source and origin of Late Paleozoic dropstones from Peninsular Malaysia: First record of Mississippian glaciogenic deposits of Gondwana in Southeast Asia. Geol. J. 2020, 55, 6361–6375. [Google Scholar] [CrossRef]
- Mtui, Y.P. Tropical Rainforest above Ground Biomass and Carbon Stock Estimation for Upper and Lower Canopies Using Terrestrial Laser Scanner and Canopy Height Model from Unmanned Aerial Vehicle (UAV) Imagery in Ayer-Hitam, Malaysia. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2017. [Google Scholar]
- Awang Noor, A.; Norini, H.; Khamurudin, M. Valuing the rain forest: The economic values of selected forest goods and services in Ayer Hitam Forest Reserve, Puchong, Selangor. Trop. Gricultural Sci. 2007, 30, 141. [Google Scholar]
- Zhao, P.; Lu, D.; Wang, G.; Liu, L.; Li, D.; Zhu, J.; Yu, S. Forest aboveground biomass estimation in Zhejiang Province using the integration of Landsat TM and ALOS PALSAR data. Int. J. Appl. Earth Obs. Geoinf. 2016, 53, 1–15. [Google Scholar] [CrossRef]
- Teh, C.B.S.; Talib, J. Soil Physics Analyses; Universiti Putra Malaysia Press: Serdang, Malaysia, 2006; Volume 1. [Google Scholar]
- Rosemary, F.; Indraratne, S.; Weerasooriya, R.; Mishra, U. Exploring the spatial variability of soil properties in an Alfisol soil catena. Catena 2017, 150, 53–61. [Google Scholar] [CrossRef]
- Gupta, R.D.; Kundu, D. Generalized exponential distribution: Existing results and some recent developments. J. Stat. Plan. Inference 2007, 137, 3537–3547. [Google Scholar] [CrossRef] [Green Version]
- Arifin, A.; Parisa, A.; Hazandy, A.; Mahmud, T.; Junejo, N.; Fatemeh, A.; Mohsen, S.; Majid, N. Evaluation of cadmium bioaccumulation and translocation by Hopea odorata grown in a contaminated soil. Afr. J. Biotechnol. 2012, 11, 7472–7482. [Google Scholar]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Zannah, T.I.; Jusop, S.; Ishaq, F.C.; Roslan, I. FTIR and XRD Analyses of Highly Weathered Ultisols and Oxisols in Peninsular Malaysia. Asian J. Agric. Food Sci. 2016, 4, 191–201. [Google Scholar]
- Hammad, H.M.; Nauman, H.M.F.; Abbas, F.; Ahmad, A.; Bakhat, H.F.; Saeed, S.; Shah, G.M.; Ahmad, A.; Cerdà, A. Carbon sequestration potential and soil characteristics of various land use systems in arid region. J. Environ. Manag. 2020, 264, 110254. [Google Scholar] [CrossRef]
- Shahzad, T.; Rashid, M.I.; Maire, V.; Barot, S.; Perveen, N.; Alvarez, G.; Mougin, C.; Fontaine, S. Root penetration in deep soil layers stimulates mineralization of millennia-old organic carbon. Soil Biol. Biochem. 2018, 124, 150–160. [Google Scholar] [CrossRef]
- Rajoo, K.; Abdu, A.; Singh, D.; Abdul-Hamid, H.; Jusop, S.; Zhen, W. Heavy metal uptake and translocation by Dipterocarpus verrucosus from sewage sludge contaminated soil. Am. J. Environ. Sci. 2013, 9, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Panhwar, Q.A.; Naher, U.A.; Shamshuddin, J.; Othman, R.; Ismail, M.R. Applying limestone or basalt in combination with bio-fertilizer to sustain rice production on an acid sulfate soil in Malaysia. Sustainability 2016, 8, 700. [Google Scholar] [CrossRef] [Green Version]
- Minasny, B.; McBratney, A. Limited effect of organic matter on soil available water capacity. Eur. J. Soil Sci. 2018, 69, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Matali, S.; Metali, F. Selected soil physico-chemical properties in the Acacia mangium plantation and the adjacent heath forest at Andulau Forest Reserve. Malays. J. Soil Sci. 2015, 19, 45–48. [Google Scholar]
- Murugan, R.; Beggi, F.; Prabakaran, N.; Maqsood, S.; Joergensen, R.G. Changes in plant community and soil ecological indicators in response to Prosopis juliflora and Acacia mearnsii invasion and removal in two biodiversity hotspots in Southern India. Soil Ecol. Lett. 2020, 2, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Keet, J.-H.; Ellis, A.G.; Hui, C.; Novoa, A.; Le Roux, J.J. Impacts of Invasive Australian Acacias on Soil Bacterial Community Composition, Microbial Enzymatic Activities, and Nutrient Availability in Fynbos Soils. Microb. Ecol. 2021, 2021, 1–18. [Google Scholar]
- Pereira, A.; Ferreira, V. Invasion of Native Riparian Forests by Acacia Species Affects In-Stream Litter Decomposition and Associated Microbial Decomposers. Microb. Ecol. 2021, 81, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.d.A. The Microbiome Related to Carbon and Nitrogen Cycling in Pure and Mixed Eucalyptus grandis and Acacia mangium Plantations. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2018. [Google Scholar]
- Fujii, K.; Hayakawa, C.; Inagaki, Y.; Kosaki, T. Effects of land use change on turnover and storage of soil organic matter in a tropical forest. Plant Soil 2020, 446, 425–439. [Google Scholar] [CrossRef]
- Li, Z.-A.; Peng, S.-L.; Rae, D.J.; Zhou, G.-Y. Litter decomposition and nitrogen mineralization of soils in subtropical plantation forests of southern China, with special attention to comparisons between legumes and non-legumes. Plant Soil 2001, 229, 105–116. [Google Scholar] [CrossRef]
- Augusto, L.; Ranger, J.; Binkley, D.; Rothe, A. Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 2002, 59, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, A.; Darvishi, L. Soil changes induced by hardwood and coniferous tree plantations establishment: Comparison with natural forest soil at Berenjestanak lowland forest in north of Iran. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 432–449. [Google Scholar]
- Moran, J.A.; Barker, M.G.; Moran, A.J.; Becker, P.; Ross, S.M. A Comparison of the Soil Water, Nutrient Status, and Litterfall Characteristics of Tropical Heath and Mixed-Dipterocarp Forest Sites in Brunei 1. Biotropica 2000, 32, 2–13. [Google Scholar] [CrossRef]
- Demir, M.; Makineci, E.; Yilmaz, E. Investigation of timber harvesting impacts on herbaceous cover, forest floor and surface soil properties on skid road in an oak (Quercus petrea L.) stand. Build. Environ. 2007, 42, 1194–1199. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Othman, F.E.; Kahar, R.S. Growth and competition between seedlings of an invasive plantation tree, Acacia mangium, and those of a native Borneo heath-forest species, Melastoma beccarianum. Ecol. Res. 2005, 20, 205–214. [Google Scholar] [CrossRef]
- Morris, T.L.; Esler, K.J.; Barger, N.N.; Jacobs, S.M.; Cramer, M.D. Ecophysiological traits associated with the competitive ability of invasive Australian acacias. Divers. Distrib. 2011, 17, 898–910. [Google Scholar] [CrossRef]
- Krisnawati, H.; Kallio, M.; Kanninen, M. Acacia mangium Willd: Ecology, Silviculture and Productivity; CIFOR: Bogor, Indonesia, 2011. [Google Scholar]
- Vijayanathan, J.; Yahya, A.Z.; Yaacob, A.; Kassim, A.S.; Chik, S.W. Impact of thinning of Acacia mangium plantation on soil chemical properties. Malays. J. Soil Sci. 2011, 15, 75–85. [Google Scholar]
- Musyoka, M.W.; Adamtey, N.; Muriuki, A.W.; Bautze, D.; Karanja, E.N.; Mucheru-Muna, M.; Fiaboe, K.K.; Cadisch, G. Nitrogen leaching losses and balances in conventional and organic farming systems in Kenya. Nutr. Cycl. Agroecosyst. 2019, 114, 237–260. [Google Scholar] [CrossRef]
- Butkovskyi, A.; Jing, Y.; Bergheim, H.; Lazar, D.; Gulyaeva, K.; Odenmarck, S.R.; Norli, H.R.; Nowak, K.M.; Miltner, A.; Kästner, M. Retention and distribution of pesticides in planted filter microcosms designed for treatment of agricultural surface runoff. Sci. Total Environ. 2021, 778, 146114. [Google Scholar] [CrossRef]
- Perumal, M.; Wasli, M.E.; Ying, H.S.; Lat, J.; Sani, H. Association between soil fertility and growth performance of planted Shorea macrophylla (de Vriese) after enrichment planting at rehabilitation sites of Sampadi Forest Reserve, Sarawak, Malaysia. Int. J. For. Res. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [Green Version]
- McLennon, E. Sustainable Agronomic Approaches for Reclaimed Wastewater Utilization: A Focus on Phosphorus Removal, Plant Tissue and Soil Nutrients Retention and Leachate Quality using Biochar and Nitrogen Application Rates under Different Forage System. Ph.D. Thesis, University of Nevada, Reno, Nevada, 2020. [Google Scholar]
- Smith, C.; Gholz, H.; de Assis Oliveira, F. Fine litter chemistry, early-stage decay, and nitrogen dynamics under plantations and primary forest in lowland Amazonia. Soil Biol. Biochem. 1998, 30, 2159–2169. [Google Scholar] [CrossRef]
- Butterfield, J. Changes in decomposition rates and Collembola densities during the forestry cycle in conifer plantations. J. Appl. Ecol. 1999, 36, 92–100. [Google Scholar] [CrossRef]
- Singwane, S.S.; Malinga, P. Impacts of pine and eucalyptus forest plantations on soil organic matter content in Swaziland-Case of Shiselweni Forests. J. Sustain. Dev. Afr. 2012, 14, 137–151. [Google Scholar]
- Wang, Q.; Wang, S. Response of labile soil organic matter to changes in forest vegetation in subtropical regions. Appl. Soil Ecol. 2011, 47, 210–216. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Rodríguez, J.; González, L.; Lorenzo, P. Here to stay. Recent advances and perspectives about Acacia invasion in Mediterranean areas. Ann. For. Sci. 2017, 74, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Turner, I.; Lucas, P.; Becker, P.; Wong, S.C.; Yong, J.; Choong, M.; Tyree, M. Tree leaf form in Brunei: A heath forest and a mixed dipterocarp forest compared 1. Biotropica 2000, 32, 53–61. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Fertility management of tropical acid soils for sustainable crop production. In Handbook of Soil Acidity; CRC Press: Boca Raton, FL, USA, 2003; pp. 359–385. [Google Scholar]
- Fisher, R.; Binkley, D. Ecology and Management of Forest Soils; John Willey & Sons. Inc.: New York, NY, USA, 2000. [Google Scholar]
- Marchante, E.; Kjøller, A.; Struwe, S.; Freitas, H. Short-and long-term impacts of Acacia longifolia invasion on the belowground processes of a Mediterranean coastal dune ecosystem. Appl. Soil Ecol. 2008, 40, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Rascher, K.G.; Große-Stoltenberg, A.; Máguas, C.; Meira-Neto, J.A.A.; Werner, C. Acacialongifolia invasion impacts vegetation structure and regeneration dynamics in open dunes and pine forests. Biol. Invasions 2011, 13, 1099–1113. [Google Scholar] [CrossRef]
- Katagiri, S.; Yamakura, T.; Lee, S.H. Properties of soils in kerangas forest on sandstone at Bako National Park, Sarawak, East Malaysia. Jpn. J. Southeast Asian Stud. 1991, 29, 35–48. [Google Scholar]
- Lorenzo, P.; Rodríguez-Echeverría, S. Soil changes mediated by invasive Australian acacias. Ecosistemas 2015, 24, 59–66. [Google Scholar] [CrossRef]
- Werner, C.; Zumkier, U.; Beyschlag, W.; Máguas, C. High competitiveness of a resource demanding invasive acacia under low resource supply. Plant Ecol. 2010, 206, 83–96. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Aguiar, A., Jr.; Barbosa, R.I.; Barbosa, J.B.; Mourão, M., Jr. Invasion of Acacia mangium in Amazonian savannas following planting for forestry. Plant Ecol. Divers. 2014, 7, 359–369. [Google Scholar] [CrossRef]
- Richardson, D.M.; Le Roux, J.J.; Wilson, J.R. Australian acacias as invasive species: Lessons to be learnt from regions with long planting histories. South. For. J. For. Sci. 2015, 77, 31–39. [Google Scholar] [CrossRef]
- Duponnois, R.; Ramanankierana, H.; Hafidi, M.; Baohanta, R.; Baudoin, E.; Thioulouse, J.; Sanguin, H.; Ba, A.; Galiana, A.; Bally, R. Native plant resources to optimize the performances of forest rehabilitation in Mediterranean and tropical environment: Some examples of nursing plant species that improve the soil mycorrhizal potential. Comptes Rendus Biol. 2013, 336, 265–272. [Google Scholar] [CrossRef] [PubMed]

| Study Site Name | Region Code | Plots | Study Site Altitude |
|---|---|---|---|
| The Acacia mangium region | AM | 7–12 | 3°00′18.2″ N, 101°38′51.2″ E, elevation 100 m |
| The native forest region | NF | 19–24 | 3°00′22″ N, 101°38′49.1″ E, elevation 100 m |
| The open ground region | OG | 1–6 | 3°00′19″ N, 101°38′54″ E, elevation 100 m |
| The transition region | TZ | 13–18 | 3°00′20″ N, 101°38′ 50.3″ E, elevation 100 m |
| Regions | Clay (%) | Silt (%) | Sand (%) | Textural Class | Depth of Organic Matter (cm) |
|---|---|---|---|---|---|
| AM | 50.88 a ± 1.4 | 33.16 a ± 1.5 | 15.98 a ± 1.2 | Clay Loam | 3.47 a |
| NF | 53.36 a ± 0.8 | 32.30 a ± 1.5 | 14.34 b ± 1.2 | Clay Loam | 7.03 a |
| OG | 50.62 a ± 2.4 | 32.1 a ± 1.5 | 17.28 a ± 1.2 | Clay Loam | 1.5 c |
| TZ | 52.53 a ± 1.1 | 33.1 a ± 1.5 | 17.53 a ± 1.2 | Clay Loam | 5.11 b |
| A A. mangium Region (AM) | B Native Forest Region (NF) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S.O.V | df | GWC | pH | EC | OM | N | P | K | Ca | Mg | GWC | pH | EC | OM | N | P | K | Ca |
| Regions | 1 | 3.5 ns | 0.1 ns | 0.0004 * | 0.5 ** | 0.0006 ns | 0.7 ** | 0.0001 ns | 0.005 ns | 0.002 ns | 17.3 ** | 0.03 ns | 0.003 ** | 0.02 ns | 0.00001 ns | 0.04 ns | 0.003 ns | 0.0006 ns |
| Replicate | 2 | 7.9 ns | 0.008 ns | 0.00001 ns | 0.0006 ns | 0.0001 ns | 0.12 ns | 0.0006 ns | 0.001 ns | 0.005 ns | 2.1 ns | 0.11 ns | 0.00006 | 0.002 ns | 0.00002 ns | 0.8 ns | 0.006 ns | 0.00015 ns |
| Error | 2 | 1.5 | 0.01 | 0.00001 | 0.0005 | 0.00045 | 0.01 | 0.0002 | 0.0003 | 0.004 | 0.06 | 0.08 | 0.00006 | 0.001 | 0.0000001 | 0.45 | 0.004 | 0.0006 |
| Total | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| C.V. | - | 7.4 | 2.9 | 4.01 | 0.56 | 14.1 | 0.97 | 6.57 | 3.06 | 21.07 | 1.5 | 6.5 | 6.9 | 1.06 | 0.8979 | 4.58 | 23.56 | 4.8 |
| C Open Ground Region (OG) | D Transition Region (TZ) | |||||||||||||||||
| S.O.V | df | GWC | pH | EC | OM | N | P | K | Ca | Mg | GWC | pH | EC | OM | N | P | K | Ca |
| Regions | 1 | 12.5 ns | 0.03 ns | 0.0001 ** | 0.002 ns | 0.00006 ns | 4.08 ** | 0.0001 ns | 0.002 ns | 0.0006 ns | 11.7 ns | 0.043 ns | 0.001 * | 0.01 ns | 0.001 * | 1.53 ** | 0.000001 ns | 0.0001 ns |
| Replicate | 2 | 0.03 ns | 0.006 ns | 0.00006 ns | 0.0002 ns | 0.00001 ns | 0.03 ns | 0.0002 ns | 0.004 ns | 0.00005 ns | 0.48 ns | 0.006 ns | 0.00006 ns | 0.001 ns | 0.00005 ns | 0.001 ns | 0.0006 ns | 0.0009 ns |
| Error | 2 | 1.7 | 0.002 | 0.000001 | 0.0008 | 0.00001 | 0.006 | 0.0002 | 0.006 | 0.0006 | 0.66 | 0.003 | 0.00006 | 0.003 | 0.00005 | 0.006 | 0.0002 | 0.0006 |
| Total | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| C.V. | - | 7.8 | 1.1 | 0.754 | 1.3 | 4.7 | 0.58 | 6.01 | 13.4 | 6.3 | 5.06 | 1.28 | 6.9 | 1.34 | 6.73 | 0.63 | 5.43 | 4.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad-Sheip, Y.; Abdul-Hamid, H.; Abiri, R.; Saleh, M.-N.; Mohamed, J.; Jalil, A.-M.; Naji, H.R. Effect of Acacia mangium Canopy on Physicochemical Characteristics and Nutrient Concentrations of the Soil at Ayer Hitam Forest Reserve, Malaysia. Forests 2021, 12, 1259. https://doi.org/10.3390/f12091259
Hamad-Sheip Y, Abdul-Hamid H, Abiri R, Saleh M-N, Mohamed J, Jalil A-M, Naji HR. Effect of Acacia mangium Canopy on Physicochemical Characteristics and Nutrient Concentrations of the Soil at Ayer Hitam Forest Reserve, Malaysia. Forests. 2021; 12(9):1259. https://doi.org/10.3390/f12091259
Chicago/Turabian StyleHamad-Sheip, Younes, Hazandy Abdul-Hamid, Rambod Abiri, Mohd-Nazre Saleh, Johar Mohamed, Abd-Majid Jalil, and Hamid R. Naji. 2021. "Effect of Acacia mangium Canopy on Physicochemical Characteristics and Nutrient Concentrations of the Soil at Ayer Hitam Forest Reserve, Malaysia" Forests 12, no. 9: 1259. https://doi.org/10.3390/f12091259
APA StyleHamad-Sheip, Y., Abdul-Hamid, H., Abiri, R., Saleh, M.-N., Mohamed, J., Jalil, A.-M., & Naji, H. R. (2021). Effect of Acacia mangium Canopy on Physicochemical Characteristics and Nutrient Concentrations of the Soil at Ayer Hitam Forest Reserve, Malaysia. Forests, 12(9), 1259. https://doi.org/10.3390/f12091259








