The Scientific Basis of the Target Plant Concept: An Overview
Abstract
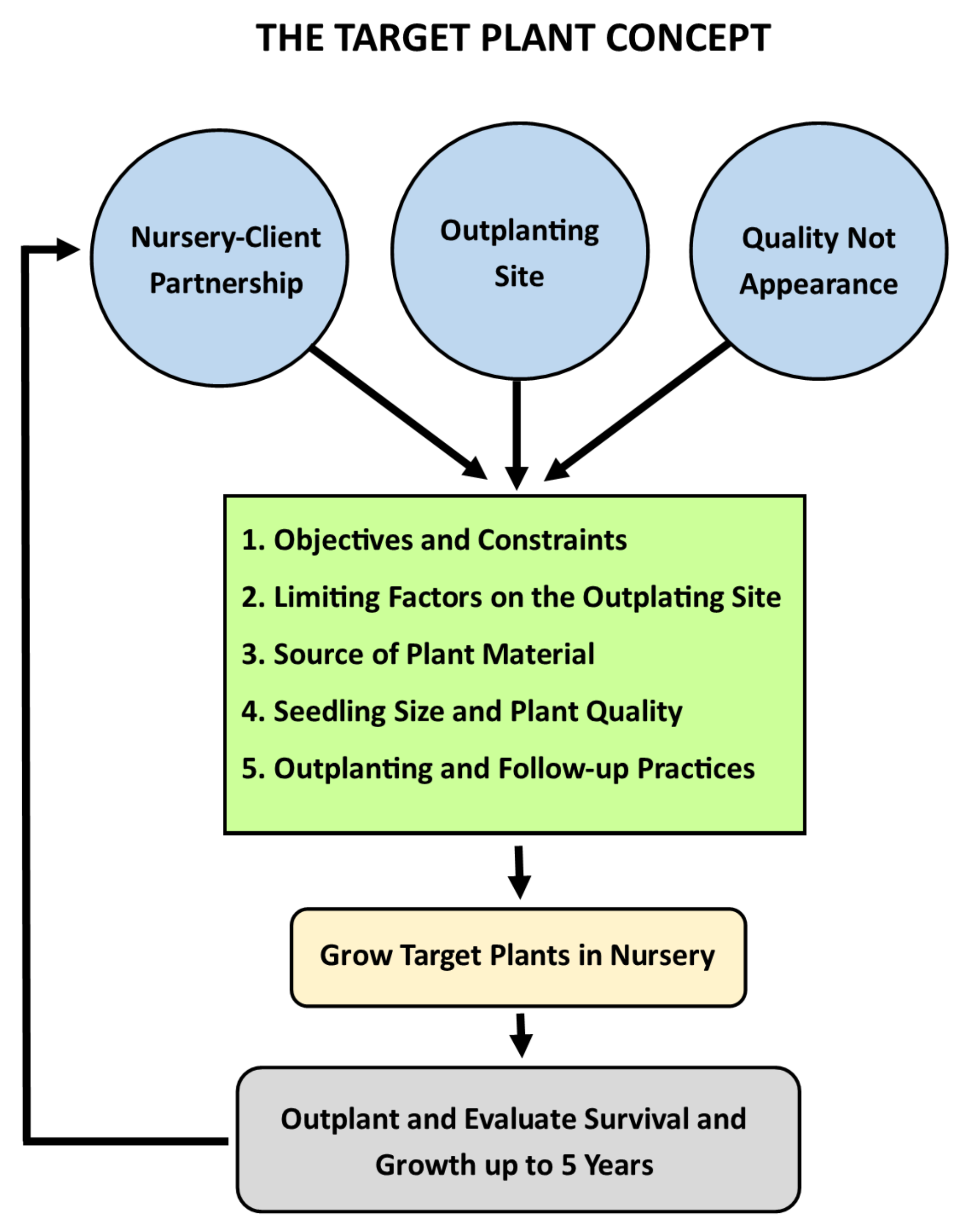
:1. Introduction
Foresters have complained for years about poor seedling survival and growth, often with little understanding of why a specific reforestation effort failed. In some cases, it was stock of inherently low quality due to poor nursery cultural practices, or seedling storage and handling conditions. In other cases, the stock was in top notch shape, but inappropriate for specific site conditions, such as dense competing vegetation, early fall frosts, or high soil temperatures. Sometimes it was all of the above! In trying to solve this problem we too often tried to compartmentalize it and fix each piece... one at a time, often unsuccessfully.
2. The Target Plant Concept as a Holistic Framework
2.1. Identifying Program Objectives and Constraints
2.2. Limiting Factors on the Outplanting Site
2.3. Managing Genetic Resources
2.4. Seedling Size and Quality
2.5. Post-Nursery Practices
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Rose, R.; Carlson, W.C.; Morgan, P. The target seedling concept. In General Technical Report RM-200, Proceedings of the Western Forest Nursery Association, Roseburg, OR, USA, 13–17 August 1990; Rose, R., Campbell, S.J., Landis, T.D., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1990; 8p. [Google Scholar]
- Landis, T.D. The target plant concept-a history and brief overview. In RMRS-P-65, Proceedings of the Forest and Conservation Nursery Association, 2010; Riley, L.E., Haase, D.L., Pinto, J.R., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 61–66. [Google Scholar]
- Dumroese, K.R.; Landis, T.D.; Pinto, J.R.; Haase, D.L.; Wilkinson, K.W.; Davis, A.S. Meeting forest restoration challenges: Using the target plant concept. Reforesta 2016, 1, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Haase, D.L.; Davis, A.S. Developing and supporting quality nursery facilities and staff are necessary to meet global forest and landscape restoration needs. Reforesta 2017, 4, 69–93. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.N.; Freer-Smith, P.; Madsen, P. Production, restoration, mitigation: A new generation of plantations. New For. 2019, 50, 153–168. [Google Scholar] [CrossRef]
- Mexal, J.G.; Landis, T.D. Target seedling concepts: Height and diameter. In General Technical Report RM-200, Proceedings of the Western Forest Nursery Association, Roseburg, OR, USA, 13–17 August 1990; Rose, R., Campbell, S.J., Landis, T.D., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1990; 19p. [Google Scholar]
- Pinto, J.R. Morphology targets: What do seedling morphological attributes tell us? In RMRS-P-65, Proceedings of the Forest and Conservation Nursery Association, 2010; Riley, L.E., Haase, D.L., Pinto, J.R., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; pp. 74–79. [Google Scholar]
- Ritchie, G.A.; Tanaka, Y. Root growth potential and the target seedling. In General Technical Report RM-200, Proceedings of the Western Forest Nursery Association, Roseburg, OR, USA, 13–17 August 1990; Rose, R., Campbell, S.J., Landis, T.D., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1990; 15p. [Google Scholar]
- Davis, A.S.; Jacobs, D.F. Quantifying root system quality of nursery seedlings and relationship to outplanting performance. New For. 2005, 30, 295–311. [Google Scholar] [CrossRef]
- Van den Driessche, R. Nursery growth of conifer seedlings using fertilizers of different solubilities and application time, and their forest growth. Can. J. For. Res. 1988, 18, 172–180. [Google Scholar] [CrossRef]
- Carlson, W.C.; Miller, D.E. Target Seedling Root System Size, Hydraulic Conductivity, and Water Use During Seedling Establishment. In General Technical Report RM-200, Proceedings of the Western Forest Nursery Association, Roseburg, OR, USA, 13–17 August 1990; Rose, R., Campbell, S.J., Landis, T.D., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1990; pp. 53–65. [Google Scholar]
- Sperry, J.S.; Adler, F.; Campbell, G.S.; Comstock, J.P. Limitation of plant water use by rhizosphere and xylem conductance: Results from a model. Plant. Cell Environ. 1998, 21, 347–359. [Google Scholar] [CrossRef]
- Davis, A.S.; Jacobs, D.F.; Dumroese, R.K. Ch 15-Challenging a paradigm: Toward integrating indigenous species into tropical plantation forestry. In Forest Landscape Restoration; Stanturf, J., Lamb, D., Madsen, P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 293–308. [Google Scholar]
- Sarkissian, A.J.; Brook, R.M.; Talhouk, S.N.; Hockley, N. Using stakeholder preferences to select native tree species for reforestation in Lebanon. New For. 2018, 49, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Dumroese, R.K.; Balloffet, N.; Crockett, J.W.; Stanturf, J.A.; Nave, L.E. A national approach to leverage the benefits of tree planting on public lands. New For. 2019, 50, 1–9. [Google Scholar] [CrossRef]
- Pinto, J.R.; Davis, A.S.; Leary, J.J.; Aghai, M.M. Stocktype and grass suppression accelerate the restoration trajectory of Acacia koa in Hawaiian montane ecosystems. New For. 2015, 46, 855–867. [Google Scholar] [CrossRef]
- Acuff, A.A. Seedling Performance Metrics: A Standardized Monitoring Approach. Tree Plant. Notes 2019, 62, 155–160. [Google Scholar]
- Jokela, E.J.; Martin, T.A.; Vogel, J.G. Twenty-five years of intensive forest management with southern pines: Important lessons learned. J. For. 2010, 108, 338–347. [Google Scholar]
- St. Clair, B.J.; Howe, G.T. Genetic maladaptation of coastal Douglas-fir seedlings to future climates. Glob. Chang. Biol. 2007, 13, 1441–1454. [Google Scholar] [CrossRef]
- Dudley, N.S.; Jones, T.C.; James, R.L.; Sniezko, R.A.; Cannon, P.; Borthakur, D. Applied disease screening and selection program for resistance to vascular wilt in Hawaiian Acacia koa. South. For. 2015, 77, 65–73. [Google Scholar] [CrossRef]
- Löf, M.; Madsen, P.; Metslaid, M.; Witzell, J.; Jacobs, D.F. Restoring forests: Regeneration and ecosystem function for the future. New For. 2019, 50, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Stanturf, J.A.; Schoenholtz, S.H.; Schweitzer, C.J.; Shepard, J.P. Achieving restoration success: Myths in bottomland hardwood forests. Restor. Ecol. 2001, 9, 189–200. [Google Scholar] [CrossRef]
- Cernansky, R. How to rebuild a forest. Nature 2018, 560, 542–544. [Google Scholar] [CrossRef]
- Collins, S.; Stritch, L.E. Caring for our natural assets: An ecosystem services perspective. In General Technical Report GTR-PNW-733, Proceedings of the National Silviculture Workshop, Portland, OR, USA, 2007; Deal, R., Ed.; USDA Forest Service, Pacific Northwest Research Station: Portland, OR, USA; pp. 1–11.
- Johnson, R.; Stritch, L.; Olwell, P.; Lambert, S.; Horning, M.E.; Cronn, R. What are the best seed sources for ecosystem restoration on BLM and USFS lands? Nativ. Plants J. 2010, 11, 117–131. [Google Scholar] [CrossRef]
- Uprety, Y.; Asselin, H.; Bergeron, Y.; Doyon, F.; Boucher, J.F. Contribution of traditional knowledge to ecological restoration: Practices and applications. Ecoscience 2012, 19, 225–237. [Google Scholar] [CrossRef]
- Kimmerer, R.W. Native knowledge for native ecosystems. J. For. 2000, 98, 4–9. [Google Scholar]
- Huntington, H.P. Using traditional ecological knowledge in science: Methods and applications. Ecol. App. 2000, 10, 1270–1274. [Google Scholar] [CrossRef]
- Shebitz, D.J.; Reichard, S.H.; Dunwiddie, P.W. Ecological and cultural significance of burning beargrass habitat on the Olympic Peninsula, Washington. Ecol. Res. 2009, 27, 306–319. [Google Scholar] [CrossRef]
- Marks-Block, T.; Lake, F.K.; Curran, L.M. Effects of understory fire management treatments on California Hazelnut, an ecocultural resource of the Karuk and Yurok Indians in the Pacific Northwest. For. Ecol. Manag. 2019, 450, 117517. [Google Scholar] [CrossRef]
- Riikonen, J.; Luoranen, J. Seedling Production and the Field Performance of Seedlings. Forests 2018, 9, 740. [Google Scholar] [CrossRef] [Green Version]
- Wan, F.; Ross-Davis, A.L.; Shi, W.; Weston, C.; Song, X.; Chang, X.; Davis, A.S.; Liu, Y.; Teng, F. Subirrigation Effects on Larch Seedling Growth, Root Morphology, and Media Chemistry. Forests 2019, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Hubbel, K.; Ross-Davis, A.; Pinto, J.; Burney, O.; Davis, A. Toward Sustainable Cultivation of Pinus occidentalis Swartz in Haiti: Effects of Alternative Growing Media and Containers on Seedling Growth and Foliar Chemistry. Forests 2018, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Hedges, S.B.; Cohen, W.B.; Timyan, J.; Yang, Z. Haiti’s biodiversity threatened by nearly complete loss of primary forest. Proc. Natl. Acad. Sci. USA 2018, 115, 11850–11855. [Google Scholar] [CrossRef] [Green Version]
- Dregne, H.E. Desertification of Arid Lands; Harwood Academic Publishers: New York, NY, USA, 1983; Volume 3, 242p. [Google Scholar]
- Mabbutt, J.A. Climate change: Some likely multiple impacts in Southern Africa. Food Policy 1994, 19, 165–191. [Google Scholar]
- Svejcar, L.N.; Kildisheva, O.A. The age of restoration: Challenges presented by dryland systems. Plant Ecol. 2017, 218, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kolb, P.F.; Robberecht, R. High temperature and drought stress effects on survival of Pinus ponderosa seedlings. Tree Phys. 1996, 16, 665–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, J.R.; Marshall, J.D.; Dumroese, R.K.; Davis, A.S.; Cobos, D.R. Photosynthetic response, carbon isotopic composition, survival, and growth of three stock types under water stress enhanced by vegetative competition. Can. J. For. Res. 2012, 42, 333–344. [Google Scholar] [CrossRef]
- Thyroff, E.C.; Burney, O.T.; Jacobs, D.F. Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration. Forests 2019, 10, 950. [Google Scholar] [CrossRef] [Green Version]
- Burdett, A.N. Physiological processes in plantation establishment and the development of specifications for forest planting stock. Can. J. For. Res. 1990, 20, 415–427. [Google Scholar] [CrossRef]
- Valdecantos, A.; Cortina, J.; Vallejo, V.R. Nutrient status and field performance of tree seedlings planted in Mediterranean degraded areas. Ann. For. Sci. 2006, 63, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Kinloch, B.B., Jr. White pine blister rust in North America: Past and prognosis. Phytopathology 2003, 93, 1044–1047. [Google Scholar] [CrossRef] [Green Version]
- Örlander, G.; Nordlander, G. Effects of field vegetation control on pine weevil (Hylobius abietis) damage to newly planted Norway spruce seedlings. Ann. For. Sci. 2003, 60, 667–671. [Google Scholar] [CrossRef] [Green Version]
- Lopushinsky, W.; Max, T.A. Effect of soil temperature on root and shoot growth and on budburst timing in conifer seedling transplants. New For. 1990, 4, 107–124. [Google Scholar] [CrossRef]
- Habte, M. Impact of simulated erosion on the abundance and activity of indigenous vesicular-arbuscular mycorrhizal endophytes in an Oxisol. Biol. Fertil. Soils 1989, 7, 164–167. [Google Scholar] [CrossRef]
- Chirino, E.; Vilagrosa, A.; Hernández, E.I.; Matos, A.; Vallejo, V.R. Effects of a deep container on morpho-functional characteristics and root colonization in Quercus suber L. seedlings for reforestation in Mediterranean climate. For. Ecol. Manag. 2008, 256, 779–785. [Google Scholar] [CrossRef]
- St. Clair, B.J.; Johnson, R. Structure of genetic variation and implications for the management of seed and planting stock. In RMRS-P-33, Proceedings of the Forest and Conservation Nursery Associations—2003; Riley, L.E., Dumroese, R.K., Landis, T.D., Eds.; Technical Coordinators, USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2003; pp. 64–71. [Google Scholar]
- St. Clair, J.B.; Kilkenny, F.F.; Johnson, R.C.; Shaw, N.L.; Weaver, G. Genetic variation in adaptive traits and seed transfer zones for Pseudoroegneria spicata (bluebunch wheat-grass) in the northwestern United States. Evol. Appl. 2013, 6, 933–948. [Google Scholar] [CrossRef]
- Richardson, B.A.; Chaney, L. Climate-based seed transfer of a widespread shrub: Population shifts, restoration strategies, and the trailing edge. Ecol. Appl. 2018, 28, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Dudley, N.; Jones, T.; Gerber, K.; Ross-Davis, A.L.; Sniezko, R.A.; Cannon, P.; Dobbs, J. Establishment of a Genetically Diverse, Disease-Resistant Acacia koa A. Gray Seed Orchard in Kokee, Kauai: Early Growth, Form, and Survival. Forests 2020, 11, 1276. [Google Scholar] [CrossRef]
- Bower, A.D.; St. Clair, J.B.; Ericson, V. Generalized provisional seed zones for native plants. Ecol. App. 2014, 24, 913–919. [Google Scholar] [CrossRef]
- Seedlot Selection Tool. Available online: https://seedlotselectiontool.org/sst/ (accessed on 20 August 2019).
- Climate Smart Restoration Tool. Available online: https://climaterestorationtool.org/csrt/. (accessed on 24 August 2019).
- Basey, A.C.; Fant, J.B.; Kramer, A.T. Producing native plant materials for restoration: 10 rules to collect and maintain genetic diversity. Nativ. Plants J. 2015, 16, 37–53. [Google Scholar] [CrossRef]
- Breed, M.F.; Stead, M.G.; Ottewell, K.M.; Gardner, M.G.; Lowe, A.J. Which provenance and where? Seed sourcing strategies for revegetation in a changing environment. Conserv. Genet. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Havens, K.; Vitt, P.; Still, S.; Kramer, A.T.; Fant, J.B.; Schatz, K. Seed sourcing for restoration in an era of climate change. Nat. Areas J. 2015, 35, 122–133. [Google Scholar] [CrossRef]
- Kramer, A.T.; Crane, B.; Downing, J.; Hamrick, J.L.; Havens, K.; Highland, A.; Jacobi, S.K.; Kaye, T.M.; Lonsdorf, E.V.; Neale, J.R.; et al. Sourcing native plants to support ecosystem function in different planting contexts. Restor. Ecol. 2019, 27, 470–476. [Google Scholar] [CrossRef]
- Meissen, J.C.; Galatowitsch, S.M.; Cornett, M.W. Risks of overharvesting seed from native tallgrass prairies. Restor. Ecol. 2015, 23, 882–891. [Google Scholar] [CrossRef]
- Nevill, P.G.; Cross, A.T.; Dixon, K.W. Ethical seed sourcing is a key issue in meeting global restoration targets. Curr. Biol. 2018, 28, R1378–R1379. [Google Scholar] [CrossRef] [Green Version]
- Rantala-Sykes, B.; Campbell, D. Should I pick that? A scoring tool to prioritize and valuate native wild seed for restoration: Scoring wild seed collection effort. Restor. Ecol. 2018, 27, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Pedrini, S.; Gibson-Roy, P.; Trivedi, C.; Gálvez-Ramírez, C.; Hardwick, K.; Shaw, N.; Frischie, S.; Laverack, G.; Dixon, K. Collection and production of native seeds for ecological restoration. Restor. Ecol. 2020, 28, S228–S238. [Google Scholar] [CrossRef]
- Williams, M.I.; Dumroese, R.K. Preparing for climate change: Forestry and assisted migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Sniezko, R.A.; Smith, J.; Liu, J.J.; Hamelin, R.C. Genetic resistance to fusiform rust in southern pines and white pine blister rust in white pines—A contrasting tale of two rust pathosystems—Current status and future prospects. Forests 2014, 5, 2050–2083. [Google Scholar] [CrossRef]
- Woodcock, P.; Marzano, M.; Quine, C. Key lessons from resistant tree breeding programmes in the Northern Hemisphere. Ann. For. Sci. 2019, 76, 1–16. [Google Scholar] [CrossRef]
- Moran, E.; Lauder, J.; Musser, C.; Stathos, A.; Shu, M. The genetics of drought tolerance in conifers. New Phytol. 2017, 216, 1034–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, S.H.; Boerjan, W.; Chiang, V.; Costanza, A.; Coleman, H.; Davis, J.M.; Lu, Me.; Mansfield, S.D.; Merkle, S.; Myburg, A.; et al. Certification for gene-edited forests. Science 2019, 365, 767–768. [Google Scholar]
- Jacobs, D.F.; Davis, A.S.; Dumroese, R.K.; Burney, O.T. Nursery Cultural Techniques Facilitate Restoration of Acacia koa Competing with Invasive Grass in a Dry Tropical Forest. Forests 2020, 11, 1124. [Google Scholar] [CrossRef]
- Haase, D.L.; Bouzza, K.; Emerton, L.; Friday, J.B.; Lieberg, B.; Aldrete, A.; Davis, A.S. The High Cost of the Low-Cost Polybag System: A Review of Nursery Seedling Production Systems. Land 2021, 10, 826. [Google Scholar] [CrossRef]
- Pinto, J.R.; Dumroese, R.K.; Davis, A.S.; Landis, T.D. Conducting seedling stock type trials: A new approach to an old question. J. For. 2011, 109, 293–299. [Google Scholar]
- Amidon, T.E.; Barnett, J.P.; Gallagher, H.P.; McGilvray, J.M. A field test of containerized seedlings under drought conditions. In General Technical Report SO-37, Proceedings of the Southern Containerized Forest Tree Seedling Conference, New Orleans, LA, USA; Guilin, R.W., Barnett, J.P., Eds.; USDA Forest Service, Southern Forest Experiment Station: New Orleans, LA, USA, 1982; pp. 139–144. [Google Scholar]
- Rose, R.; Haase, D.L.; Kroiher, F.; Sabin, T. Root volume and growth of ponderosa pine and Douglas-fir seedlings: A summary of eight growing seasons. West. J. Appl. For. 1997, 12, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Overton, W.S.; Ching, K.K. Analysis of differences in height growth among populations in a nursery selection study of Douglas-fir. For. Sci. 1978, 24, 497–509. [Google Scholar]
- Newton, M.; Cole, E.C.; White, D.E. Tall planting stock for enhanced growth and domination of brush in the Douglas-fir region. New For. 1993, 7, 107–121. [Google Scholar] [CrossRef]
- Thiffault, N.; Jobidon, R.; Munson, A.D. Performance and physiology of large containerized and bare-root spruce seedlings in relation to scarification and competition in Québec (Canada). Ann. For. Sci. 2003, 60, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Simpson, D.G.; Thompson, C.F.; Sutherland, C.D. Field performance potential of interior spruce seedlings: Effects of stress treatments and prediction by root growth potential and needle conductance. Can. J. For. Res. 1994, 24, 576–586. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Growth and nutritional response of hardwood seedlings to controlled-release fertilization at outplanting. For. Ecol. Manag. 2005, 214, 28–39. [Google Scholar] [CrossRef]
- Pinto, J.R.; Marshall, J.D.; Dumroese, R.K.; Davis, A.S.; Cobos, D.R. Establishment and growth of container seedlings for reforestation: A function of stocktype and edaphic conditions. For. Ecol. Manag. 2011, 261, 1876–1884. [Google Scholar] [CrossRef]
- Park, B.B.; Han, S.H.; Hernandez, J.O.; An, J.Y.; Nyam-Osor, B.; Jung, M.H.; Lee, P.S.-H.; Lee, S.I. The Use of Deep Container and Heterogeneous Substrate as Potentially Effective Nursery Practice to Produce Good Quality Nodal Seedlings of Populus sibirica Tausch. Forests 2021, 12, 418. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Seedling quality: History, application, and plant attributes. Forests 2018, 9, 283. [Google Scholar] [CrossRef] [Green Version]
- Van den Driessche, R. Influence of container nursery regimes on drought resistance of seedlings following planting. I. Survival and growth. Can. J. For. Res. 1991, 21, 555–565. [Google Scholar] [CrossRef]
- Landis, T.D.; Tinus, R.W.; McDonald, S.E.; Barnett, J.P. Atmospheric Environment, Volume 3. In The Container Tree Nursery Manual. Agricultural Handbook; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1992; 145p. [Google Scholar]
- Reely, J.A.; Nelson, A.S. Root Growth Potential and Microsite Effects on Conifer Seedling Establishment in Northern Idaho. Forests 2021, 12, 597. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Aghai, M.M.; Bouazza, K.; Davis, A.S. Improving restoration success through research-driven initiatives: Case studies targeting Pinus pinea reforestation stock development in Lebanon. Plant Ecol. 2017, 218, 39–53. [Google Scholar] [CrossRef]
- Tigabu, M.; Daneshvar, A.; Jingjing, R.; Wu, P.; Ma, X.; Odén, P.C. Multivariate Discriminant Analysis of Single Seed Near Infrared Spectra for Sorting Dead-Filled and Viable Seeds of Three Pine Species: Does One Model Fit All Species? Forests 2019, 10, 469. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Liu, Y.; Liu, H.; Dumroese, R.K. Interaction of Biochar Type and Rhizobia Inoculation Increases the Growth and Biological Nitrogen Fixation of Robinia pseudoacacia Seedlings. Forests 2020, 11, 711. [Google Scholar] [CrossRef]
- Mariotti, B.; Martini, S.; Raddi, S.; Tani, A.; Jacobs, D.F.; Oliet, J.A.; Maltoni, A. Coconut Coir as a Sustainable Nursery Growing Media for Seedling Production of the Ecologically Diverse Quercus Species. Forests 2020, 11, 522. [Google Scholar] [CrossRef]
- Apostol, K.G.; Dumroese, R.K.; Pinto, J.R.; Davis, A.S. Response of conifer species from three latitudinal populations to light spectra generated by light-emitting diodes and high-pressure sodium lamps. Can. J. For. Res. 2015, 45, 1711–1719. [Google Scholar] [CrossRef]
- Sloan, J.L.; Burney, O.T.; Pinto, J.R. Drought-conditioning of quaking aspen (Populus tremuloides Michx.) seedlings during nursery production modifies seedling anatomy and physiology. Front. Plant Sci. 2020, 11, 1325. [Google Scholar] [CrossRef] [PubMed]
- McKay, H.M. A review of the effect of stresses between lifting and planting on nursery stock quality and performance. New For. 1997, 13, 369–399. [Google Scholar] [CrossRef]
- McKay, H.M.; Gardiner, B.A.; Mason, W.L.; Nelson, D.G.; Hollingsworth, M.K. The gravitational forces generated by dropping plants and the response of Sitka spruce seedlings to dropping. Can. J. For. Res. 1993, 23, 2443–2451. [Google Scholar] [CrossRef]
- Landis, T.D.; Dumroese, R.K.; Haase, D.L. Seedling Processing, Storage, and Outplanting, Volume 7. In The Container Tree Nursery Manual. Agricultural Handbook; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2010; 200p. [Google Scholar]
- Landis, T.D.; Tinus, R.W.; Barnett, J.P. Seedling Propagation, Volume 6. In The Container Tree Nursery Manual. Agricultural Handbook; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1999; 166p. [Google Scholar]
- Luoranen, J.; Rikala, R.; Konttinen, K.; Smolander, H. Extending the planting period of dormant and growing Norway spruce container seedlings to early summer. Silva. Fenn. 2005, 39, 481–496. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.; Blanton, S.; Bielech, J.P. Summer planting performance of white spruce 1+ 0 container seedlings affected by nursery short-day treatment. New For. 2008, 35, 187–205. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; South, D.B. Fall acclimation and the lift/store pathway: Effect on reforestation. Open For. Sci. J. 2014, 7, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.; McNassar, B.; Kildisheva, O.; Davis, A. Stocktype and Vegetative Competition Influences on Pseudotsuga menziesii and Larix occidentalis Seedling Establishment. Forests 2018, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Cogliastro, A.; Paquette, A. Thinning effect on light regime and growth of underplanted red oak and black cherry in post-agricultural forests of south-eastern Canada. New For. 2012, 43, 941–954. [Google Scholar] [CrossRef]
- Devine, W.D.; Harrington, C.A.; Leonard, L.P. Post-planting treatments increase growth of Oregon white oak (Quercus garryana Doug. ex Hook.) seedlings. Restor. Ecol. 2007, 15, 212–222. [Google Scholar] [CrossRef]
- Kloetzel, S. Revegetation and restoration planting tools: An in-the-field perspective. Nativ. Plants J. 2004, 5, 34–42. [Google Scholar] [CrossRef]
- Bulmer, C.E.; Simpson, D.G. Soil Compaction Reduced the Growth of Lodgepole Pine and Douglas-fir Seedlings in Raised Beds after Two Growing Seasons. Soil Sci. Soc. Am. J. 2010, 74, 2162–2174. [Google Scholar] [CrossRef]
- Taylor, T.S.; Loewenstein, E.F.; Chappelka, A.H. Effect of animal browse protection and fertilizer application on the establishment of planted Nuttall oak seedlings. New For. 2006, 32, 133–143. [Google Scholar] [CrossRef]
- Hackworth, Z.; Lhotka, J.; Cox, J.; Barton, C.; Springer, M. First-Year Vitality of Reforestation Plantings in Response to Herbivore Exclusion on Reclaimed Appalachian Surface-Mined Land. Forests 2018, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Barrere, J.; Petersson, L.K.; Boulanger, V.; Collet, C.; Felton, A.M.; Löf, M.; Saïd, S. Canopy openness and exclusion of wild ungulates act synergistically to improve oak natural regeneration. For. Ecol. Manag. 2021, 487, 118976. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Steinbeck, K. Tree shelters improve the survival and growth of planted Engelmann spruce seedlings in southwestern Colorado. West. J. Appl. For. 2001, 16, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Oliet, J.A.; Artero, F.; Cuadros, S.; Puértolas, J.; Luna, L.; Grau, J.M. Deep planting with shelters improves performance of different stocktype sizes under arid Mediterranean conditions. New For. 2012, 43, 925–939. [Google Scholar] [CrossRef]
- Oliet, J.A.; Blasco, R.; Valenzuela, P.; de Blas, M.M.; Puértolas, J. Should we use meshes or solid tube shelters when planting in Mediterranean semiarid environments? New For. 2019, 50, 267–282. [Google Scholar] [CrossRef]
- Ward, J.S.; Gent, M.P.; Stephens, G.R. Effects of planting stock quality and browse protection-type on height growth of northern red oak and eastern white pine. For. Ecol. Manag. 2000, 127, 205–216. [Google Scholar] [CrossRef]
- Helgerson, O.T. Heat damage in tree seedlings and its prevention. New For. 1989, 3, 333–358. [Google Scholar] [CrossRef]
- Scowcroft, P.G.; Haraguchi, J.E.; Fujii, D.M. Understory structure in a 23-year-old Acacia koa forest and 2-year growth responses to silvicultural treatments. For. Ecol. Manag. 2008, 255, 1604–1617. [Google Scholar] [CrossRef]
- Ayala-Jacobo, L.M.; Woeste, K.E.; Jacobs, D.F. Cold acclimation increases freeze tolerance in Acacia koa, a tropical tree species occurring over a wide elevational gradient. Forests 2021, 12, 1089. [Google Scholar] [CrossRef]
- Jiménez, M.N.; Fernández-Ondoño, E.; Ripoll, M.A.; Navarro, F.B.; Gallego, E.; De Simón, E.; Lallena, A.M. Influence of different post-planting treatments on the development in Holm oak afforestation. Trees 2007, 21, 443–455. [Google Scholar] [CrossRef]
- Cuesta, B.; Benayas, J.R.; Gallardo, A.; Villar-Salvador, P.; González-Espinosa, M. Soil chemical properties in abandoned Mediterranean cropland after succession and oak reforestation. Acta Oecol. 2012, 38, 58–65. [Google Scholar] [CrossRef]
- Sloan, J.L.; Jacobs, D.F. Fertilization at planting influences seedling growth and vegetative competition on a post-mining boreal reclamation site. New For. 2013, 44, 687–701. [Google Scholar] [CrossRef]
- Rose, K.M.E.; Baribault, T.W.; Jacobs, D.F. Alternative field fertilization techniques to promote restoration of leguminous Acacia koa on contrasting tropical sites. For. Ecol. Manag. 2016, 376, 126–134. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, A.S.; Pinto, J.R. The Scientific Basis of the Target Plant Concept: An Overview. Forests 2021, 12, 1293. https://doi.org/10.3390/f12091293
Davis AS, Pinto JR. The Scientific Basis of the Target Plant Concept: An Overview. Forests. 2021; 12(9):1293. https://doi.org/10.3390/f12091293
Chicago/Turabian StyleDavis, Anthony S., and Jeremiah R. Pinto. 2021. "The Scientific Basis of the Target Plant Concept: An Overview" Forests 12, no. 9: 1293. https://doi.org/10.3390/f12091293
APA StyleDavis, A. S., & Pinto, J. R. (2021). The Scientific Basis of the Target Plant Concept: An Overview. Forests, 12(9), 1293. https://doi.org/10.3390/f12091293





