Infestation of Early- and Late-Flushing Trees by Spring Caterpillars: An Associational Effect of Neighbouring Trees
Abstract
:1. Introduction
2. Materials and Methods
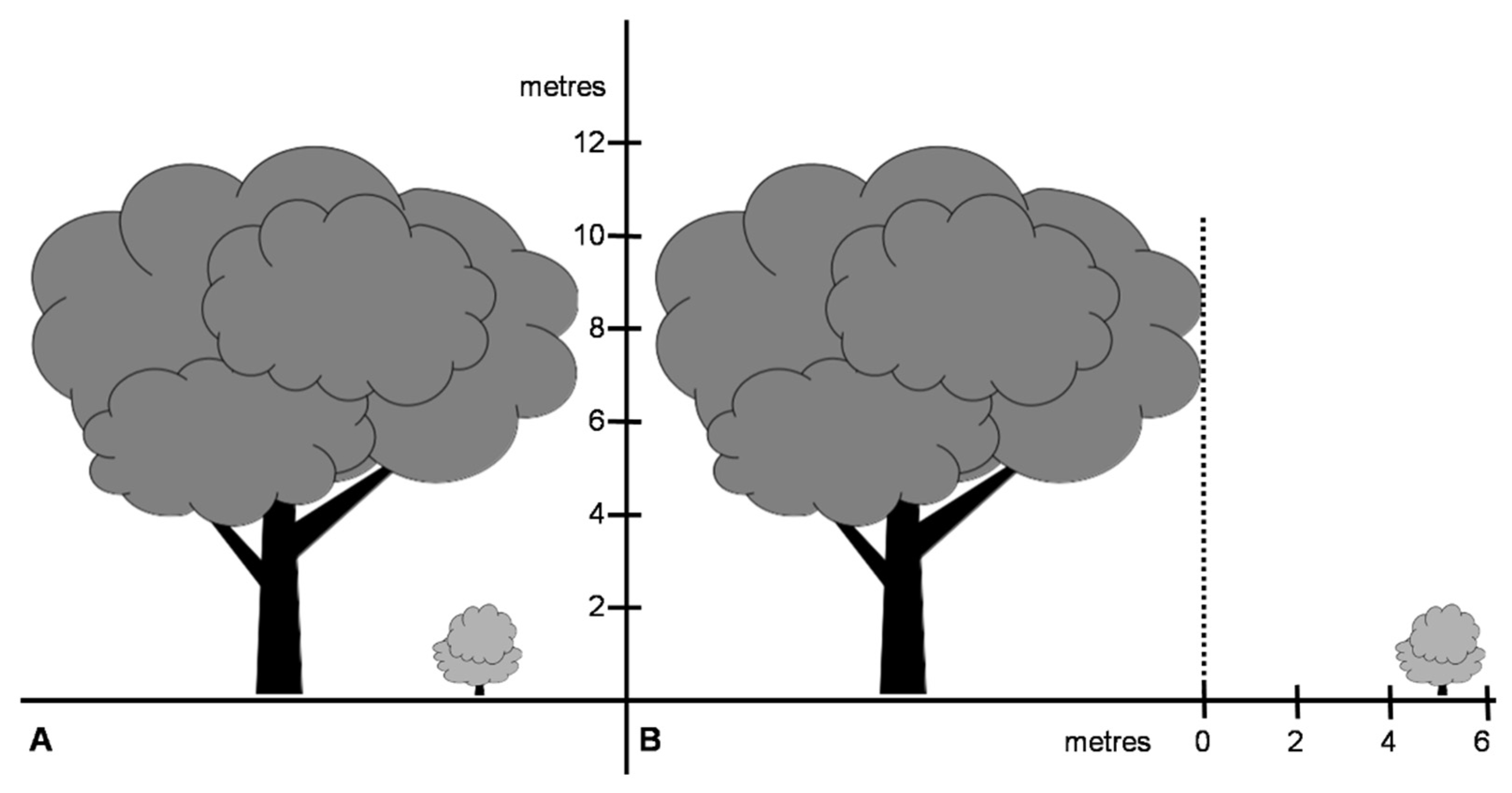
2.1. Studied Area
2.2. Data Collecting
2.3. Data Analyses
3. Results
3.1. Caterpillars on Mature Trees
3.2. Caterpillars on Young Trees
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elton, C.S. The forest canopy: Herbivores. In The Pattern of Animal Communities, 1st ed.; Springer: Dordrecht, The Netherlands, 1966; pp. 190–209. [Google Scholar] [CrossRef]
- Patočka, J. Die Raupen und Puppen der Eichenschmetterlinge Mitteleuropas, 1st ed.; Paul Parey: Hamburg/Berlin, Germany, 1980; p. 188. [Google Scholar]
- Csóka, G. Oak Defoliating Insects in Hungary. In Proceedings of the Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects, Banská Štiavnica, Slovakia, 18–23 August 1996; McManus, M.L., Liebhold, A.M., Eds.; USDA Forest Service General Technical Report NE-247. USDA: Madison, WI, USA, 1998; pp. 334–335. [Google Scholar]
- Topp, W.; Kulfan, J.; Mergel, S.; Zach, P. Massenvermehrung von phyllophagen Schmetterlingen in Laubwäldern des Rheinlands. Anz. Schädlingskd. Pfl. Umwelt. 1998, 71, 88–93. [Google Scholar] [CrossRef]
- Patočka, J.; Krištín, A.; Kulfan, J.; Zach, P. Die Eichenschädlinge und Ihre Feinde, 1st ed.; Institut für Waldökologie der Slowakischen Akademie der Wissenschaften: Zvolen, Slovakia, 1999; p. 396. [Google Scholar]
- Ruohomäki, K.; Tanhuanpää, M.; Ayres, M.P.; Kaitaniemi, P.; Tammaru, T.; Haukioja, E. Causes of cyclicity of Epirrita autumnata (Lepidoptera, Geometridae): Grandiose theory and tedious practice. Popul. Ecol. 2000, 42, 211–223. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiński, P. Late leaf development in pedunculate oak (Quercus robur): An antiherbivore defence? Scand. J. For. Res. 2008, 23, 386–394. [Google Scholar] [CrossRef]
- Glavendekić, M.M.; Medarević, M.J. Insect defoliators and their influence on oak forests in the Djerdap National Park, Serbia. Arch. Biol. Sci. 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Kulfan, M. Structure of lepidopterocenoses on oaks Quercus dalechampii and Q. cerris in Central Europe and estimation of the most important species. Munis Entmol. Zool. 2012, 7, 732–741. [Google Scholar]
- Kulfan, J.; Sarvašová, L.; Parák, M.; Dzurenko, M.; Zach, P. Can late flushing trees avoid attack by moth larvae in temperate forests? Plant Protect. Sci. 2018, 54, 272–283. [Google Scholar] [CrossRef] [Green Version]
- Zúbrik, M.; Pilarska, D.; Kulfan, J.; Barta, M.; Hajek, A.E.; Bittner, T.D.; Hirka, A. Phytophagous larvae occurring in Central and Southeastern European oak forests as a potential host of Entomophaga maimaiga (Entomophthorales: Entomophthoraceae)—A field study. J. Invertebr. Pathol. 2018, 155, 52–54. [Google Scholar] [CrossRef]
- Sarvašová, L.; Kulfan, J.; Saniga, M.; Zúbrik, M.; Zach, P. Winter geometrid moths in oak forests: Is monitoring a single species reliable to predict defoliation risk? Forests 2020, 11, 288. [Google Scholar] [CrossRef] [Green Version]
- Du Merle, P. Phenological resistance of oaks to the green leafroller, Tortrix viridana (Lepidoptera: Tortricidae). In Mechanisms of Woody Plant Defences against Insects, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1988; pp. 215–226. [Google Scholar] [CrossRef]
- Hunter, M.D. Differential susceptibility to variable plant phenology and its role in competition between two insect herbivores on oak. Ecol. Entomol. 1990, 15, 401–408. [Google Scholar] [CrossRef]
- Hunter, M.D. A variable insect–plant interaction: The relationship between tree budburst phenology and population levels of insect herbivores among trees. Ecol. Entomol. 1992, 17, 91–95. [Google Scholar] [CrossRef]
- Van Dongen, S.; Backeljau, T.; Matthysen, E.; Dhondt, A.A. Synchronization of hatching date with budburst of individual host trees (Quercus robur) in the winter moth (Operophtera brumata) and its fitness consequences. J. Anim. Ecol. 1997, 66, 113–121. [Google Scholar] [CrossRef]
- Hunter, A.F.; Elkinton, J.S. Effects of synchrony with host plant on populations of a spring feeding Lepidopteran. Ecology 2000, 81, 1248–1261. [Google Scholar] [CrossRef]
- Tikkanen, O.-P.; Lyytikäinen-Saarenmaa, P. Adaptation of a generalist moth, Operophtera brumata, to variable budburst phenology of host plants. Entomol. Exp. Appl. 2002, 103, 123–133. [Google Scholar] [CrossRef]
- Tikkanen, O.-P.; Julkunen-Tiitto, R. Phenological variation as protection against defoliating insects: The case of Quercus robur and Operophtera brumata. Oecologia 2003, 136, 244–251. [Google Scholar] [CrossRef]
- Van Asch, M.; Visser, M.E. Phenology of forest caterpillars and their host trees: The importance of synchrony. Annu. Rev. Entomol. 2007, 52, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, A.; Pureswaran, D.; Bauce, É.; Despland, E. How does synchrony with host plant affect the performance of an outbreaking insect defoliator? Oecologia 2017, 184, 847–857. [Google Scholar] [CrossRef]
- Keena, M.A.; Shi, J. Effects of temperature on first instar Lymantria (Lepidoptera: Erebidae) survival and development with and without food. Environ. Entomol. 2019, 48, 655–666. [Google Scholar] [CrossRef]
- Feeny, P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Schultz, J.C.; Nothnagle, P.J.; Baldwin, I.T. Seasonal and individual variation in leaf quality of two northern hardwoods tree species. Am. J. Bot. 1982, 69, 753–759. [Google Scholar] [CrossRef]
- Raupp, M.J.; Werren, J.H.; Sadof, C.S. Effects of short-term phenological changes in leaf suitability on the survivorship, growth and development of gypsy moth (Lepidoptera: Lymantriidae) larvae. Environ. Entomol. 1988, 17, 316–319. [Google Scholar] [CrossRef]
- Hunter, A.F.; Lechowicz, M.J. Foliage quality changes during canopy development of some northern hardwood trees. Oecologia 1992, 89, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.C.; Despland, E. Effects of synchronization with host plant phenology occur in the larval development of a spring folivore. Can. J. Zool. 2006, 84, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Martemyanov, V.V.; Pavlushin, S.V.; Dubovskiy, I.M.; Yushkova, Y.V.; Morosov, S.V.; Chernyak, E.I.; Efimov, V.M.; Ruuhola, T.; Glupov, V.V. Asynchrony between host plant and insects-defoliator within a tritrophic system: The role of herbivore innate immunity. PLoS ONE 2015, 10, e0130988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbehenn, R.V.; Kapila, M.; Kileen, S.; Nusbaum, C.P. Acquiring nutrients from tree leaves: Effects of leaf maturity and development type on a generalist caterpillar. Oecologia 2017, 184, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E. The life history of the Bruce spanworm, Opheroptera bruceata (Hulst), (Lepidoptera: Geometridae). Can. Entomol. 1962, 94, 1103–1107. [Google Scholar] [CrossRef]
- Mrkva, R. Bionomie píďalky podzimní (Operophtera brumata L.)—Motýl a vajíčko. Acta Univ. Agric. (Brno) Ser. C 1968, 37, 223–246. (In Czech) [Google Scholar]
- Edland, T. Wind dispersal of the winter moth larvae Operophtera brumata L. (Lep., Geometridae) and its relevance to control measures. Nor. Entomol. Tidsskr. 1971, 18, 103–107. [Google Scholar]
- Leonard, D.E. Air-borne dispersal of larvae of the gypsy moth and its influence on concepts of control. J. Econ. Entomol. 1971, 64, 638–641. [Google Scholar] [CrossRef]
- West, C. The Effects on Phytophagous Insects of Variation in Defence Mechanisms within a Plant. Ph.D. Thesis, University of Oxford, Oxford, UK, 1985. [Google Scholar]
- Barbosa, P.; Krischik, V.; Lance, D. Life-history traits of forest-inhabiting flightless Lepidoptera. Am. Midl. Nat. 1989, 122, 262–274. [Google Scholar] [CrossRef]
- Tammaru, T.; Kaitaniemi, P.; Ruohomäki, K. Oviposition choices of Epirrita autumnata (Lepidoptera: Geometridae) in relation to its eruptive population dynamics. Oikos 1995, 74, 296–304. [Google Scholar] [CrossRef]
- Diss, A.L.; Kunkel, J.G.; Montgomery, M.E.; Leonard, D.E. Effects of maternal nutrition and egg provisioning on parameters of larval hatch, survival and dispersal in the gypsy moth, Lymantria dispar L. Oecologia 1996, 106, 470–477. [Google Scholar] [CrossRef]
- Elkinton, J.; Boettner, G.; Liebhold, A.; Gwiazdowski, R. Biology, Spread, and Biological Control of Winter Moth in the Eastern United States; FHTET-2014-07; U.S. Department of Agriculture, Forest Service, Forest Health Technology Team: Morgantown, WV, USA, 2015; p. 22. Available online: https://www.fs.fed.us/nrs/pubs/jrnl/2015/fhtet-2014-07_elkinton_2015_001.pdf (accessed on 17 March 2019).
- Murakami, M.; Wada, N. Difference in leaf quality between canopy trees and seedlings affects migration and survival of spring-feeding moth larvae. Can. J. For. Res. 1997, 27, 1351–1356. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Stockel, J.; Roehrich, R.; Rodríguez-Molina, M.C. The relation between dispersal and survival of Lobesia botrana larvae and their density in vine inflorescences. Entomol. Exp. Appl. 1997, 84, 109–114. [Google Scholar] [CrossRef]
- Sugiura, S.; Yamazaki, K. The role of silk threads as lifelines for caterpillars: Pattern and significance of lifeline-climbing behaviour. Ecol. Entomol. 2006, 31, 52–57. [Google Scholar] [CrossRef]
- Jones, R.E. Search Behaviour: A study of three caterpillar species. Behaviour 1977, 60, 237–259. [Google Scholar] [CrossRef]
- Moore, R.; Warrington, S.; Whittaker, J.B. Herbivory by insects on oak trees in pure stands compared with paired mixtures. J. Appl. Ecol. 1991, 28, 290–304. [Google Scholar] [CrossRef]
- Alalouni, U.; Brandl, R.; Auge, H.; Schädler, M. Does insect herbivory on oak depend on the diversity of tree stands? Basic Appl. Ecol. 2014, 15, 685–692. [Google Scholar] [CrossRef]
- Bognounou, F.; De Grandpré, L.; Pureswaran, D.S.; Kneeshaw, D. Temporal variation in plant neighborhood effects on the defoliation of primary and secondary hosts by an insect pest. Ecosphere 2017, 8, e01759. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Giffard, B.; Péré, C.; Jactel, H. Plant apparency, an overlooked driver of associational resistance to insect herbivory. J. Ecol. 2013, 101, 418–429. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Giffard, B.; Valdés-Correcher, E.; Hampe, A. Tree diversity effects on leaf insect damage on pedunculate oak: The role of landscape context and forest stratum. For. Ecol. Manag. 2019, 433, 287–294. [Google Scholar] [CrossRef]
- Guyot, V.; Jactel, H.; Imbaud, B.; Burnel, L.; Castagneyrol, B.; Heinz, W.; Deconchat, M.; Vialatte, A. Tree diversity drives associational resistance to herbivory at both forest edge and interior. Ecol. Evol. 2019, 9, 9040–9051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damestoy, T.; Jactel, H.; Belouard, T.; Schmuck, H.; Plomion, C.; Castagneyrol, B. Tree species identity and forest composition affect the number of oak processionary moth captured in pheromone traps and the intensity of larval defoliation. Agric. For. Entomol. 2020, 22, 169–177. [Google Scholar] [CrossRef]
- Barbosa, P.; Hines, J.; Kaplan, I.; Martinson, H.; Szczepaniec, A.; Szendrei, Z. Associational resistance and associational susceptibility: Having right or wrong neighbors. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Jactel, H.; Brockerhoff, E.G. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef]
- Guyot, V.; Castagneyrol, B.; Vialatte, A.; Deconchat, M.; Jactel, H. Tree diversity reduces pest damage in mature forests across Europe. Biol. Lett. 2016, 12, 20151037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jactel, H.; Moreira, X.; Castagneyrol, B. Tree diversity and forest resistance to insect pests: Patterns, mechanisms and prospects. Annu. Rev. Entomol. 2020, 66, 277–296. [Google Scholar] [CrossRef]
- Vehviläinen, H.; Koricheva, J.; Ruohomäki, K. Tree species diversity influences herbivore abundance and damage: Meta-analysis of long-term forest experiments. Oecologia 2007, 152, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, N.N.; Vanhellemont, M.; Baeten, L.; Dillen, M.; Verheyen, K. The effects of local neighbourhood diversity on pest and disease damage of trees in a young experimental forest. For. Ecol. Manag. 2014, 334, 1–9. [Google Scholar] [CrossRef]
- Moreira, X.; Glauser, G.; Abdala-Roberts, L. Interactive effects of plant neighbourhood and ontogeny on insect herbivory and plant defensive traits. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Van Schrojenstein Lantman, I.M.; Hertzog, L.R.; Vandegehuchte, M.L.; Martel, A.; Verheyen, K.; Lens, L.; Bonte, D. Leaf herbivory is more impacted by forest composition than by tree diversity or edge effects. Basic Appl. Ecol. 2018, 29, 79–88. [Google Scholar] [CrossRef]
- Underwood, N.; Inouye, B.D.; Hambäck, P.A. A conceptual framework for associational effects: When do neighbors matter and how would we know? Q. Rev. Biol. 2014, 89, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Moreira, X.; Abdala-Roberts, L.; Rasmann, S.; Castagneyrol, B.; Mooney, K.A. Plant diversity effects on insect herbivores and their natural enemies: Current thinking, recent findings, and future directions. Curr. Opin. Insect. Sci. 2016, 14, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nealis, V.G.; Régnière, J. Insect host relationships influencing disturbance by the spruce budworm in a boreal mixedwood forest. Can. J. For. Res. 2004, 34, 1870–1882. [Google Scholar] [CrossRef] [Green Version]
- Schafellner, C.; Kramer, W.; Schopf, A. Three trophic level interaction: The influence of host plants on the performance of gypsy moth (Lymantria dispar) and its parasitoid, Glyptapanteles liparidis (Hymenoptera, Braconidae). IOBC WPRS Bull. 2005, 28, 193–200. [Google Scholar]
- Korpeľ, Š. Die Urwälder der Westkarpaten, 1st ed.; Fischer Verlag: Stuttgart, Germany, 1995; p. 310. [Google Scholar]
- Wesołowski, T.; Rowiński, P. Tree defoliation by winter moth Operophtera brumata L. during an outbreak affected by structure of forest landscape. For. Ecol. Manag. 2006, 221, 299–305. [Google Scholar] [CrossRef]
- Wesołowski, T.; Rowiński, P. Timing of bud burst and tree-leaf development in a multispecies temperate forest. For. Ecol. Manag. 2006, 237, 387–393. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Dufrêne, E.; Pontailler, J.Y.; Louvet, J.M.; Kremer, A.; Michalet, R. Leaf phenology sensitivity to temperature in European trees: Do within-species populations exhibit similar responses? Agric. For. Meteorol. 2009, 149, 735–744. [Google Scholar] [CrossRef]
- Pividori, M.; Giannetti, F.; Barbati, A.; Chirici, G. European forest types: Tree species matrix. In European Atlas of Forest Tree Species, 1st ed.; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 34–35. [Google Scholar]
- Cole, E.F.; Sheldon, B.C. The shifting phenological landscape: Within and between-species variation in leaf emergence in a mixed-deciduous woodland. Ecol. Evol. 2017, 7, 1135–1147. [Google Scholar] [CrossRef] [Green Version]
- Van Dongen, S.; Backeljau, T.; Matthysen, E.; Dhondt, A.A. Effects of forest fragmentation on the population structure of the winter moth Operophtera brumata L. (Lepidoptera. Geometridae). Acta Oecol. 1994, 15, 193–206. [Google Scholar]
- Tscharntke, T.; Brandl, R. Plant-insect interactions in fragmented landscapes. Annu. Rev. Entomol. 2004, 49, 405–430. [Google Scholar] [CrossRef]
- Rossetti, M.R.; Tscharntke, T.; Aguilar, R.; Batáry, P. Responses of insect herbivores and herbivory to habitat fragmentation: A hierarchical meta-analysis. Ecol. Lett. 2017, 20, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.F.; Lourenço, R.; Lopes, C.; Oliveira, A.; Ribeiro-Silva, J.; Rabaça, J.E.; Pinto-Correia, T.; Figueiredo, D.; Mira, A.; Marques, J.T. The influence of management and environmental factors on insect attack on cork oak canopy. For. Ecol. Manag. 2019, 453, 117582. [Google Scholar] [CrossRef]
- Van Dongen, S.; Scott, T. Effects of forest fragmentation and local habitat structure on densities of winter moth (Operophtera brumata L.). Belg. J. Zool. 2002, 132, 165–170. [Google Scholar]
- Summerville, K.S.; Crist, T.O. Determinants of lepidopteran community composition and species diversity in eastern deciduous forests: Roles of season, eco-region and patch size. Oikos 2003, 100, 134–148. [Google Scholar] [CrossRef]
- Summerville, K.S.; Crist, T.O. Contrasting effects of habitat quantity and quality on moth communities in fragmented landscapes. Ecography 2004, 27, 3–12. [Google Scholar] [CrossRef]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef]
- Schmidt, N.B.C.; Roland, J. Moth diversity in a fragmented habitat: Importance of functional groups and landscape scale in the boreal forest. Ann. Entomol. Soc. Am. 2006, 99, 1110–1120. [Google Scholar] [CrossRef]
- Valdés-Correcher, E.; Van Halder, I.; Barbaro, L.; Castagneyrol, B.; Hampe, A. Insect herbivory and avian insectivory in novel native oak forests: Divergent effects of stand size and connectivity. For. Ecol. Manag. 2019, 445, 146–153. [Google Scholar] [CrossRef]
- Viswanathan, A.; Ghazoul, J.; Lewis, O.; Honwad, G.; Bagchi, R. Effects of forest fragment area on interactions between plants and their natural enemies: Consequences for plant diversity at multiple spatial scales. Front. For. Glob. Chang. 2019, 2, 88. [Google Scholar] [CrossRef]
- Csóka, G.; Szabóky, C. Checklist of herbivorous insects of native and exotic oaks in Hungary I (Lepidoptera). Acta Silv. Lign. Hung. 2005, 1, 59–72. [Google Scholar]
- Patočka, J.; Kulfan, J. Lepidoptera of Slovakia: Bionomics and Ecology/Motýle Slovenska: Bionómia a Ekológia, 1st ed.; VEDA: Bratislava, Slovakia, 2009; p. 312. [Google Scholar]
- Goliašová, K.; Michalková, E. Flóra Slovenska V/3 [The Flora of Slovakia V/3], 1st ed.; VEDA: Bratislava, Slovakia, 2006; p. 344. [Google Scholar]
- Patočka, J. Húsenice na Duboch v ČSR [Caterpillars on oaks in Czechoslovakia], 1st ed.; Štátne Pôdohospodárske Nakladateľstvo: Bratislava, Czechoslovakia, 1954; p. 264.
- Bergmeier, E.; Petermann, J.; Schröder, E. Geobotanical survey of wood-pasture habitats in Europe: Diversity, threats and conservation. Biodivers. Conserv. 2010, 19, 2995–3014. [Google Scholar] [CrossRef] [Green Version]
- Ádám, R.; Ódor, P.; Bölöni, J. The effects of stand characteristics on the understory vegetation in Quercus petraea and Q. cerris dominated forests. Community Ecol. 2013, 14, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Roloff, A.; Korn, S.; Gillner, S. The Climate-Species-Matrix to select tree species for urban habitats considering climate change. Urban For. Urban Green. 2009, 8, 295–308. [Google Scholar] [CrossRef]
- Kätzel, R.; Becker, F.; Schröder, J.; Glatthorn, J.; Höltken, A.; Löffler, S. Flaum-und Zerr-Eiche in Brandenburg—Alternative Baumarten im Klimawandel? [Downy oak and Turkey oak in Brandenburg—Alternative Tree Species in Climate Change?]. Wissenstransfer Die Prax. 2012, 49, 23–36. Available online: http://webdoc.sub.gwdg.de/ebook/serien/yo/EfS/49.pdf#page=24 (accessed on 28 April 2019). (In German).
- De Rigo, D.; Enescu, C.M.; Houston Durrant, T.; Caudullo, G. Quercus cerris in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species, 1st ed.; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 148–149. Available online: https://ies-ows.jrc.ec.europa.eu/efdac/download/Atlas/pdf/Quercus_cerris.pdf (accessed on 14 April 2019).
- Reif, A.; Xystrakis, F.; Gärtner, S.; Sayer, U. Floristic change at the drought limit of European beech (Fagus sylvatica L.) to Downy oak (Quercus pubescens) forest in the temperate climate of Central Europe. Not. Bot. Horti Agrobot. Cluj Napoca 2017, 45, 646–654. [Google Scholar] [CrossRef] [Green Version]
- Früchtenicht, E.; Neumann, L.; Klein, N.; Bonal, D.; Brüggemann, W. Response of Quercus robur and two potential climate change winners—Quercus pubescens and Quercus ilex—To two years summer drought in a semi-controlled competition study: I—Tree water status. Environ. Exp. Bot. 2018, 152, 107–117. [Google Scholar] [CrossRef]
- Mevy, J.-P.; Loriod, B.; Liu, X.; Corre, E.; Torres, M.; Büttner, M.; Haguenauer, A.; Reiter, I.M.; Fernandez, C.; Gauquelin, T. Response of Downy oak (Quercus pubescens Willd.) to climate change: Transcriptome assembly, differential gene analysis and targeted metabolomics. Plants 2020, 9, 1149. [Google Scholar] [CrossRef]
- Hlásny, T.; Holuša, J.; Štěpánek, P.; Turčáni, M.; Polčák, N. Expected impacts of climate change on forests: Czech Republic as a case study. J. For. Sci. 2011, 57, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Rubtsov, V.V.; Utkina, I.A. Long-term dynamics of Operophtera brumata L. in the oak stands of forest-steppe. Contemp. Probl. Ecol. 2011, 4, 777–783. [Google Scholar] [CrossRef]
- Wada, N.; Murakami, M.; Yoshida, K. Effects of herbivore-bearing adult trees of the oak Quercus crispula on the survival of their seedlings. Ecol. Res. 2000, 15, 219–227. [Google Scholar] [CrossRef]
- Bochníček, O.; Hrušková, K.; Zvara, I. Klimatický atlas Slovenska/Climate Atlas of Slovakia, 1st ed.; Slovenský Hydrometeorologický ústav: Bratislava, Slovak, 2015; p. 131.
- Basset, Y.; Springate, N.D.; Aberlenc, H.P.; Delvare, G. A review of methods for sampling arthropods in tree canopies. In Canopy Arthropods, 1st ed.; Stork, N.E., Adis, J., Didham, R.K., Eds.; Chapman & Hall: London, UK, 1997; pp. 27–52. [Google Scholar]
- Lepidoptera and Their Ecology. Available online: www.pyrgus.de (accessed on 15 July 2016).
- Lepiforum: Bestimmung von Schmetterlingen (Lepidoptera) und Ihren Präimaginalstadien [Lepiforum: Identification of Butterflies and Moths (Lepidoptera) and Their Preimaginal Stages]. Available online: http://lepiforum.org/wiki (accessed on 10 July 2016).
- Pastorális, G.; Kalivoda, H.; Panigaj, Ľ. Zoznam motýľov (Lepidoptera) zistených na Slovensku. [Checklist of Lepidoptera recorded in Slovakia]. Folia Faun. Slov. 2013, 18, 101–232. Available online: http://www.ffs.sk/pdf/FFS-18-15-Pastoralis-et-al-2013.pdf (accessed on 28 April 2019).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Kruskal, J.B. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 1964, 29, 1–27. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 6 December 2019).
- Canty, A.; Ripley, B. Boot: Bootstrap R (S–Plus) Functions. R Package. Version 1.3-18. Available online: https://cran.r-project.org/web/packages/boot/index.html (accessed on 19 December 2019).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; p. 260. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; p. 498. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package. Version 2.5-4. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 December 2019).
- Kulfan, J. Zur Struktur und Saisondynamik von Raupenzönosen (Lepidoptera) an Eichen [On the structure and seasonal dynamics of the caterpillar communities (Lepidoptera) on oaks]. Biologia (Bratislava) 1992, 47, 653–661. (In German) [Google Scholar]
- Kulfan, M.; Holecová, M.; Fajčík, J. Caterpillar (Lepidoptera) communities on European Turkey oak (Quercus cerris) in Malé Karpaty Mts (SW Slovakia). Biologia 2006, 61, 573–578. [Google Scholar] [CrossRef]
- Ovcharov, D.; Dojchev, D.; Todorov, A. Differences in attack from leaf chewing pests (families Tortricidae and Geometridae, order Lepidoptera) by some Quercus species. In Lesotekhnicheski Universitet. Yubileen Sbornik Nauchni Dokladi: 75 Godini Visshe Lesotekhnichesko Obrazovanie v Blgariya. Sektsiya Ekologiya i Opazvane na Okolnata Sreda; University of Forestry: Sofia, Bulgaria, 2000; pp. 269–278. [Google Scholar]
- Gripenberg, S.; Morriën, E.; Cudmore, A.; Salminen, J.P.; Roslin, T. Resource selection by female moths in a heterogeneous environment: What is a poor girl to do? J. Anim. Ecol. 2007, 76, 854–865. [Google Scholar] [CrossRef]
- Holliday, N.J. Population ecology of winter moth (Operophtera brumata) on apple in relation to larval dispersal and time of bud burst. J. Appl. Ecol. 1977, 14, 803–813. [Google Scholar] [CrossRef]
- Graf, B.; Borer, F.; Höpli, H.U.; Hohn, H.; Dorn, S. The winter moth Operophtera brumata L. (Lep., Geometridae) on apple and cherry: Spatial and temporal aspects of recolonization in autumn. J. Appl. Entomol. 1995, 119, 295–301. [Google Scholar] [CrossRef]
- Tiberi, R.; Benassai, D.; Niccoli, A. Influence of different host plants on the biology and behaviour of the green oak leaf roller, Tortrix viridana L.: First results. IOBC/WPRS Bull. 2005, 28, 211–217. Available online: https://www.iobc-wprs.org/pub/bulletins/iobc-wprs_bulletin_2005_28_08.pdf#page=231 (accessed on 28 April 2019).
- Capinera, J.L.; Barbosa, P. Dispersal of first-instar gypsy moth larvae in relation to population quality. Oecologia 1976, 26, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.P.; Clarke, A.R.; Malcolm, S.B. Ecology and behavior of first instar larval Lepidoptera. Annu. Rev. Entomol. 2002, 47, 361–393. [Google Scholar] [CrossRef]
- Lance, D.; Barbosa, P. Host tree influences on the dispersal of first instar gypsy moth, Lymantria dispar. Oikos 1982, 38, 1–7. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Elkinton, J.S.; Wallner, W.E. Effect of burlap bands on between-tree movement of late-instar gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae). Environ. Entomol. 1986, 15, 373–379. [Google Scholar] [CrossRef]
- Doane, C.; Leonard, D. Orientation and dispersal of late-stage larvae of Porthetria dispar (Lepidoptera: Lymantriidae). Can. Entomol. 1975, 107, 1333–1338. [Google Scholar] [CrossRef]
- Humphrey, J.W.; Swaine, M.D. Factors affecting the natural regeneration of Quercus in Scottish oakwoods. II. Insect defoliation of trees and seedlings. J. Appl. Ecol. 1997, 34, 585–593. [Google Scholar] [CrossRef]
- Hajek, A. Larval behavior in Lymantria dispar increases risk of fungal infection. Oecologia 2001, 126, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Greeney, H.F.; Dyer, L.A.; Smilanich, A.M. Feeding by lepidopteran larvae is dangerous: A review of caterpillars’ chemical, physiological, morphological, and behavioral defenses against natural enemies. Invertebr. Surviv. J. 2012, 9, 7–34. Available online: https://www.isj.unimore.it/index.php/ISJ/article/view/256/171 (accessed on 28 April 2019).
- Pepi, A.A.; Broadley, H.J.; Elkinton, J.S. Density-dependent effects of larval dispersal mediated by host plant quality on populations of an invasive insect. Oecologia 2016, 182, 499–509. [Google Scholar] [CrossRef]
- Humphreys, R.K.; Ruxton, G.D. Dropping to escape: A review of an under-appreciated antipredator defence. Biol. Rev. 2019, 94, 575–589. [Google Scholar] [CrossRef]
- Wittman, J.T.; Aukema, B.H. Foliage type and deprivation alters the movement behavior of late instar European gypsy moth Lymantria dispar (Lepidoptera: Erebidae). J. Insect Behav. 2019, 32, 24–37. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Migration of terrestrial arthropods in relation to habitat. Biol. Rev. 1962, 37, 171–211. [Google Scholar] [CrossRef]
- Mitchell, R.G. Dispersal of early instars of the Douglas-fir tussock moth. Ann. Entomol. Soc. Am. 1979, 72, 291–297. [Google Scholar] [CrossRef]
- Tikkanen, O.-P.; Woodcock, B.; Watt, A.; Lock, K. Are polyphagous geometrid moths with flightless females adapted to budburst phenology of local host species? Oikos 2006, 112, 83–90. [Google Scholar] [CrossRef]
- van Asch, M.; Salis, L.; Holleman, L.J.M.; van Lith, B.; Visser, M.E. Evolutionary response of the egg hatching date of a herbivorous insect under climate change. Nat. Clim. Chang. 2013, 3, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Hagstrum, D.W.; Subramanyam, B. Immature insects: Ecological roles of mobility. Am. Entomol. 2010, 56, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Vindstad, O.P.L.; Jepsen, J.U.; Yoccoz, N.G.; Bjørnstad, O.N.; Mesquita, M.D.S.; Ims, R.A. Spatial synchrony in sub-arctic geometrid moth outbreaks reflects dispersal in larval and adult life cycle stages. J. Anim. Ecol. 2019, 88, 1134–1145. [Google Scholar] [CrossRef] [Green Version]
- Saunders, D.A.; Hobbs, R.J.; Margules, C.R. Biological consequences of ecosystem fragmentation: A review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Ozanne, C.M.P.; Speight, M.R.; Hambler, C.; Evans, H.F. Isolated trees and forest patches: Patterns in canopy arthropod abundance and diversity in Pinus sylvestris (Scots Pine). For. Ecol. Manag. 2000, 137, 53–63. [Google Scholar] [CrossRef]
- Maleque, M.A.; Ishii, H.T.; Maeto, K. The use of arthropods as indicators of ecosystem integrity in forest management. J. For. 2006, 104, 113–117. [Google Scholar] [CrossRef]
- Bernaschini, M.L.; Trumper, E.; Valladares, G.; Salvo, A. Are all edges equal? Microclimatic conditions, geographical orientation and biological implications in a fragmented forest. Agric. Ecosyst. Environ. 2019, 280, 142–151. [Google Scholar] [CrossRef]
- Fortin, M.; Mauffette, Y. Forest edge effects on the biological performance of the forest tent caterpillar (Lepidoptera: Lasiocampidae) in sugar maple stands. Ecoscience 2001, 8, 164–172. [Google Scholar] [CrossRef]
- Wirth, R.; Meyer, S.T.; Leal, I.R.; Tabarelli, M. Plant herbivore interactions at the forest edge. In Progress in Botany, 1st ed.; Lüttge, U., Beyschlag, W., Murata, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 69, pp. 423–448. [Google Scholar] [CrossRef]
- White, P.J.; McGill, B.J.; Lechowicz, M.J. Human-disturbance and caterpillars in managed forest fragments. Biodivers. Conserv. 2011, 20, 1745–1762. [Google Scholar] [CrossRef]
- Maldonado-López, Y.; Cuevas-Reyes, P.; Stone, G.N.; Nieves-Aldrey, J.L.; Oyama, K. Gall wasp community response to fragmentation of oak tree species: Importance of fragment size and isolated trees. Ecosphere 2015, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Macek, J.; Dvořák, J.; Traxler, L.; Červenka, V. Motýli a housenky střední Evropy. Noční motýli II.—Můrovití. [Butterflies and Caterpillars of Central Europe. Moths II.–Noctuidae], 1st ed.; Academia: Praha, Czech, 2008; p. 490. (In Czech) [Google Scholar]
- Hausmann, A.; Viidalepp, J. The Geometrid Moths of Europe, 1st ed.; Apollo Books: Stenstrup, Denmark, 2012; Volume 3, p. 743. [Google Scholar]
- Müller, B.; Erlacher, S.; Hausmann, A.; Rajaei, H.; Sihvonen, P.; Skou, P. The Geometrid Moths of Europe. Volume 6: Ennominae II, 1st ed.; E.J. Brill: Leiden, The Netherlands; Boston, MA, USA, 2019; p. 906. [Google Scholar]
- Mitchell, C.; Brennan, R.M.; Cross, J.V.; Johnson, S.N. Arthropod pests of currant and gooseberry crops in the UK: Their biology, management and future prospects. Agric. For. Entomol. 2011, 13, 221–237. [Google Scholar] [CrossRef]
- Stancă-Moise, C. A study about the pest insects in the apple trees orchards, with local sorts, specific to Sibiel village (Sibiu county), in the conditions of the years 2015–2016. Sci. Pap. Ser. Manag. Econom. Eng. Agric. Rural Dev. 2017, 17, 379–383. [Google Scholar]
- Offenberg, J.; Nielsen, J.S.; Damgaard, C. Wood ant (Formica polyctena) services and disservices in a Danish apple plantation. Sociobiology 2019, 66, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Samnegård, U.; Alins, G.; Boreux, V.; Bosch, J.; García, D.; Happe, A.K.; Klein, A.-M.; Miñarro, M.; Mody, K.; Porcel, M.; et al. Management trade-offs on ecosystem services in apple orchards across Europe: Direct and indirect effects of organic production. J. Appl. Ecol. 2019, 56, 802–811. [Google Scholar] [CrossRef] [Green Version]
- Adamska, I.; Dziegielewska, M. Colonization of selected rose varieties by pests and pathogens. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2016, 330, 7–17. [Google Scholar] [CrossRef]
- Dzięgielewska, M.; Adamska, I. The health of the forest stand along urban routes of different traffic intensity in Szczecin. Prog. Plant Prot. 2016, 56, 191–198. (In Polish) [Google Scholar]
- White, J.A.; Whitham, T.G. Associational susceptibility of cottonwood to a box elder herbivore. Ecology 2000, 81, 1795–1803. [Google Scholar] [CrossRef]
- Plath, M.; Dorn, S.; Riedel, J.; Barrios, H.; Mody, K. Associational resistance and associational susceptibility: Specialist herbivores show contrasting responses to tree stand diversification. Oecologia 2012, 169, 477–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, G.; Steiner, A.; Trusch, R. Ennominae (Geometridae). In Die Schmetterlinge Baden-Württembergs, 1st ed.; Ebert, G., Ed.; Verlag Eugen Ulmer: Stuttgart, Germany, 2003; Volume 9—Nachtfalter VII, pp. 294–579. [Google Scholar]
- Szabó, S.; Árnyas, E.M.; Tóthmérész, B.; Varga, Z. Long-term light trap study on the macro-moth (Lepidoptera: Macroheterocera) fauna of the Aggtelek National Park. Acta Zool. Acad. Sci. Hung. 2007, 53, 257–269. [Google Scholar]
- Kulfan, J.; Sarvašová, L.; Parák, M.; Zach, P. Effects of a host tree on movement and distribution of winter geometrid moths (Lepidoptera): Thickness of trunks and branches. Folia Oecol. 2019, 46, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Shutt, J.D.; Burgess, M.D.; Phillimore, A.B. A spatial perspective on the phenological distribution of the spring woodland caterpillar peak. Am. Nat. 2019, 194, E109–E121. [Google Scholar] [CrossRef]
- Bussotti, F.; Borghini, F.; Celesti, C.; Leonzio, C.; Bruschi, P. Leaf morphology and macronutrients in broadleaved trees in central Italy. Trees 2000, 14, 361–368. [Google Scholar] [CrossRef]
- Velichkova Wolkerstorfer, S.; Wonisch, A.; Stankova, T.; Tsvetkova, N.; Tausz, M. Seasonal variations of gas exchange, photosynthetic pigments, and antioxidants in Turkey oak (Quercus cerris L.) and Hungarian oak (Quercus frainetto Ten.) of different age. Trees 2011, 25, 1043–1052. [Google Scholar] [CrossRef]
- Neuvonen, S.; Haukioja, E.; Molarius, A. Delayed inducible resistance against a leaf-chewing insect in four deciduous tree species. Oecologia 1987, 74, 363–369. [Google Scholar] [CrossRef]
- Zúbrik, M.; Kunca, A.; Kulfan, J.; Rell, S.; Nikolov, C.; Galko, J.; Vakula, J.; Gubka, A.; Leontovyč, R.; Konôpka, B.; et al. Occurrence of gypsy moth (Lymantria dispar L.) in the Slovak Republic and its outbreaks during 1945–2020. Cent. Eur. For. J. 2021, 67, 55–71. [Google Scholar] [CrossRef]
- Visser, M.E.; Holleman, L.J. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. B 2001, 268, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Marçais, B.; Desprez-Loustau, M.L. European oak powdery mildew: Impact on trees, effects of environmental factors, and potential effects of climate change. Ann. For. Sci. 2014, 71, 633–642. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarvašová, L.; Zach, P.; Parák, M.; Saniga, M.; Kulfan, J. Infestation of Early- and Late-Flushing Trees by Spring Caterpillars: An Associational Effect of Neighbouring Trees. Forests 2021, 12, 1281. https://doi.org/10.3390/f12091281
Sarvašová L, Zach P, Parák M, Saniga M, Kulfan J. Infestation of Early- and Late-Flushing Trees by Spring Caterpillars: An Associational Effect of Neighbouring Trees. Forests. 2021; 12(9):1281. https://doi.org/10.3390/f12091281
Chicago/Turabian StyleSarvašová, Lenka, Peter Zach, Michal Parák, Miroslav Saniga, and Ján Kulfan. 2021. "Infestation of Early- and Late-Flushing Trees by Spring Caterpillars: An Associational Effect of Neighbouring Trees" Forests 12, no. 9: 1281. https://doi.org/10.3390/f12091281
APA StyleSarvašová, L., Zach, P., Parák, M., Saniga, M., & Kulfan, J. (2021). Infestation of Early- and Late-Flushing Trees by Spring Caterpillars: An Associational Effect of Neighbouring Trees. Forests, 12(9), 1281. https://doi.org/10.3390/f12091281





